Application of the Thermodynamic Cycle to Assess the Energy Efficiency of Amine-Based Absorption of Carbon Capture
Abstract
:1. Introduction
2. Methodology
2.1. Framework of Thermodynamic Research
2.2. Thermodynamic Cycle Construction
2.2.1. Thermodynamic Properties
2.2.2. Processes
2.2.3. Construction from the Ideal Cycle to the Actual Cycle
- The absorption and the desorption are set to an isothermal process.
- During the pre-heating and cooling process, the CO2 loading remains unchanged, that is, no CO2 desorption occurs.
- The absorption and the desorption process are in a gas–liquid equilibrium state.
- All kinds of heat loss in the cycle are not considered.
- The solution does not react with other types of gases in the flue gas except CO2, and the flue gas is assumed to be an ideal gas.
2.3. Performance Indicators
2.3.1. Regeneration Heat
2.3.2. COPCO2
2.3.3. The Second-Law Efficiency
3. Results and Discussion
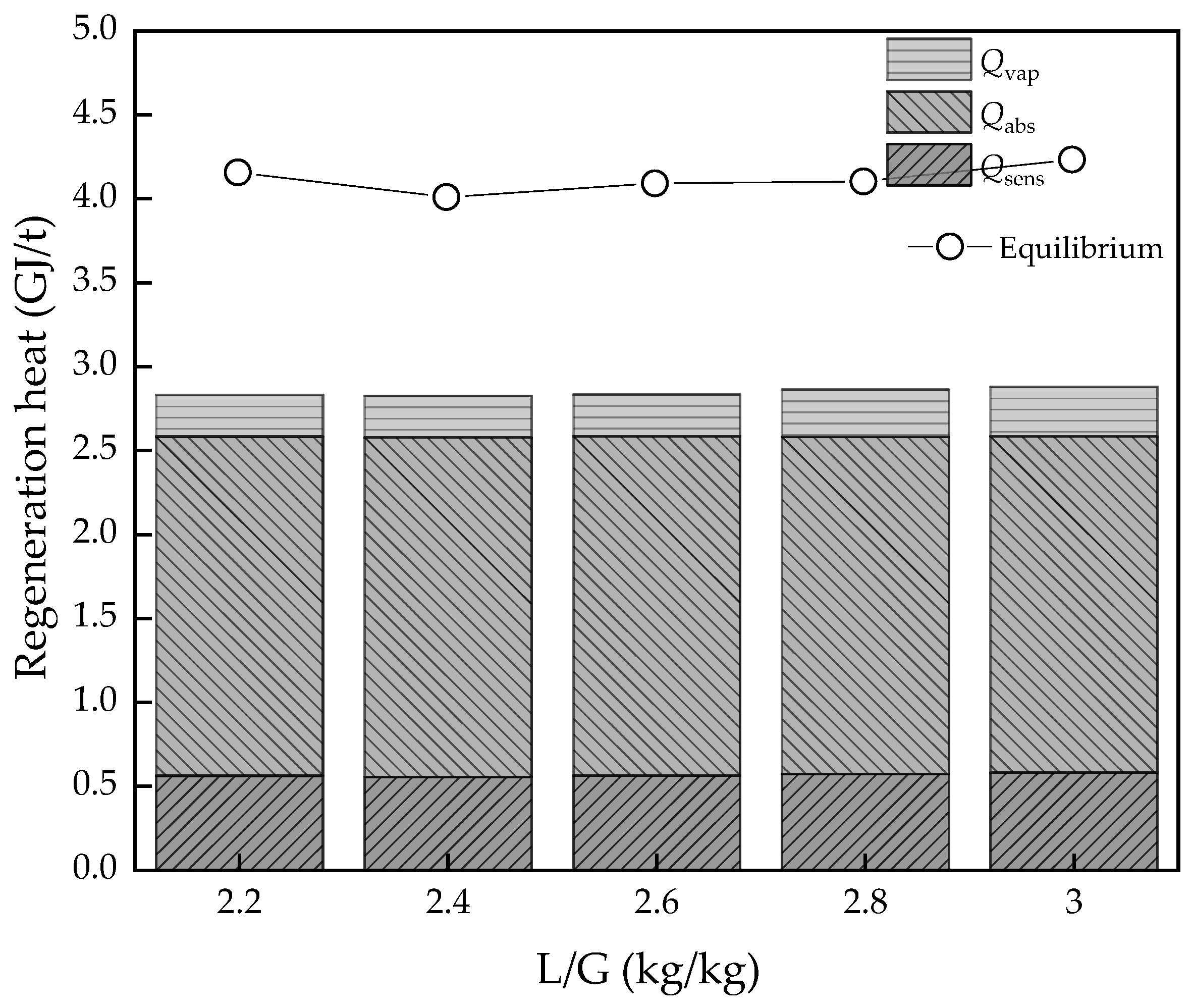
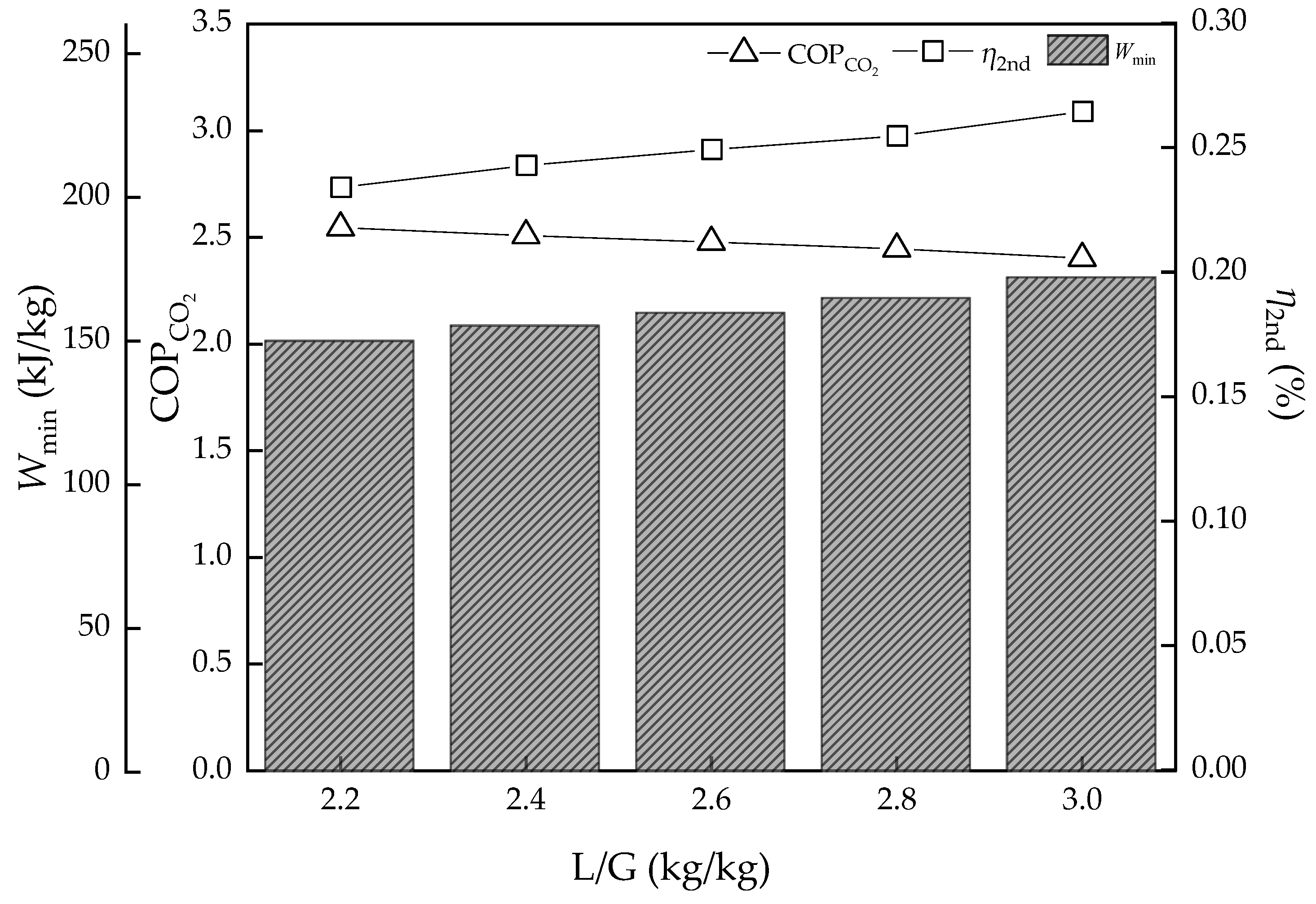
3.1. Effect of the Ratio of Liquid:Gas (L/G)
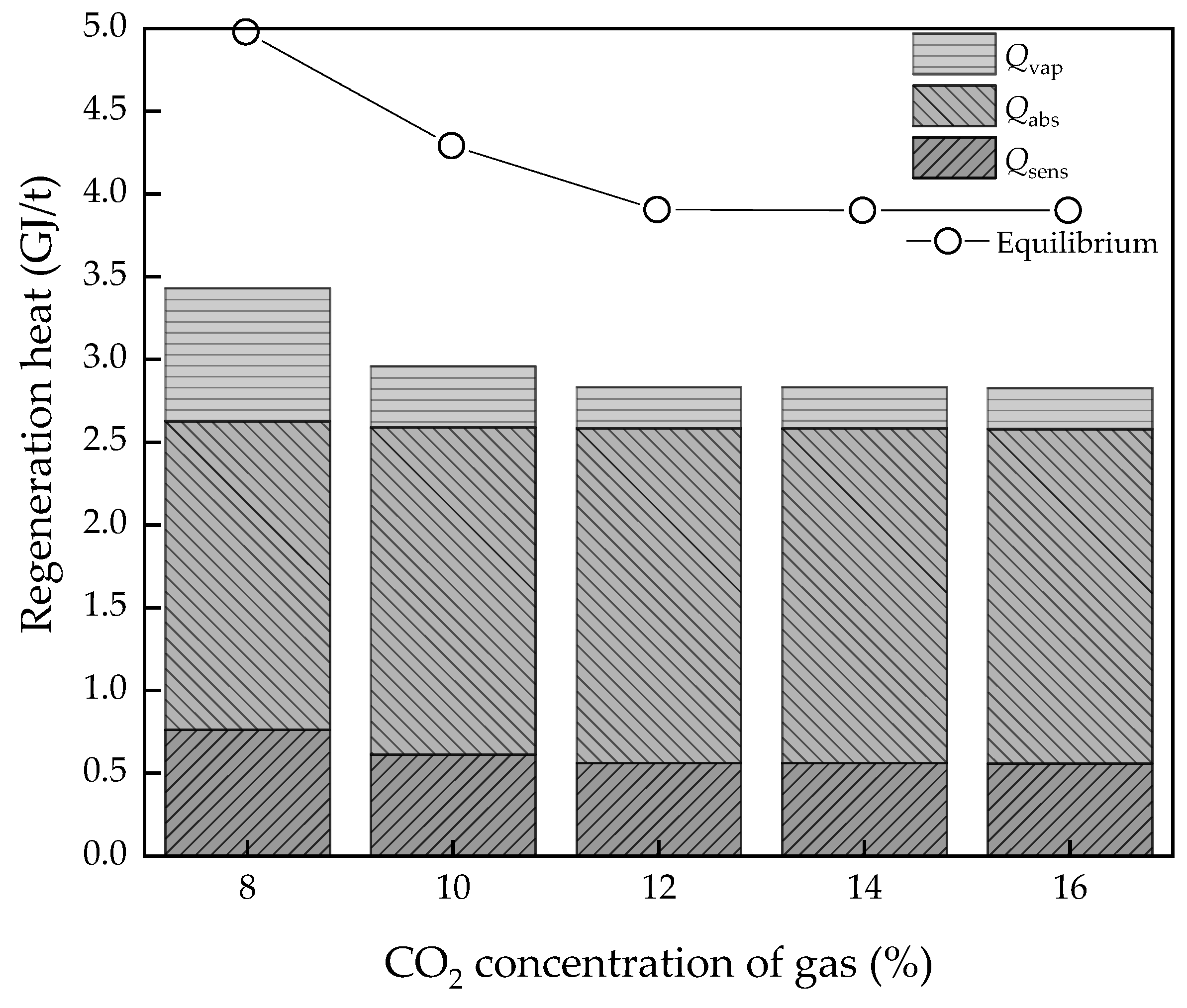
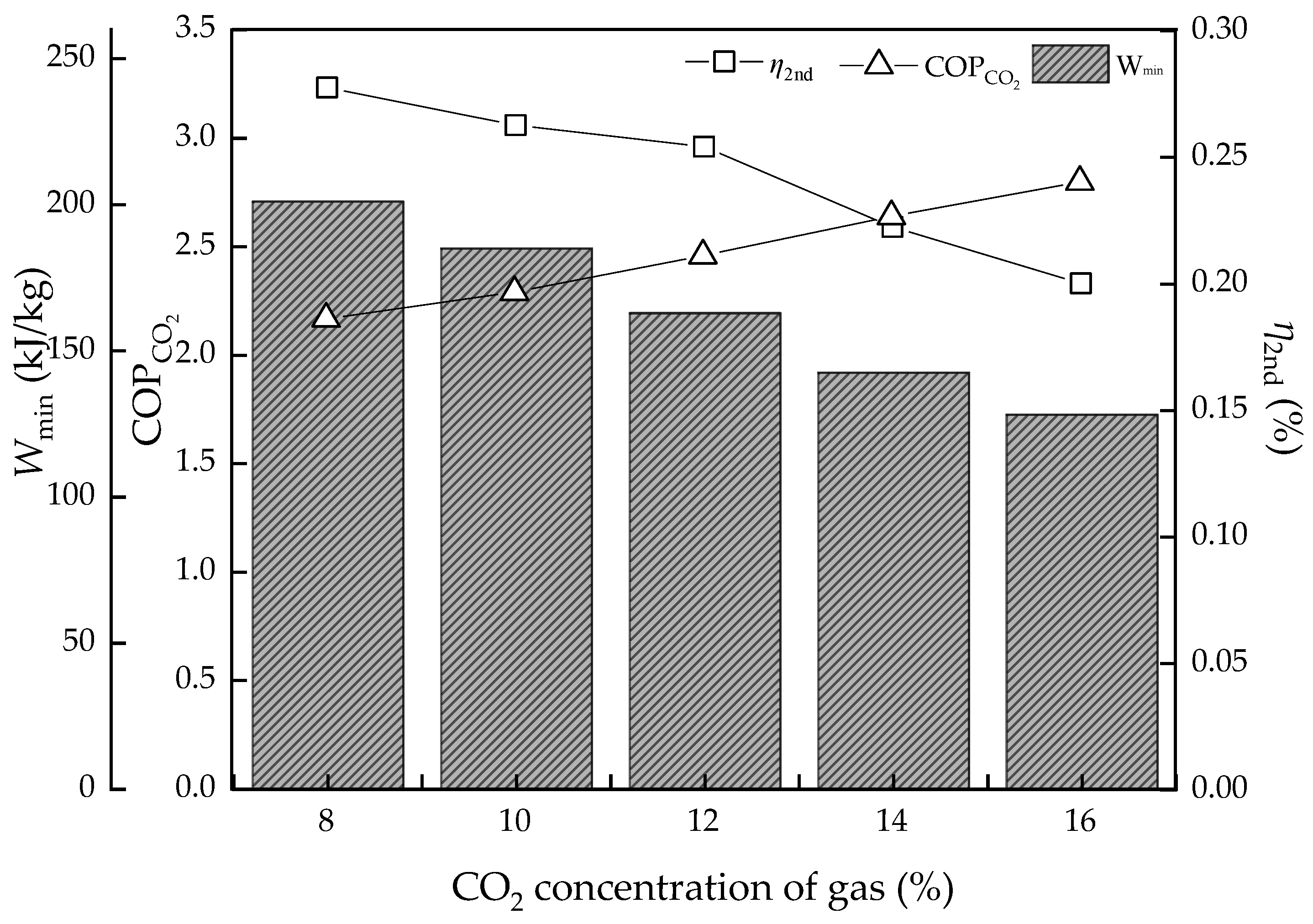
3.2. Effect of CO2 Concentration of Gas
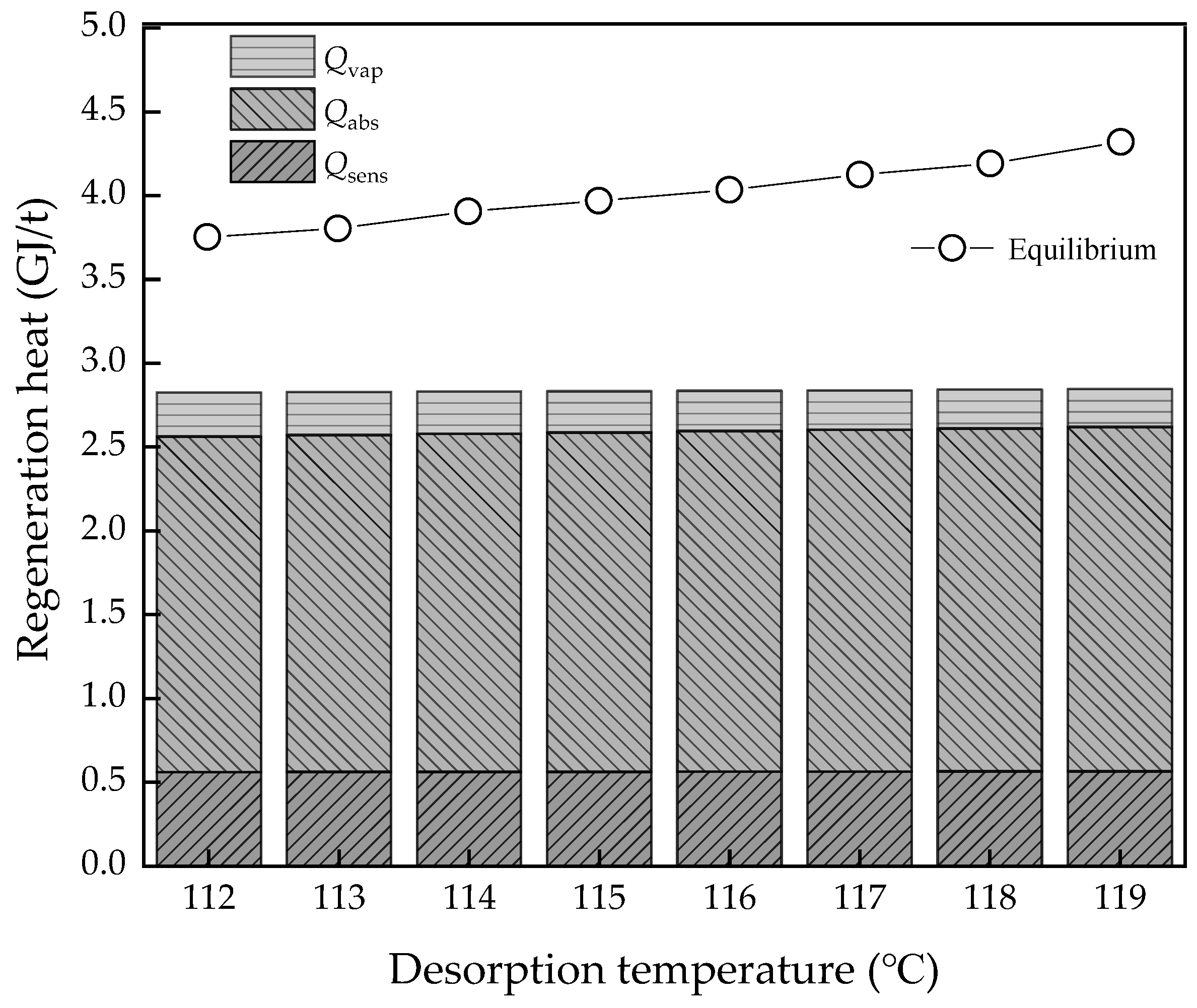
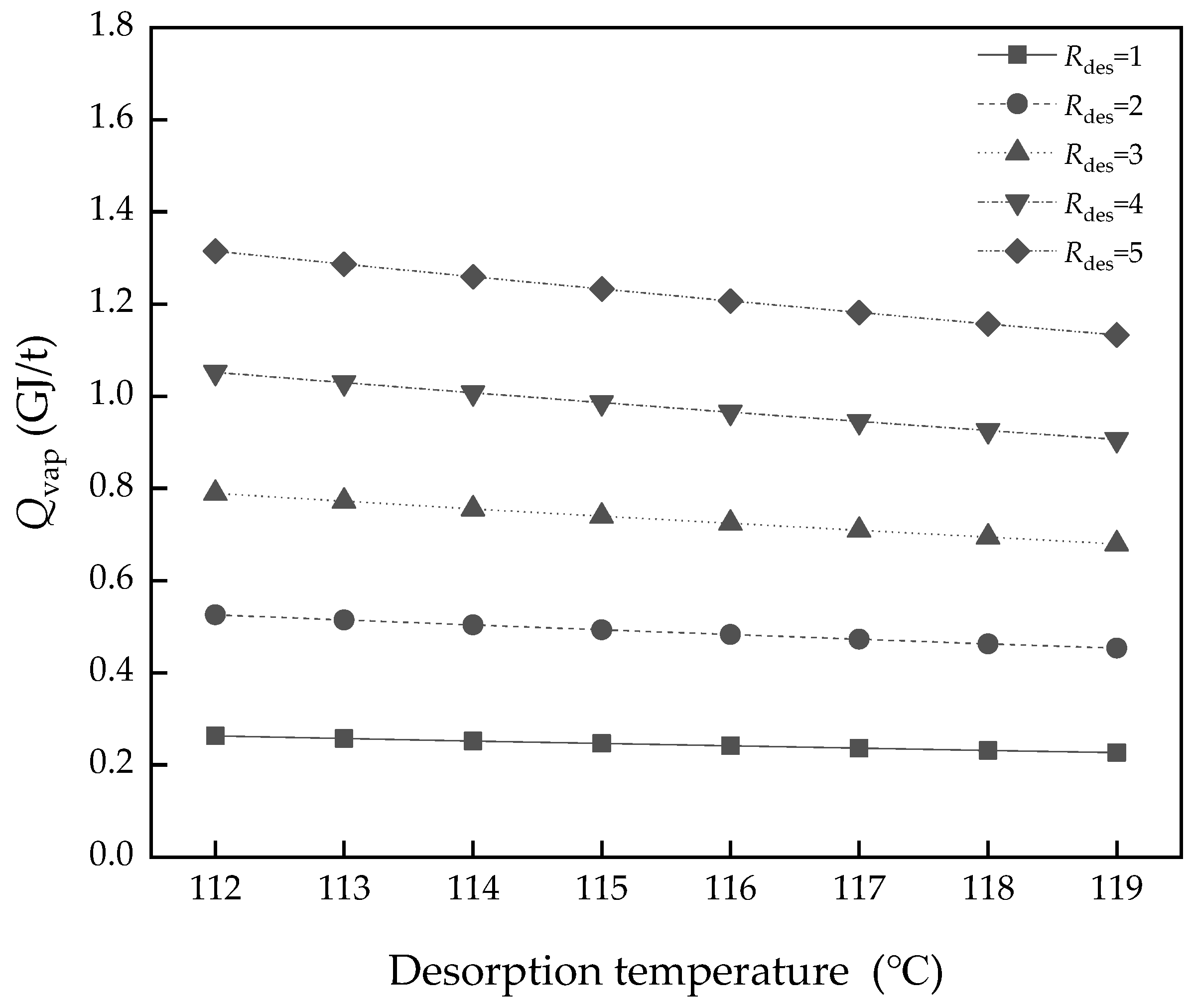
3.3. Effect of Desorption Temperature
3.4. Effect of Rdes and Pinch Temperature of the Heat Exchanger
4. Conclusions
- A new indicator, COPCO2, was proposed firstly, which would be integrated into the current assessment framework of CO2 absorption systems to be more complete.
- As for 30 wt% MEA solvent, the lowest regeneration heat was 2.82 GJ/t when Rdes = 1 and ΔTheat-ex = 10 K and the highest energy conversion efficiency was 2.80 in these cases.
- The L/G had the best value, as too high and too low are both bad for energy consumption of regeneration heat. However, for potential energy efficiency improvement, the lower the L/G value, the better, on the assumption that the solvent could achieve the goal of the removal rate. As for the CO2 concentration of flue gas, the higher the value, the better energy performance and efficiency. However, real performance is limited by the solvent properties, which may not achieve the ideal conditions; the lowest regeneration heat was about 2.82 and 3.89 GJ/t, respectively, while the COPCO2 continued to increase. The desorption temperature was not a sensitive parameter to energy performance in an ideal condition. However, in the actual situation, the higher the temperature, the higher the heat loss.
- The operating parameters, Rdes and ΔTheating-ex, were a compromise between cost and performance. The better performance of the heat exchanger will bring a lot of energy saving in Qsens, which decreased from 1.38 to 0.28 GJ/t when ΔTheating-ex varied from 25 to 5 K in L/G = 2.4.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Symbols | sol | MEA solution | |
| P | Pressure | Greek letters | |
| T | Temperature | α | CO2 loading |
| ΔHabs | Heat of CO2 absorption | Δα | CO2 capacity |
| Hvap | Heat of water evaporation | η2nd | Second law efficiency |
| Cp | Specific heat of solution | η | Capture rate |
| Q | Energy consumption | Acronym | |
| x | Molar fraction | CCS | Carbon capture |
| R | Partial pressure ratio | L/G | Ratio of liquid-gas |
| M | Molar mass | ILs | Ionic liquids |
| XCO2 | Concentration of CO2 in flue gas | VLE | Vaper-Liquid-Equilibrium |
| F | Molar flow rate of flue gas | MEA | Monoethanolamine |
| ms | Mass flow rate of solution | MDEA | Methyldiethanolamine |
| q | Mass flow rate of captured CO2 | PZ | Piperazine |
| COPCO2 | Energy conversion efficiency of CCS | AMP | 2-amino-2-methyl-1-propanol |
| Subscripts | MAPA | N-methyl-1,3-propane-diamine | |
| abs | Absorption | DEEA | 2-(diethylamino)-ethanol |
| des | Desorption | AEEA | 2-((2-aminoethyl) amino) ethanol |
| sen | Sensible | TETA | Triethylenetetramine |
| vap | Water evaporation | DEAPD | 3-(Diethylamino)-1,2-propanediol |
| re | Regeneration | TMPDA | Tetramethyl-1,3-propanediamine |
| solv | MEA solvent | DMCA | N, N-dimethylcyclohexylamine |
References
- A Daily Record of Atmospheric Carbon Dioxide from Scripps Institution of Oceanography at UC San Diego. Available online: https://scripps.ucsd.edu/programs/keelingcurve/ (accessed on 13 May 2019).
- Rizvi, R.H.; Newaj, R.; Chaturvedi, O.P.; Prasad, R.; Handa, A.K.; Alam, B. Carbon sequestration and CO2 absorption by agroforestry systems: An assessment for Central Plateau and Hill region of India. J. Earth Syst. Sci. 2019, 128, 56. [Google Scholar] [CrossRef]
- Aerenson, T.; Tebaldi, C.; Sanderson, B.; Lamarque, J.F. Changes in a suite of indicators of extreme temperature and precipitation under 1.5 and 2 degrees warming. Environ. Res. Lett. 2018, 13, 035009. [Google Scholar] [CrossRef]
- Special Report on Global Warming of 1.5 °C (Report). Incheon, South Korea; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 7 October 2018. Available online: https://www.ipcc.ch/sr15/ (accessed on 13 May 2019).
- International Energy Agency. Energy Technology Perspectives; International Energy Agency: Paris, France, 2017; Available online: https://webstore.iea.org/energy-technology-perspectives-2017 (accessed on 13 May 2019).
- Kanniche, M.; Le Moullec, Y.; Authier, O.; Hagi, H.; Bontemps, D.; Neveux, T.; Louis-Louisy, M. Up-to-date CO2 Capture in Thermal Power Plants. Energy Procedia 2017, 114, 95–103. [Google Scholar] [CrossRef]
- Mantripragada, H.C.; Zhai, H.; Rubin, E.S. Boundary Dam or Petra Nova–Which is a better model for CCS energy supply? Int. J. Greenh Gas Control 2019, 82, 59–68. [Google Scholar] [CrossRef]
- Zhao, R.; Shuai, D.; Li, Z.; Liu, Y.; Tan, Y. Energy-saving pathway exploration of CCS integrated with solar energy: Literature research and comparative analysis. Energy Convers. Manag. 2015, 102, 66–80. [Google Scholar] [CrossRef]
- Idem, R.; Wilson, M.; Tontiwachwuthikul, P.; Chakma, A.; Veawab, A.; Aroonwilas, A.; Gelowitz, D. Pilot Plant Studies of the CO2 Capture Performance of Aqueous MEA and Mixed MEA/MDEA Solvents at the University of Regina CO2 Capture Technology Development Plant and the Boundary Dam CO2 Capture Demonstration Plant. Ind. Eng. Chem. Res. 2006, 45, 2414–2420. [Google Scholar] [CrossRef]
- Li, X.; Wang, S.; Chen, C. Experimental Study of Energy Requirement of CO2 Desorption from Rich Solvent. Energy Procedia 2013, 37, 1836–1843. [Google Scholar] [CrossRef]
- Sakwattanapong, R.; Aroonwilas, A.A.; Veawab, A. Behavior of Reboiler Heat Duty for CO2 Capture Plants Using Regenerable Single and Blended Alkanolamines. Ind. Eng. Chem. Res. 2005, 44, 4465–4473. [Google Scholar] [CrossRef]
- Artanto, Y.; Jansen, J.; Pearson, P.; Do, T.; Cottrell, A.; Meuleman, E.; Feron, P. Performance of MEA and amine-blends in the CSIRO PCC pilot plant at Loy Yang Power in Australia. Fuel 2012, 101, 264–275. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, F.; Cui, Z.; Liu, C.; Yue, H.; Tang, S.; Liu, Y.; Lu, H.; Liang, B. Enhancing the energetic efficiency of MDEA/PZ-based CO2 capture technology for a 650 MW power plant: Process improvement. Appl. Energy 2016, 185, 362–375. [Google Scholar] [CrossRef]
- Wai, S.K.; Nwaoha, C.; Saiwan, C.; Idem, R.; Supap, T. Absorption heat, solubility, absorption and desorption rates, cyclic capacity, heat duty, and absorption kinetic modeling of AMP–DETA blend for post–combustion CO2 capture. Sep. Purif. Technol. 2018, 194, 89–95. [Google Scholar] [CrossRef]
- Mangalapally, H.P.; Hasse, H. Pilot plant study of two new solvents for post combustion carbon dioxide capture by reactive absorption and comparison to monoethanolamine. Chem. Eng. Sci. 2011, 66, 5512–5522. [Google Scholar] [CrossRef]
- Rabensteiner, M.; Kinger, G.; Koller, M.; Hochenauer, C. Pilot plant study of aqueous solution of piperazine activated 2-amino-2-methyl-1-propanol for post combustion carbon dioxide capture. Int. J. Greenh. Gas. Control 2016, 51, 106–117. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; Yan, J.; Tu, S.T. CO2 capture using amine solution mixed with ionic liquid. Ind. Eng. Chem. Res. 2014, 53, 2790–2799. [Google Scholar] [CrossRef]
- Bernard, F.L.; Dalla Vecchia, F.; Rojas, M.F.; Ligabue, R.; Vieira, M.O.; Costa, E.M.; Chaban, V.V.; Einloft, S. Anticorrosion Protection by Amine-Ionic Liquid Mixtures: Experiments and Simulations. J. Chem. Eng. Data 2016, 61, 1803–1810. [Google Scholar] [CrossRef]
- Akinola, T.E.; Oko, E.; Wang, M. Study of CO2 removal in natural gas process using mixture of ionic liquid and MEA through process simulation. Fuel 2019, 236, 135–146. [Google Scholar] [CrossRef]
- Zacchello, B.; Oko, E.; Wang, M.; Fethi, A. Process simulation and analysis of carbon capture with an aqueous mixture of ionic liquid and monoethanolamine solvent. Int. J. Coal Sci. Technol. 2017, 4, 25–32. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, X.; Xin, Z.; Dong, H.; Zhang, S. Thermodynamic Modeling and Assessment of Ionic Liquid-Based CO2 Capture Processes. Ind. Eng. Chem. Res. 2014, 53, 11805–11817. [Google Scholar]
- Mobley, P.D.; Rayer, A.V.; Tanthana, J.; Gohndrone, T.R.; Soukri, M.; Coleman, L.J.; Lail, M. CO2 Capture Using Fluorinated Hydrophobic Solvents. Ind. Eng. Chem. Res. 2017, 56, 11958–11966. [Google Scholar] [CrossRef]
- Yu, Y.S.; Lu, H.F.; Zhang, T.T.; Zhang, Z.X.; Wang, G.X.; Rudolph, V. Determining the Performance of an Efficient Nonaqueous CO2 Capture Process at Desorption Temperatures below 373 K. Ind. Eng. Chem. Res. 2013, 52, 12622–12634. [Google Scholar] [CrossRef]
- Guo, H.; Li, C.; Shi, X.; Li, H.; Shen, S. Nonaqueous amine-based absorbents for energy efficient CO2 capture. Appl. Energy 2019, 239, 725–734. [Google Scholar] [CrossRef]
- Zhu, K.; Lu, H.; Liu, C.; Wu, K.; Jiang, W.; Cheng, J.; Tang, S.; Yue, H.; Liu, Y.; Liang, B. Investigation on the Phase-Change Absorbent System MEA + Solvent A (SA) + H2O Used for the CO2 Capture from Flue Gas. Ind. Eng. Chem. Res. 2019, 58, 3811–3821. [Google Scholar] [CrossRef]
- Zhang, W.; Jin, X.; Tu, W.; Ma, Q.; Mao, M.; Cui, C. Development of MEA-based CO2 phase change absorbent. Appl. Energy 2017, 195, 316–323. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, Q.; Agar, D.W. Improvement of Lipophilic-Amine-based Thermomorphic Biphasic Solvent for Energy-Efficient Carbon Capture. Energy Procedia 2012, 23, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Pinto, D.D.D.; Knuutila, H.; Fytianos, G.; Haugen, G.; Mejdell, T.; Svendsen, H.F. CO2 post combustion capture with a phase change solvent. Pilot plant campaign. Int. J. Greenh. Gas Control 2014, 31, 153–164. [Google Scholar] [CrossRef]
- Shen, Y.; Jiang, C.; Zhang, S.; Chen, J.; Wang, L.; Chen, J. Biphasic solvent for CO2 capture: Amine property-performance and heat duty relationship. Appl. Energy 2018, 230, 726–733. [Google Scholar] [CrossRef]
- Liu, F.; Fang, M.; Dong, W.; Wang, T.; Xia, Z.; Wang, Q.; Luo, Z. Carbon dioxide absorption in aqueous alkanolamine blends for biphasic solvents screening and evaluation. Appl. Energy 2019, 233–234, 468–477. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, Y.; Shao, P.; Chen, J.; Wang, L. Kinetics, Thermodynamics, and Mechanism of a Novel Biphasic Solvent for CO2 Capture from Flue Gas. Environ. Sci. Technol. 2018, 52, 3660–3668. [Google Scholar] [CrossRef]
- Cuccia, L.; Dugay, J.; Bontemps, D.; Louis-Louisy, M.; Morand, T.; Kanniche, M.; Bellosta, V.; Vial, J. Monitoring of the blend monoethanolamine/methyldiethanolamine/water for post-combustion CO2 capture. Int. J. Greenh. Gas Control 2019, 80, 43–53. [Google Scholar] [CrossRef]
- Gladis, A.; Lomholdt, N.F.; Fosbøl, P.L.; Woodley, J.M.; von Solms, N. Pilot scale absorption experiments with carbonic anhydrase-enhanced MDEA-Benchmarking with 30 wt% MEA. Int. J. Greenh. Gas Control 2019, 82, 69–85. [Google Scholar] [CrossRef]
- Hadri, N.E.; Dang, V.Q.; Goetheer, E.L.V.; Zahra, M.R.M.A. Aqueous amine solution characterization for post-combustion CO2 capture process. Appl. Energy 2016, 185, 1433–1449. [Google Scholar] [CrossRef]
- Bougie, F.; Iliuta, M.C. Sterically Hindered Amine-Based Absorbents for the Removal of CO2 from Gas Streams. J. Chem. Eng. Data 2012, 57, 635–669. [Google Scholar] [CrossRef]
- Ahmady, A.; Hashim, M.A.; Aroua, M.K. Experimental Investigation on the Solubility and Initial Rate of Absorption of CO2 in Aqueous Mixtures of Methyldiethanolamine with the Ionic Liquid 1-Butyl-3-methylimidazolium Tetrafluoroborate. J. Chem. Eng. Data 2010, 55, 5733–5738. [Google Scholar] [CrossRef]
- Haider, M.B.; Hussain, Z.; Kumar, R. CO2 absorption and kinetic study in ionic liquid amine blends. J. Mol. Liq. 2016, 224, 1025–1031. [Google Scholar] [CrossRef]
- Kumar, S.; Cho, J.H.; Moon, I. Ionic liquid-amine blends and CO2 BOLs: Prospective solvents for natural gas sweetening and CO2 capture technology-A review. Int. J. Greenh. Gas Control 2014, 20, 87–116. [Google Scholar] [CrossRef]
- Gao, J.; Cao, L.; Dong, H.; Zhang, X.; Zhang, S. Ionic liquids tailored amine aqueous solution for pre-combustion CO2 capture: Role of imidazolium-based ionic liquids. Appl. Energy 2015, 154, 771–780. [Google Scholar] [CrossRef]
- Camper, D.; Bara, J.E.; Gin, D.L.; Noble, R.D. Room-temperature ionic liquid-amine solutions: Tunable solvents for efficient and reversible capture of CO2. Ind. Eng. Chem. Res. 2008, 47, 8496–8498. [Google Scholar] [CrossRef]
- Zhai, H.; Rubin, E.S. Systems Analysis of Ionic Liquids for Post-combustion CO2 Capture at Coal-fired Power Plants. Energy Procedia 2014, 63, 1321–1328. [Google Scholar] [CrossRef]
- Ramdin, M.; De Loos, T.W.; Vlugt, T.J.H. State-of-the-art of CO2 capture with ionic liquids. Ind. Eng. Chem. Res. 2012, 51, 8149–8177. [Google Scholar] [CrossRef]
- Chao, G.; Chen, S.; Zhang, Y.; Wang, G. Solubility of CO2 in Nonaqueous Absorption System of 2-(2-Aminoethylamine) ethanol + Benzyl Alcohol. J. Chem. Eng. Data 2014, 59, 1796–1801. [Google Scholar]
- Dinda, S.; Goud, V.V.; Patwardhan, A.V.; Pradhan, N.C. Kinetics of reactive absorption of carbon dioxide with solutions of 1,6-hexamethylenediamine in polar protic solvents. Sep. Purif. Technol. 2010, 75, 1–7. [Google Scholar] [CrossRef]
- Chen, S.; Hu, G.; Smith, K.H.; Mumford, K.A.; Zhang, Y.; Stevens, G.W. Kinetics of CO2 Absorption in an Ethylethanolamine Based Solution. Ind. Eng. Chem. Res. 2017, 56, 12305–12315. [Google Scholar] [CrossRef]
- Yu, C.H.; Wu, T.W.; Tan, C.S. CO2 capture by piperazine mixed with non-aqueous solvent diethylene glycol in a rotating packed bed. Int. J. Greenh. Gas Control 2013, 19, 503–509. [Google Scholar] [CrossRef]
- Leimbrink, M.; Sandkämper, S.; Wardhaugh, L.; Maher, D.; Green, P.; Puxty, G.; Conway, W.; Bennett, R.; Botma, H.; Feron, P.; et al. Energy-efficient solvent regeneration in enzymatic reactive absorption for carbon dioxide capture. Appl. Energy 2017, 208, 263–276. [Google Scholar] [CrossRef]
- Akachuku, A.; Osei, P.A.; Decardi-Nelson, B.; Srisang, W.; Pouryousefi, F.; Ibrahim, H.; Idem, R. Experimental and kinetic study of the catalytic desorption of CO2 from CO2 -loaded monoethanolamine (MEA) and blended monoethanolamine – Methyl-diethanolamine (MEA-MDEA) solutions. Energy 2019, 179, 475–489. [Google Scholar] [CrossRef]
- Lai, Q.; Toan, S.; Assiri, M.A.; Cheng, H.; Russell, A.G.; Adidharma, H.; Radosz, M.; Fan, M. Catalyst-TiO(OH)2 could drastically reduce the energy consumption of CO2 capture. Nat. Commun. 2018, 9, 2672. [Google Scholar] [CrossRef]
- Rabensteiner, M.; Kinger, G.; Koller, M.; Hochenauer, C. Three years of working experience with different solvents at a realistic post combustion capture pilot plant. Energy Procedia 2014, 63, 1578–1584. [Google Scholar] [CrossRef]
- Stec, M.; Tatarczuk, A.; Więcław-Solny, L.; Krótki, A.; Ciązko, M.; Tokarski, S. Pilot plant results for advanced CO2 capture process using amine scrubbing at the Jaworzno II Power Plant in Poland. Fuel 2015, 151, 50–56. [Google Scholar] [CrossRef]
- Krótki, A.; Więcław-Solny, L.; Tatarczuk, A.; Stec, M.; Wilk, A.; Śpiewak, D.; Spietz, T. Laboratory Studies of Post-combustion CO2 Capture by Absorption with MEA and AMP Solvents. Arab. J. Sci. Eng. 2016, 41, 371–379. [Google Scholar] [CrossRef]
- Wiecław-Solny, L.; Tatarczuk, A.; Stec, M.; Krótki, A. Advanced CO2 capture pilot plant at tauron’s coal-fired power plant: Initial results and further opportunities. Energy Procedia 2014, 63, 6318–6322. [Google Scholar] [CrossRef]
- Knudsen, J.N.; Andersen, J.; Jensen, J.N.; Biede, O. Evaluation of process upgrades and novel solvents for the post combustion CO2 capture process in pilot-scale. Energy Procedia 2011, 4, 1558–1565. [Google Scholar] [CrossRef]
- Knudsen, J.N.; Jensen, J.N.; Vilhelmsen, P.J.; Biede, O. Experience with CO2 capture from coal flue gas in pilot-scale: Testing of different amine solvents. Energy Procedia 2009, 1, 783–790. [Google Scholar] [CrossRef]
- Moser, P.; Schmidt, S.; Sieder, G.; Garcia, H.; Stoffregen, T.; Stamatov, V. The post-combustion capture pilot plant Niederaussem-Results of the first half of the testing programme. Energy Procedia 2011, 4, 1310–1316. [Google Scholar] [CrossRef]
- Feron, P.H.; Cousins, A.; Gao, S.; Liu, L.; Wang, J.; Wang, S.; Niu, H.; Yu, H.; Li, K.; Cottrell, A. Experimental performance assessment of a mono-ethanolamine-based post-combustion CO2 capture at a coal-fired power station in China. Greenh. Gas Sci. Technol. 2017, 7, 486–499. [Google Scholar] [CrossRef]
- Mangalapally, H.P.; Hasse, H. Pilot plant study of post-combustion carbon dioxide capture by reactive absorption: Methodology, comparison of different structured packings, and comprehensive results for monoethanolamine. Chem. Eng. Res. Des. 2011, 89, 1216–1228. [Google Scholar] [CrossRef]
- Nakamura, S.; Yamanaka, Y.; Matsuyama, T.; Okuno, S.; Sato, H. IHI s amine-based CO2 capture technology for coal fired power plant. Energy Procedia 2013, 37, 1897–1903. [Google Scholar] [CrossRef]
- Vitse, F.; Baburao, B.; Dugas, R.; Czarnecki, L.; Schubert, C. Technology and pilot plant results of the advanced amine process. Energy Procedia 2011, 4, 5527–5533. [Google Scholar] [CrossRef] [Green Version]
- House, K.Z.; Harvey, C.F.; Aziz, M.J.; Schrag, D.P. The energy penalty of post-combustion CO2 capture storage and its implications for retrofitting the U.S. installed base. Energy Environ. Sci. 2009, 2, 193–205. [Google Scholar] [CrossRef]
- Ruthven, D.M. CO2 capture: Value functions, separative work and process economics. Chem. Eng. Sci. 2014, 114, 128–133. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, F.; Hu, G.; Mirza, N.R.; Stevens, G.W.; Mumford, K.A. Modelling of a post-combustion carbon dioxide capture absorber using potassium carbonate solvent in Aspen Custom Modeller. Chin. J. Chem. Eng. 2018, 26, 2327–2336. [Google Scholar] [CrossRef]
- Mores, P.; Scenna, N.; Mussati, S. A rate based model of a packed column for CO2 absorption using aqueous monoethanolamine solution. Int. J. Greenh. Gas Control 2012, 6, 21–36. [Google Scholar] [CrossRef]
- Wang, J.; Sun, T.; Zhao, J.; Deng, S.; Li, K.; Xu, Y.; Fu, J. Thermodynamic considerations on MEA absorption: Whether thermodynamic cycle could be used as a tool for energy efficiency analysis. Energy 2019, 168, 380–392. [Google Scholar] [CrossRef]
- Simon, L.L.; Elias, Y.; Puxty, G.; Artanto, Y.; Hungerbuhler, K. Rate based modeling and validation of a carbon-dioxide pilot plant absorbtion column operating on monoethanolamine. Chem. Eng. Res. Des. 2011, 89, 1684–1692. [Google Scholar] [CrossRef]
- Kvamsdal, H.M.; Rochelle, G.T. Effects of the temperature bulge in CO2 absorption from flue gas by aqueous monoethanolamine. Ind. Eng. Chem. Res. 2008, 47, 867–875. [Google Scholar] [CrossRef]
- Qing, X. Thermodynamics of CO2 Loaded Aqueous Amines. Ph.D. Thesis, University of Texas Libraries, Austin, TX, USA, 2011. [Google Scholar]
- Xu, Q.; Rochelle, G. Total pressure and CO2 solubility at high temperature in aqueous amines. Energy Procedia 2011, 4, 117–124. [Google Scholar] [CrossRef]
- Wang, T.; Yu, W.; Le Moullec, Y.; Liu, F.; Xiong, Y.; He, H.; Lu, J.; Hsu, E.; Fang, M.; Luo, Z. Solvent regeneration by novel direct non-aqueous gas stripping process for post-combustion CO2 capture. Appl. Energy 2017, 205, 23–32. [Google Scholar] [CrossRef]
- Morris, D.R.; Szargut, J. Standard chemical exergy of some elements and compounds on the planet earth. Energy 1986, 11, 733–755. [Google Scholar] [CrossRef]
- Zhao, R.; Deng, S.; Liu, Y.; Zhao, Q.; He, J.; Zhao, L. Carbon pump: Fundamental theory and applications. Energy 2017, 119, 1131–1143. [Google Scholar] [CrossRef]
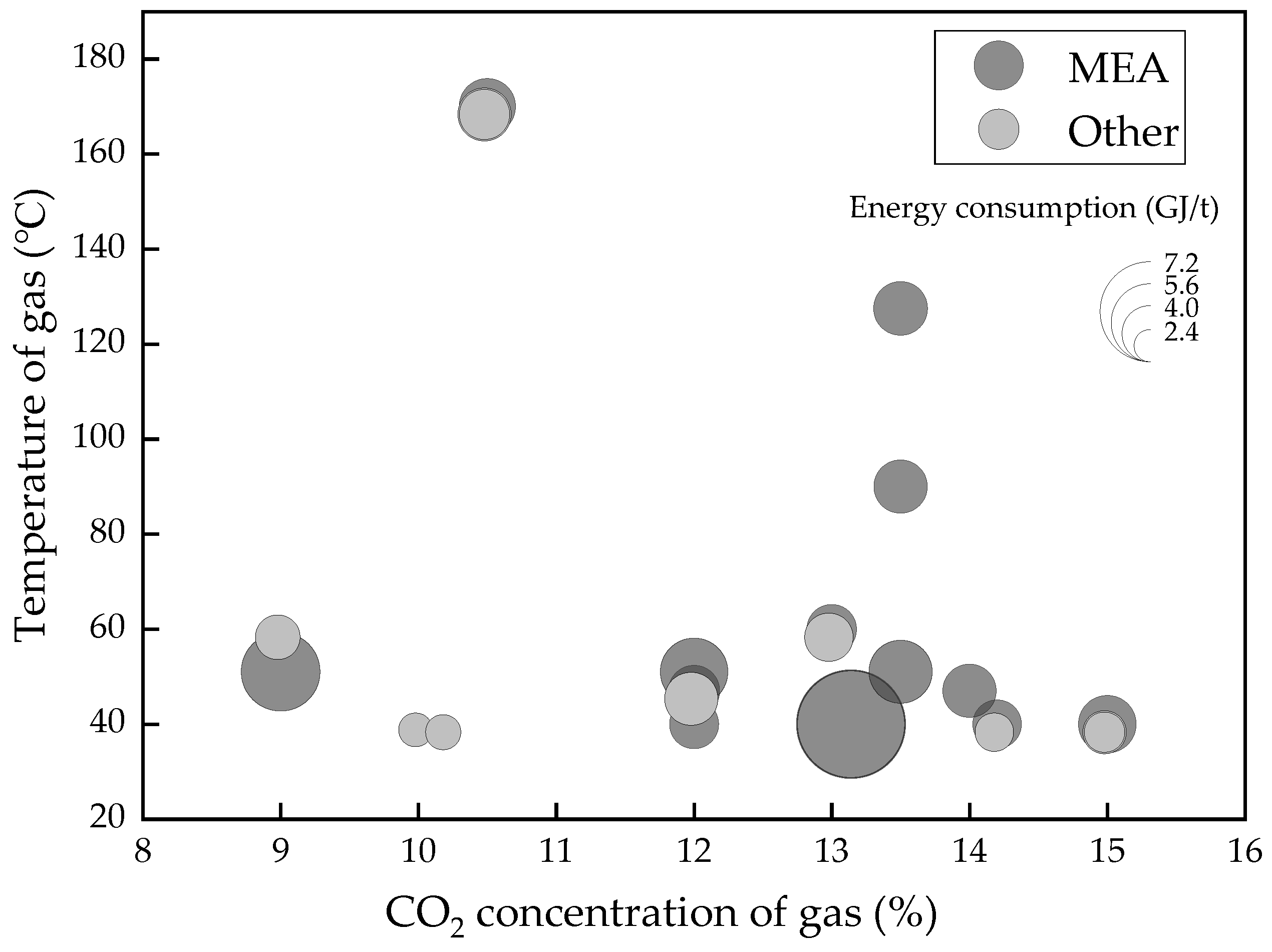

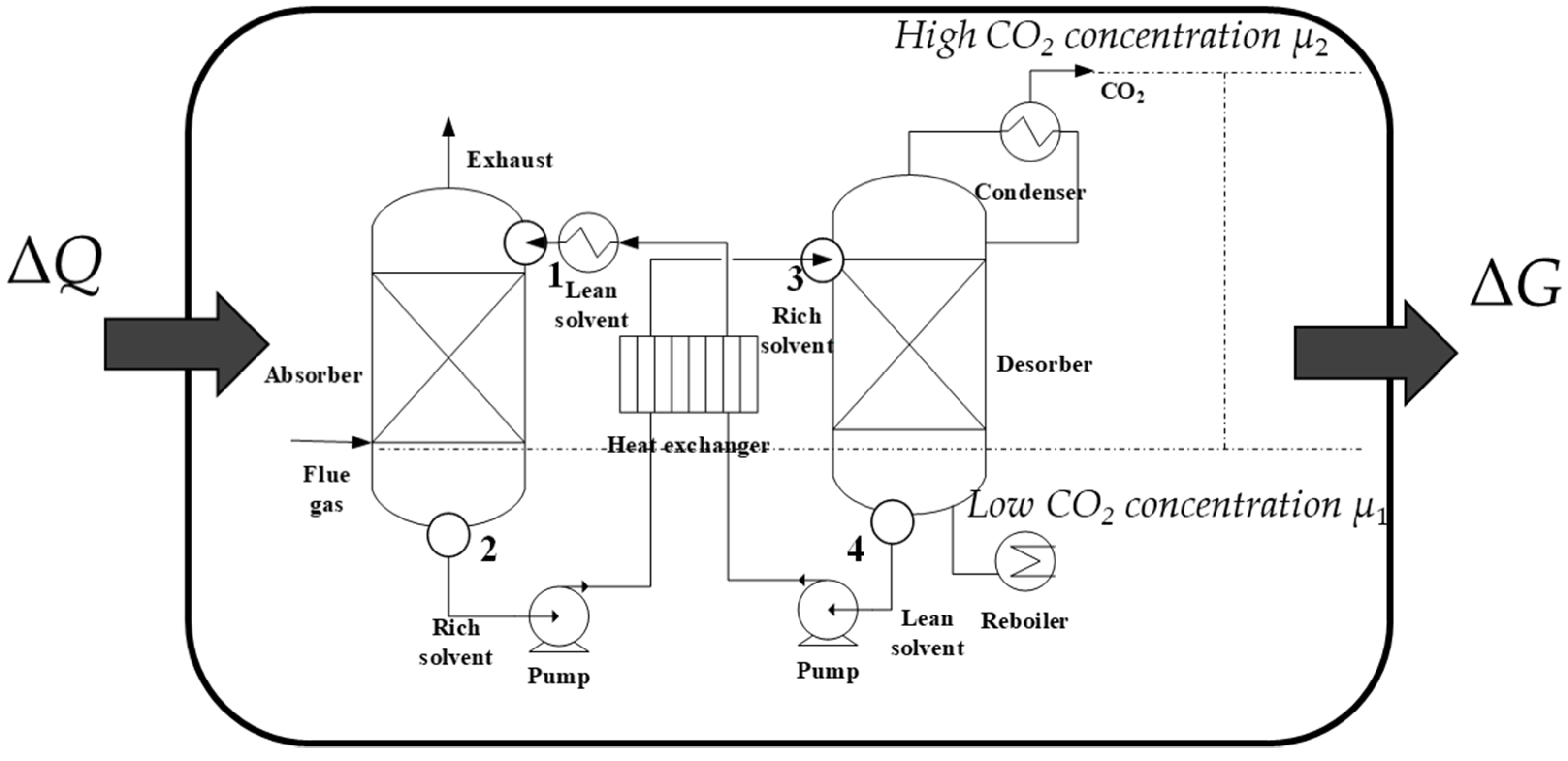
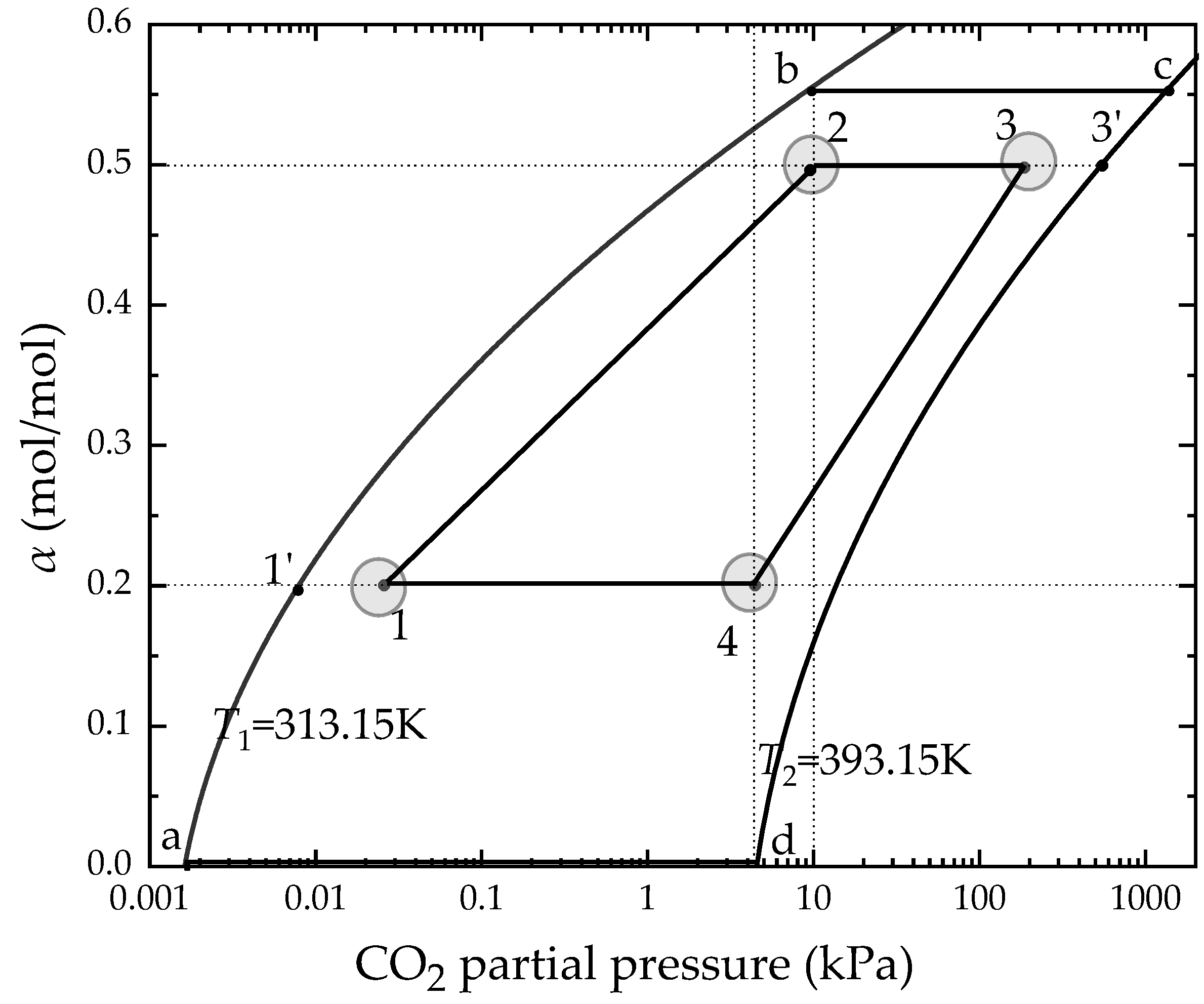
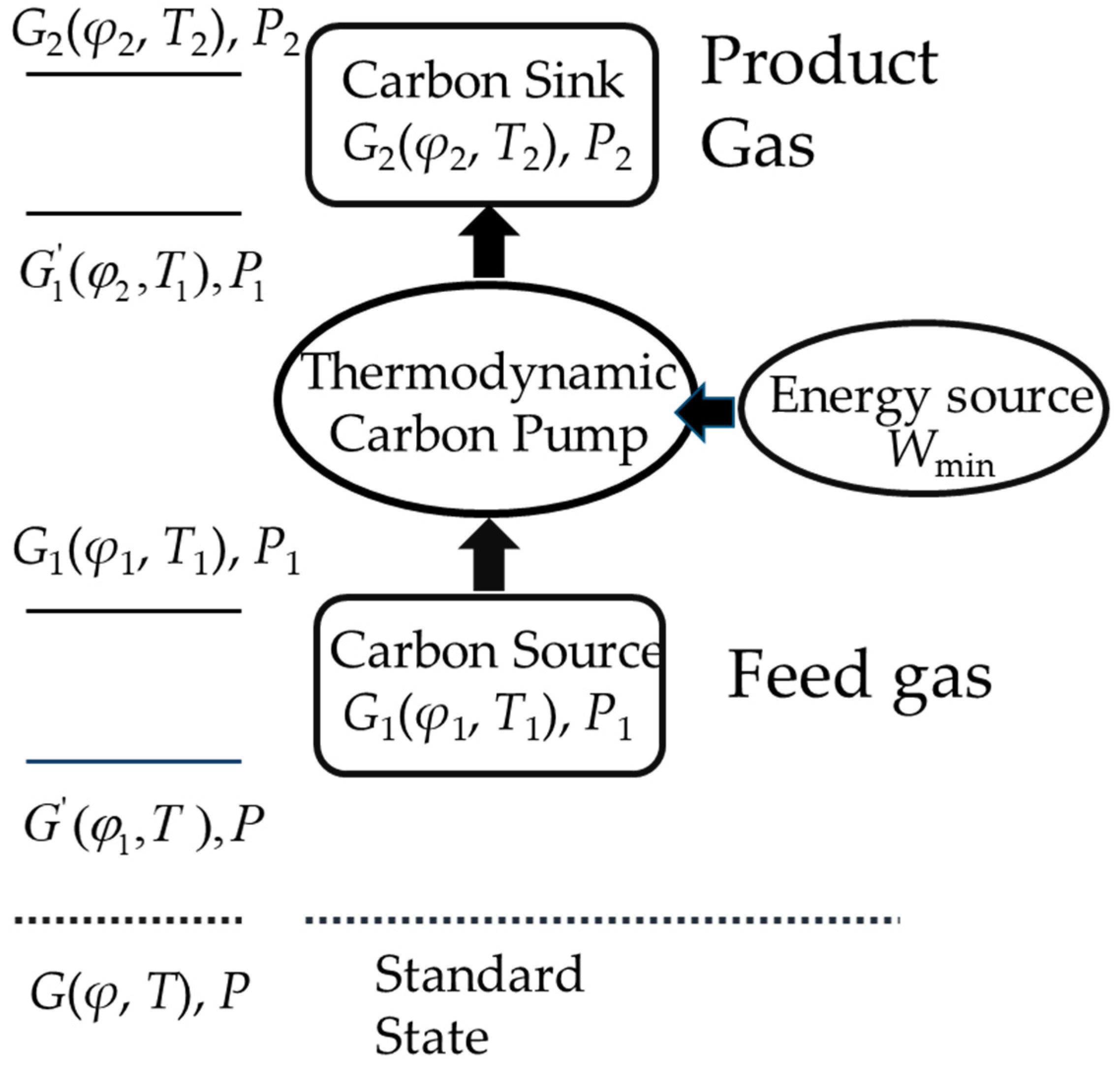


| Classification | Solvent | Energy Consumption (GJ/t) | Energy Performance Compared to MEA (Lower Percentage) | Ref |
|---|---|---|---|---|
| Blend of amines | ||||
| MEA + MDEA | 2.0–3.7 | [9,10,11] | ||
| MEA + AMP | 4.0–6.1 | [12] | ||
| MDEA + PZ | 2.24 | 27% | [13] | |
| AMP + DETA | 35.6–27.7% | [14] | ||
| AMP + PZ | 3.0–3.2 | 10–20% | [15,16] | |
| MEA + [Bmim][BF4] | 10–37.2% | [17,18] | ||
| MEA + [Bpy][BF4] | 7.44–15% | [19,20,21] | ||
| Water-lean/free | ||||
| 2-fluorophenethylamine + Octafluoropentanol | 2.2–3 | 40–50% | [22] | |
| MEA + Methanol | 2.28 | 24% | [23] | |
| 2-methoxyethanol + MEA | 55% | [24] | ||
| Phase change absorbents | ||||
| MEA + SA | 2.55 | 43.6% | [25] | |
| DMX | 2.1 | [26] | ||
| TBS | 2.5 | [27] | ||
| MAPA + DEEA | 2.2 | [28] | ||
| TETA + DEEA | 2.46 | 35% | [29] | |
| DEEA + AEEA | 2.58 | [30] | ||
| DEAPD + TETA | 2.7 | 29% | [29] | |
| TMPDA + TETA | 1.83 | 52% | [29] | |
| DMCA + TETA | 2.07–3.92 | [29,31] | ||
| Solvent | Energy Consumption (GJ/t) | Temperature of Flue Gas (°C) | CO2 Concentration of Flue Gas (%) | Ref |
|---|---|---|---|---|
| MEA | 3.5 | 40 | 12 | [50] |
| MEA | 3.82 | 127.5 | 13.5 | [51] |
| MEA | 7.7 | 40 | 13.14 | [52] |
| MEA | 3.8 | 90 | 13.5 | [53] |
| MEA | 3.53 | 60 | 13 | [54] |
| CESAR1 | 2.9 | 60 | 13 | [54] |
| CESAR2 | 3.46 | 60 | 13 | [54] |
| MEA | 3.62 | 47 | 12 | [55] |
| CASTOR1 | 3.58 | 47 | 12 | [55] |
| CASTOR2 | 3.80 | 47 | 12 | [55] |
| MEA | 3.48 | 40 | 14.2 | [56] |
| GUSTAV200 | 2.77 | 40 | 14.2 | [56] |
| MEA | 5.6 | 51 | 9.0 | [57] |
| MEA | 4.8 | 51 | 12 | [57] |
| MEA | 4.5 | 51 | 13.5 | [57] |
| MEA | 3.8 | 47 | 14 | [58] |
| MEA | 4.1 | 40 | 15 | [59] |
| SOLVENTA | 3.1 | 40 | 15 | [59] |
| SOLVENTB | 2.9 | 40 | 15 | [59] |
| SOLVENT1 | 2.8 | 60 | 9 | [60] |
| SOLVENT2 | 3.2 | 60 | 9 | [60] |
| MEA | 4.0 | 170 | 10.5 | [12] |
| BLEND1 | 3.8 | 170 | 10.5 | [12] |
| BLEND2 | 3.6 | 170 | 10.5 | [12] |
| Regeneration Heat | COPCO2 | The Second-Law Efficiency |
|---|---|---|
| The intuitive energy consumption of absorption CCS. | The potential capacity of energy conversion of CCS; the highest energy efficiency. | The develop level of existing CCS technology compared to ideal situation. |
| Design Parameters | Value |
|---|---|
| Flow of gas (L/min) | 500 |
| Mass fraction of MEA (%) | 30 |
| Temperature of gas (°C) | 40 |
| Number of stages | 20 |
| Pinch temperature of heat exchanger (K) | 10 |
| Property calculation method | E-NRTL |
| Mole fraction of CO2 (%) | 8 to16 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Deng, S.; Zhao, L.; Yuan, X.; Fu, J.; Li, S.; Liang, Y.; Wang, J.; Zhao, J. Application of the Thermodynamic Cycle to Assess the Energy Efficiency of Amine-Based Absorption of Carbon Capture. Energies 2019, 12, 2504. https://doi.org/10.3390/en12132504
Xu Y, Deng S, Zhao L, Yuan X, Fu J, Li S, Liang Y, Wang J, Zhao J. Application of the Thermodynamic Cycle to Assess the Energy Efficiency of Amine-Based Absorption of Carbon Capture. Energies. 2019; 12(13):2504. https://doi.org/10.3390/en12132504
Chicago/Turabian StyleXu, Yaofeng, Shuai Deng, Li Zhao, Xiangzhou Yuan, Jianxin Fu, Shuangjun Li, Yawen Liang, Junyao Wang, and Jun Zhao. 2019. "Application of the Thermodynamic Cycle to Assess the Energy Efficiency of Amine-Based Absorption of Carbon Capture" Energies 12, no. 13: 2504. https://doi.org/10.3390/en12132504
APA StyleXu, Y., Deng, S., Zhao, L., Yuan, X., Fu, J., Li, S., Liang, Y., Wang, J., & Zhao, J. (2019). Application of the Thermodynamic Cycle to Assess the Energy Efficiency of Amine-Based Absorption of Carbon Capture. Energies, 12(13), 2504. https://doi.org/10.3390/en12132504





