Fireside Corrosion on Heat Exchanger Surfaces and Its Effect on the Performance of Gas-Fired Instantaneous Water Heaters
Abstract
:1. Introduction
2. Background
3. Experimental Procedures
3.1. Establishment of the Test Platform
3.1.1. Test Samples and Gas Source
3.1.2. Test System
3.2. Measurement Methods
- Fins of the outlet side of heat exchangers of the five gas-fired instantaneous water heaters were examined macroscopically without disassembly and recorded with a digital camera every 400 cycles;
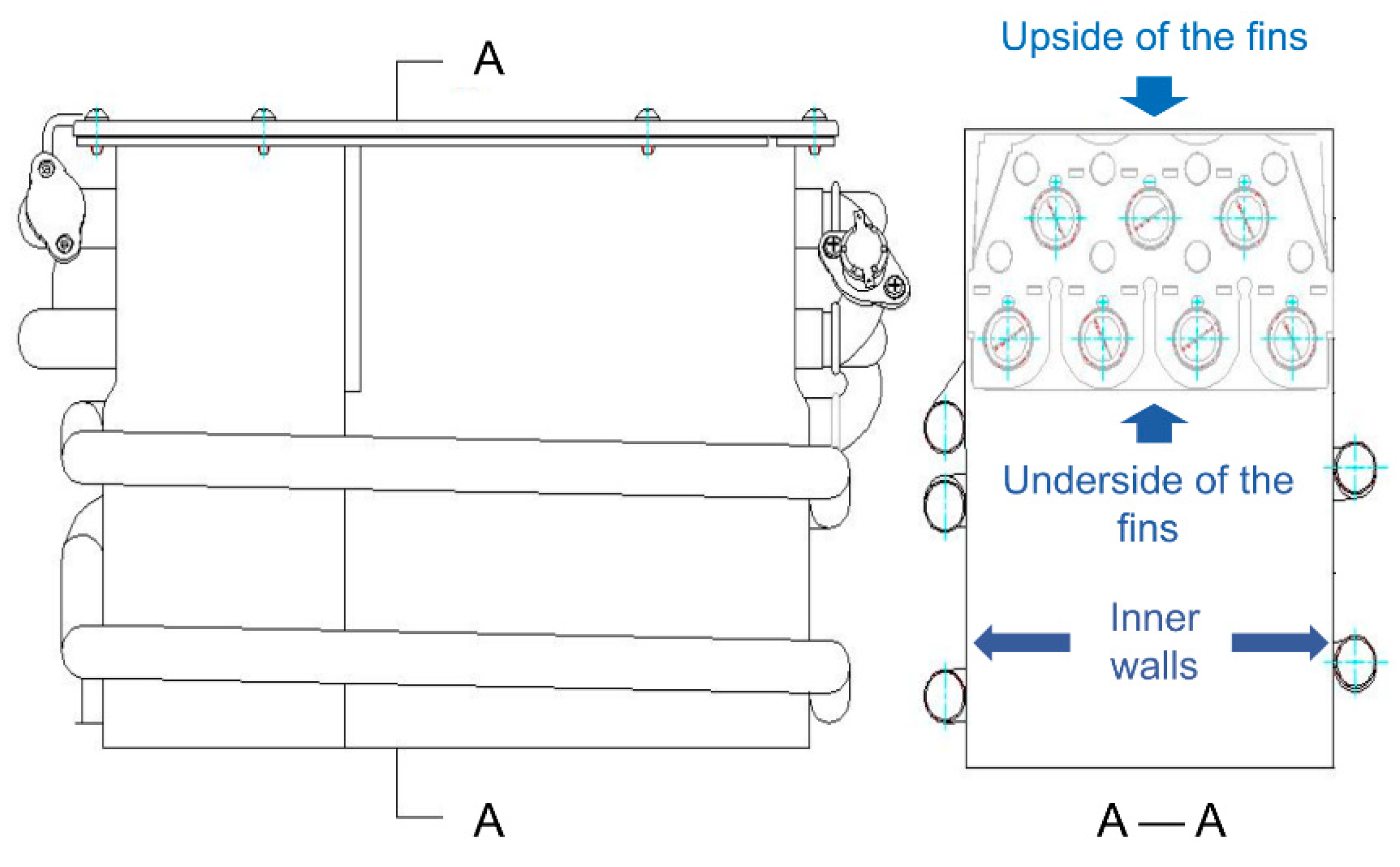
- The water heaters were disassembled after 1000 and 2000 test cycles, the condition of the heat exchangers was observed, and corrosion products on the inner wall of the combustion chamber and gums of the fins were sampled. Figure 4 shows the section view of the heat exchanger and the estimated positions in the detection are marked on the map;
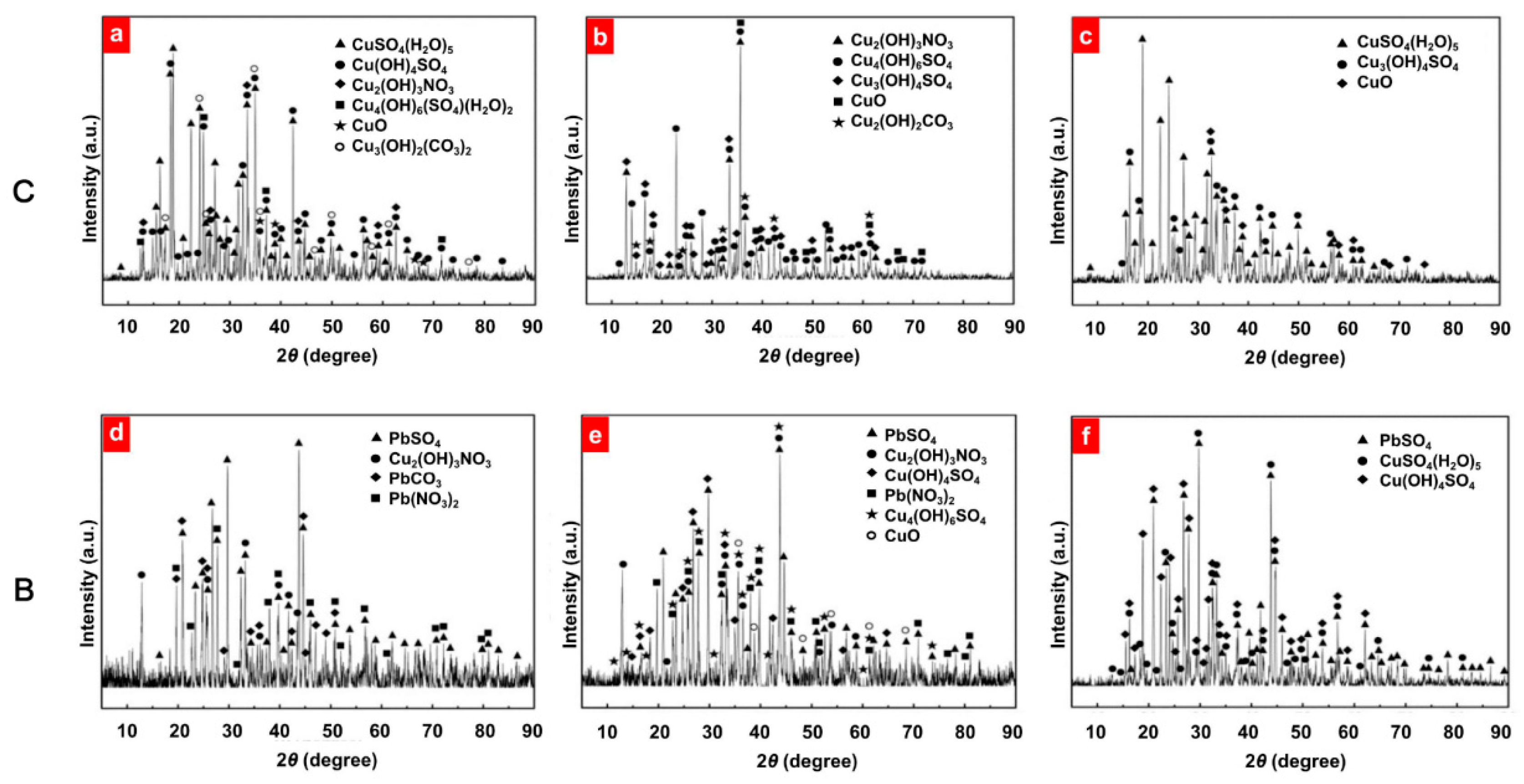
- Corrosion samples were examined by SEM, EDS and XRD;
- The heat exchangers were cleaned and the corrosion products were removed, and the weight loss of the heat exchanger was then measured after 2000 cycles, so the corrosion rate could be calculated.
- Before the test, the environmental parameters (such as temperature, atmospheric pressure, etc.) and the temperature of the inlet water were measured.
- During the test, the water temperature should be measured by mercury temperature after 15 min of launching it in order to ensure stable combustion of the water heater. The current value of the gas flow meter should be recorded when gas flow measurement starts by using a stopwatch. The outlet pipe should be placed in the water tank and the measurement should be finished after 1.5 min.
- The thermal efficiency of the gas-fired instantaneous water heater can be calculated as follows [6]:ηt = M × Cp(tw2 − tw1)/(V × Q1) × (273.15 + tg)/273.15 × 101.3/(Pamb + Pg − S) × 100.
4. Results and Discussion
4.1. Analysis of Results from Macroscopic Inspection
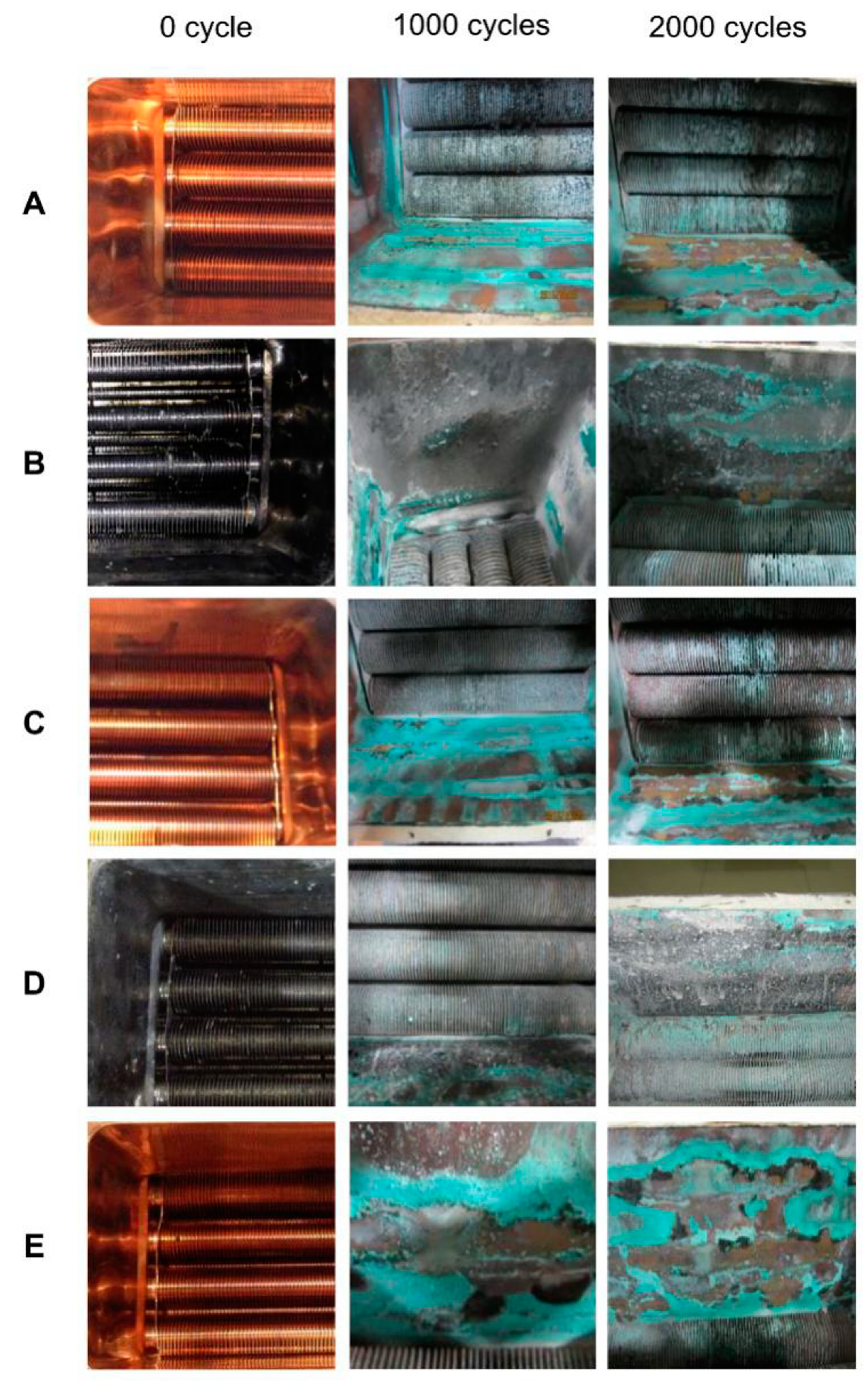
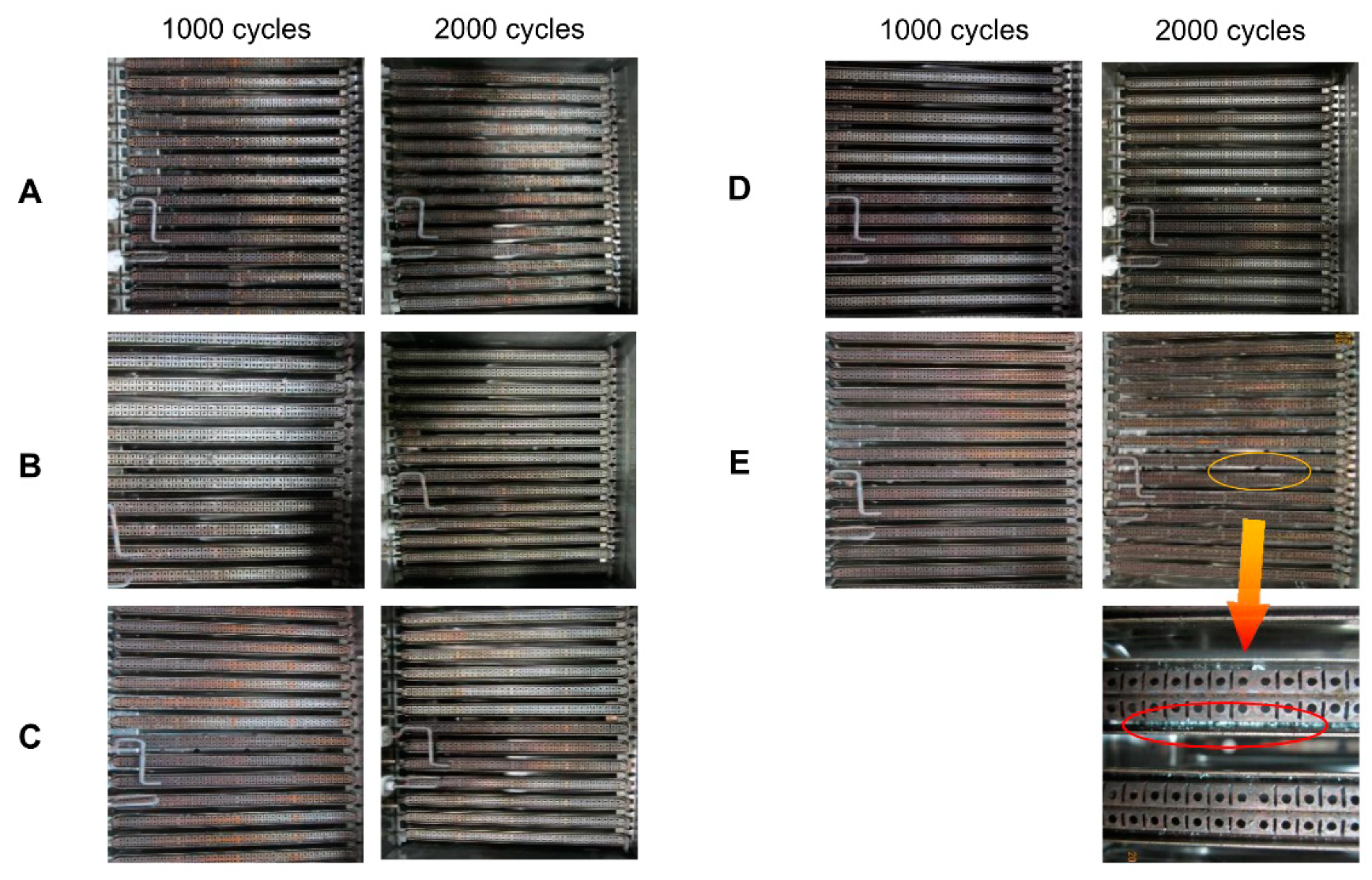
4.1.1. Macroscopic Inspection of Four Walls of Combustion Chambers
4.1.2. Macroscopic Inspection of the Underside of the Fins of the Heat Exchangers
4.1.3. Macro Inspection of the Upside of the Fin of the Heat Exchangers
4.1.4. Macroscopic Inspection of the Combustors
4.2. Analysis of the Microscopic Test
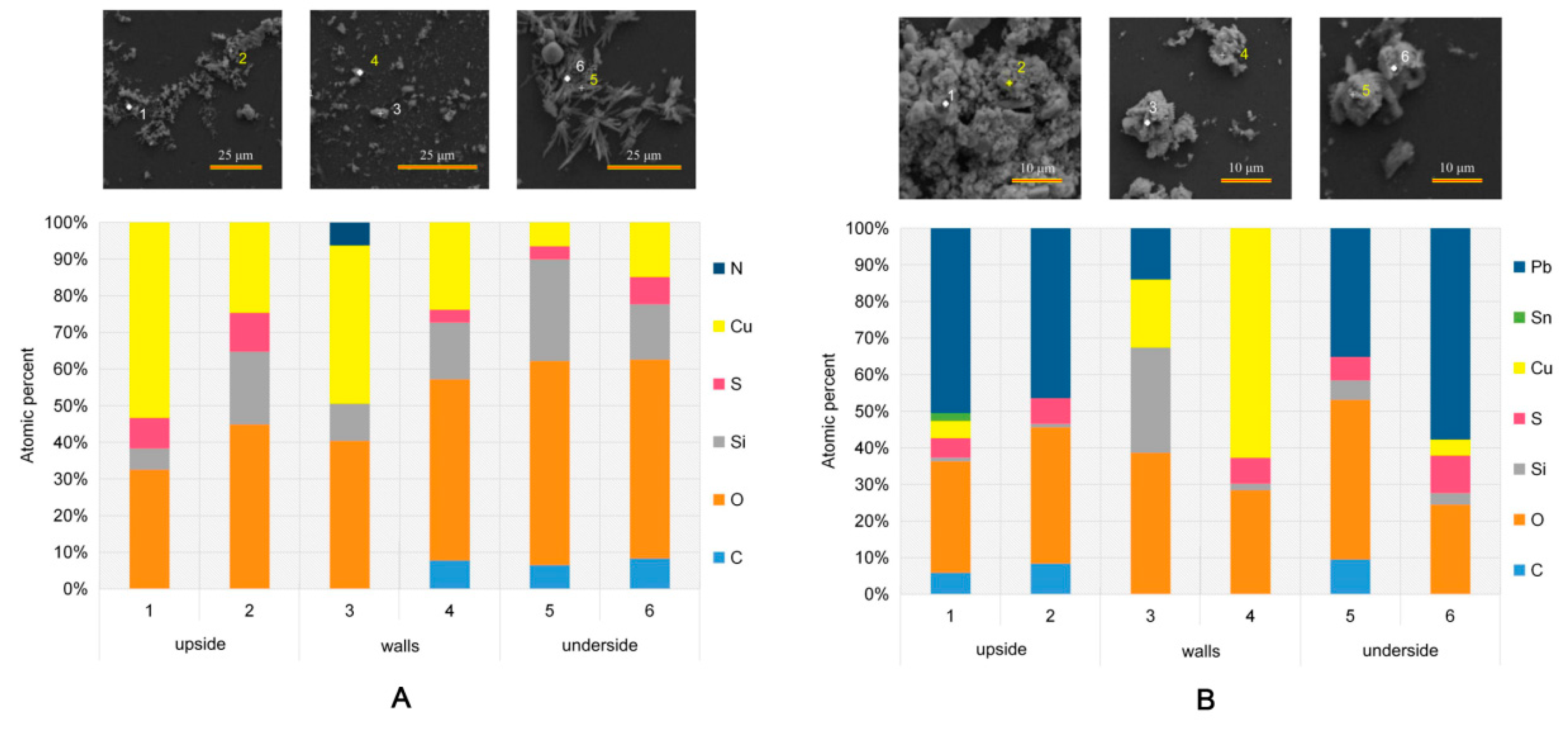
4.2.1. SEM and EDS
4.2.2. X-Ray Diffraction
4.3. Analysis of Corrosion Rates
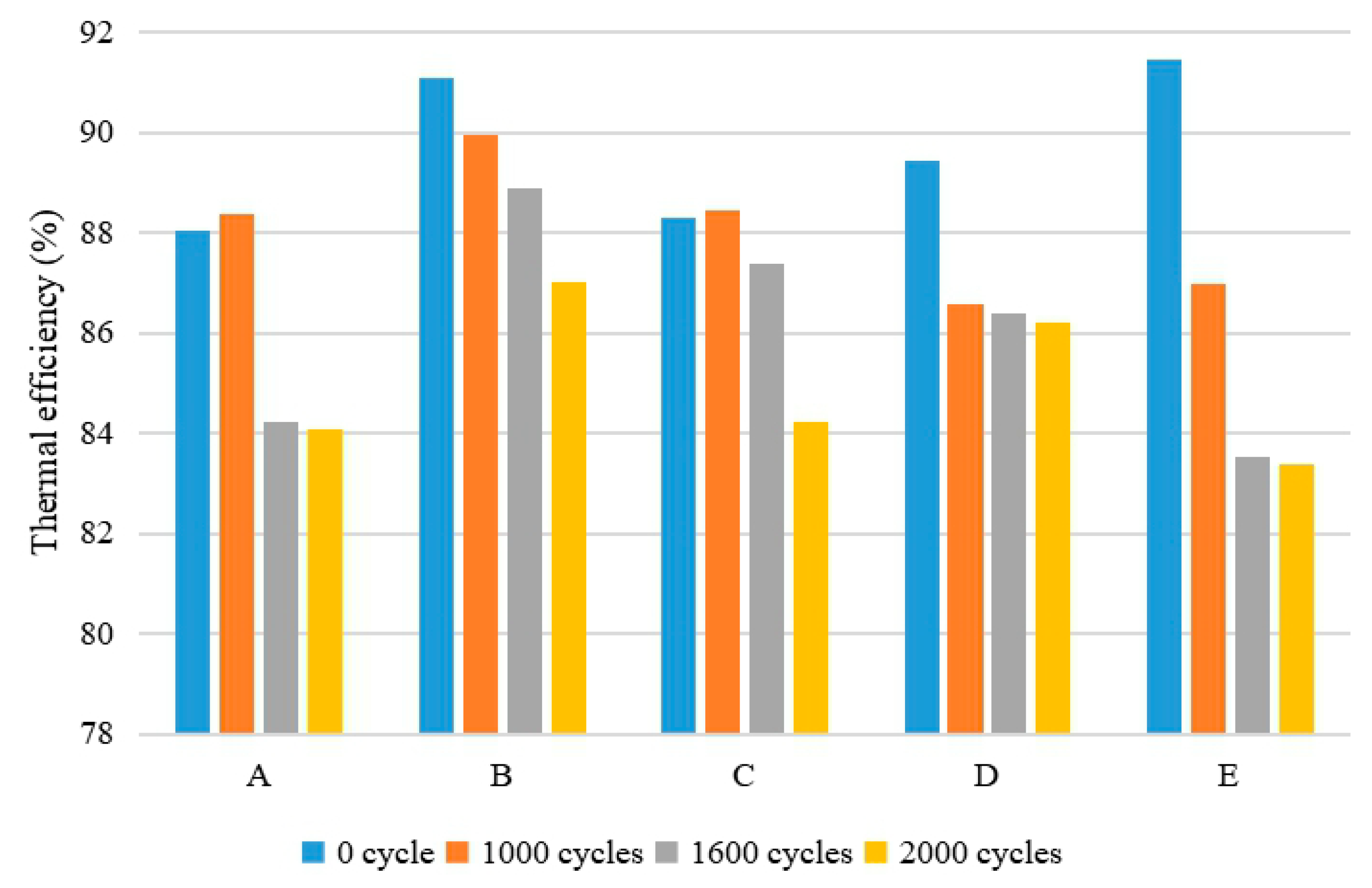
4.4. Analysis of Thermal Efficiency
4.5. Experimental Uncertainties
4.6. Suggestions for Mitigating the Fireside Corrosion for Gas-Fired Instantaneous Water Heaters
- Improve the quality of the gas. Gas meeting the first-class standard of civil fuel used in the gas-fired instantaneous water heater had positive effects on the reduction of the sulfide and sulfuric acid on the fireside of the heat exchanger;
- Increase the volume of excess air. According to a flue gas analyzer test, the volume fraction of oxygen in the flue gas refers to 12.5% and the excess air coefficient in the flue gas to 2.47 transferred by the volume fraction of oxygen. According to the equations in Table 4 and the composition of the natural gas (see Table 8), the water dew point was 41.51 °C and the acid dew point was 125.5 °C. However, the temperature on the surface of heat exchanger near the combustion chambers was about 35 °C, which was lower than the dew points. Even the temperature away from the combustion chambers was 70 °C, which was relatively higher than the water dew point required to make the flue gas weak corrosive. The formula in Table 4 illustrated that the dew point decreased with the increase of the excess air coefficient. Based on this, by increasing the excess air coefficient, the dew point could be reduced below the surface temperature of the heat exchanger to decrease corrosion. However, the increase of the excess air coefficient also had a negative impact on the heat loss of flue gas and thermal efficiency. Therefore, the excess air coefficient should be proper considering both the thermal efficiency and the longevity of the heat exchanger;
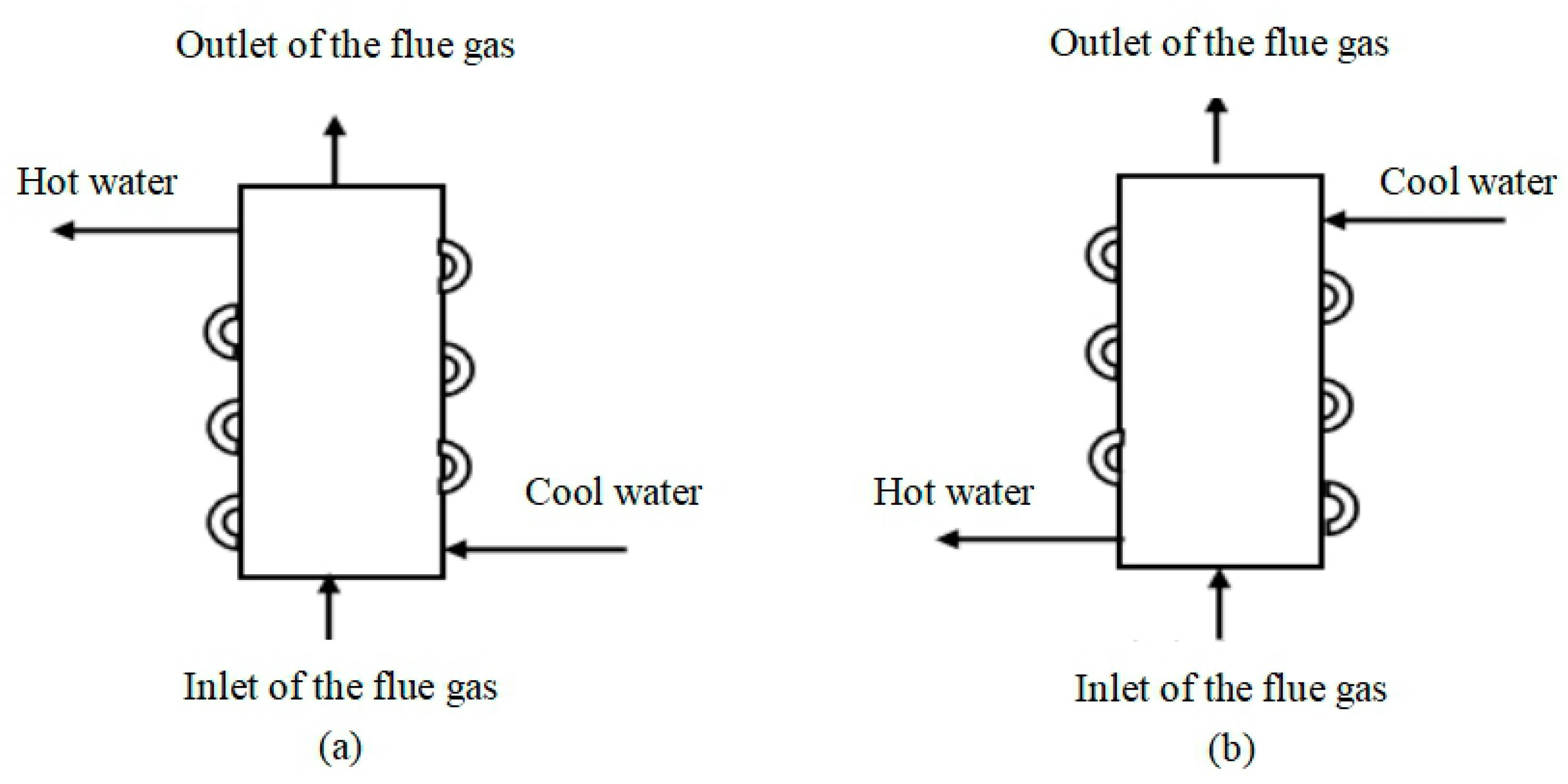
- Change the inlet and outlet position of the heat exchanger. The tube arrangement of heat exchangers in the tests is shown in Figure 15a. After the combustion of the gas in the combustion chamber, the high-temperature flue gas flowed upward from the bottom of the heat exchanger. If the inlet pipe was located at the place of the fins of the heat exchanger, and the outdoor wall of combustion was wrapped around the outlet pipe of the hot water, as is shown in Figure 15b, the average temperature difference between the water and the flue gas would be decreased. As a result, a lower amount of condensed water and acid would be generated on the inner wall of the combustion chamber, and the corrosion would be weakened;
- Choose a corrosion-resistant coating. To prevent an electrochemical reaction between the copper and acid solution, a coating could be plated on the surface of the heat exchanger, including a metal coating (protecting at the cost of sacrifice or forming a dense passivation layer, e.g., a nickel coating [48]) and non-metal coating (isolating the metal from acid solution, e.g., a fluoropolymer composite coating [49]). Furthermore, high-temperature resistance was also an important indicator of the coating and thus Fusible Ni-SA-c [50] could address this requirement to prolong the service life of the heat exchanger.
5. Conclusions
- It was found from macroscopic inspection that after 2000 cycles, the gap of fins of outlets of the flue gas was blocked by a flaky gray deposit whose amount increased with the increase of the heat input. The green solid particles on the walls of the heat exchanger were much more numerous than those for 1000 h, and the light green attachments covered a wider range. The fins of heat exchangers with a lead coating were corroded and even the copper surface was exposed;
- The microscopic examination showed that the corrosion products of heat exchangers plated without lead were made up of Cu4(OH)6SO4, CuSO4(H2O)5 and Cu3(OH)4SO4, while those of heat exchangers plated with lead were made up of PbSO4, Cu4(OH)6SO4 and CuSO4(H2O)5;
- The corrosion rate was calculated by changes of weight of the heat exchangers during the tests. The corrosion rate decreased with the increase of the heat input. Furthermore, the total mass lost was higher in the case of plating lead, but the reduction of copper was lower;
- The thermal efficiency of heaters declined to different degrees, because the accumulation of flue gas side corrosion products has bad effects on the heat transfer performance of heat exchangers. The thermal efficiency at the low heat input was always a little higher than that at the high heat input, regardless of the type of heaters. With regard to the same heat input, the thermal efficiency of the heat exchangers with a lead coating was higher than that of those without a lead coating. Even with the experimental uncertainties, the thermal efficiency of the heaters maintained the trend above.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| A | Tuned coefficient |
| B | Tuned coefficient |
| C | Tuned coefficient |
| D | Tuned coefficient |
| F1 | Empirical elemental concentration of carbon correction factors |
| F2 | Empirical excess air correction factors |
| α | Excess air fraction |
| ξ | Elemental concentration of carbon |
| S | Sulfur volume fraction for gas |
| T | Acid dew point temperature for combustion flue gases, °C |
| ηt | Thermal efficiency, % |
| M | Mass per minutes, kg/min |
| Cp | Heat capacity at constant pressure |
| tw2 | The temperature of the hot water, °C |
| tw1 | Influent temperature, °C |
| V | Consumption of water heater gas, m3/min |
| Q1 | Lower heating value of gas, MJ/m3 |
| t | Temperature, °C |
| P | Pressure, kPa |
| Ps | Partial vapor pressure, Pa |
| uc | Combined uncertainty, % |
| xi | Input quantity |
| u(xi) | Standard uncertainty of an input quantity, % |
| ci | Sensitivity coefficient |
| U | Expanded uncertainty, % |
| k | Coverage factor, k = 2 |
| Subscripts | |
| n | Atom number of carbon per cubic meter |
| m | Atom number of hydrogen per cubic meter |
| g | Gas |
| amb | Atmospheric |
References
- World Green Building Council. 2018 Global Status Report: Towards a Zero-Emission, Efficient and Resilient Buildings and Construction Sector; World Green Building Council: London, UK, 2018. [Google Scholar]
- Building Energy Research Center of Tsinghua University. China Building Energy Consumption Report (2017); Building Energy Research Center of Tsinghua University: Beijing, China, 2017. [Google Scholar]
- BP. Statistical Review of World Energy; British Petroleum: London, UK, 2018. [Google Scholar]
- BP. Energy Outlook, 2018 ed.; British Petroleum: London, UK, 2018. [Google Scholar]
- IEC 60335:2010+A1:2013 Household and Similar Electrical Appliances—Safety—Part 1: General Requirements, 5.1 ed.; DOE, IEA Publications: Washington, DC, USA, 2014.
- Domestic Gas Instantaneous Water Heater (GB6932–2015); Administration of Quality Supervision, Inspection and Quarantine (AQSIQ) Publications: Beijing, China, 2017.
- Minimum Allowable Values of Energy Efficiency and Energy Efficiency Grades for Domestic GAS INSTAntaneous Water Heaters and Gas Fired Heating and Hot Water Combi—Boilers (GB 20665–2015); Administration of Quality Supervision, Inspection and Quarantine (AQSIQ) Publications: Beijing, China, 2016.
- EN 26:1997/A3:2006:E Gas-Fired Instantaneous Water Heaters for the Production of Domestic Hot Water, Fitted With Atmospheric Burners; CEN/TC 48 Publications: Brussels, Belgium, 2006.
- Steinhagen, R.; Müller-Steinhagen, H.; Maani, K. Problems and costs due to heat exchanger fouling in New Zealand industries. Heat Transf. Eng. 1993, 14, 19–30. [Google Scholar] [CrossRef]
- Li, X.G.; Zhang, D.W.; Liu, Z.Y.; Li, Z.; Du, C.W.; Dong, C.F. Materials science: Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef] [PubMed]
- Corrosion Cost and Preventive Strategies in the United States; Federal Highway Administration Publications: Washington, DC, USA, 2002.
- Survey of Corrosion Cost in Japan; Committee on Cost of Corrosion in Japan Publications: Tokyo, Japan, 2001.
- Hou, B.R.; Lu, D.Z. Corrosion Cost and Preventive Strategies in China. Bull. Chin. Acad. Sci. 2018, 33, 601–609. (In Chinese) [Google Scholar]
- Srikanth, S.; Ravikumar, B.; Das, S.K.; Gopalakrishna, K.; Nandakumar, K.; Vijayan, P. Analysis of failures in boiler tubes due to fireside corrosion in a waste heat recovery boiler. Eng. Fail. Anal. 2003, 10, 59–66. [Google Scholar] [CrossRef]
- Malik, A.U.; Al-Muaili, F.; Al-Ayashi, M.; Meroufel, A. An investigation on the corrosion of flue gas sensor in boiler stack. Case Stud. Eng. Fail. Anal. 2003, 1, 200–207. [Google Scholar] [CrossRef]
- Li, M.J.; Tang, S.Z.; Wang, F.L.; Zhao, Q.X.; Tao, W.Q. Gas-side fouling, erosion and corrosion of heat exchangers for middle/low temperature waste heat utilization: A review on simulation and experiment. Appl. Therm. Eng. 2017, 126, 737–761. [Google Scholar] [CrossRef]
- Lampert, D. Low-temperature corrosion in feed-heaters heated by flue-gas. Brown Boveri Rev. 1978, 65, 691–695. [Google Scholar]
- Okoro, S.C.; Montgomery, M.; Frandsen, F.J.; Pantleon, K. Effect of water vapor on high-temperature corrosion under conditions mimicking biomass firing. Energy Fuels 2015, 29, 5802–5815. [Google Scholar] [CrossRef]
- Shoemaker, L.; Crum, J.; Maitra, D. Recent Experience with Stainless Steels in Wet Limestone FGD Air Pollution Control Service; NACE International Publications: Houston, TX, USA, 2011. [Google Scholar]
- Pan, P.Y.; Chen, H.; Liang, Z.Y.; Zhao, Q.X. Desulfurized flue gas corrosion coupled with deposits in a heating boiler. Corrosion Sci. 2018, 131, 126–136. [Google Scholar] [CrossRef]
- Khodamorad, S.H.; Alinezhad, N.; Fatmehsari, D.H.; Ghahtan, K. Stress corrosion cracking in Type.316 plates of a heat exchanger. Case Stud. Eng. Fail. Anal. 2016, 5, 59–66. [Google Scholar] [CrossRef]
- Angell, P.; Urbanic, K. Sulphate-reducing bacterial activity as a parameter to predict localized corrosion of stainless alloys. Corros. Sci. 2000, 42, 897–912. [Google Scholar] [CrossRef]
- Stein-Brzozowska, G.; Maier, J.; Scheffknecht, G.; Cumbo, D.; Masci, S.; Tosi, E.; Corraggio, G.; Faleni, M.; Biasci, L. Fireside corrosion of applied and modern superheater-alloys under oxy-fuel conditions. Energy Procedia 2013, 37, 1448–1461. [Google Scholar] [CrossRef]
- Wang, X.; Fang, M.; Zhang, L.; Ding, H.; Liu, Y.; Huang, Z.; Huang, S.; Yang, J. Solid particle erosion of alumina ceramics at elevated temperature. Mater. Chem. Phys. 2013, 139, 765–769. [Google Scholar] [CrossRef]
- Shida, Y.; Fujikawa, H. Particle erosion behaviour of boiler tube materials at elevated temperature. Wear 1985, 103, 281–296. [Google Scholar] [CrossRef]
- Kladkaew, N.; Idem, R.; Tontiwachwuthikul, P.; Saiwan, C. Studies on corrosion and corrosion inhibitors for amine based solvents for CO2 absorption from power plant flue gases containing CO2, O2 and SO2. Energy Procedia 2011, 4, 1761–1768. [Google Scholar] [CrossRef]
- Paz, M.D.; Zhao, D.; Karlsson, S.; Liske, J.; Jonsson, T. Investigating corrosion memory: The influence of previous boiler operation on current corrosion rate. Fuel Process. Technol. 2017, 156, 348–356. [Google Scholar] [CrossRef]
- Su, J.X.; Ma, M.Y.; Wang, T.J.; Guo, X.M.; Hou, L.G.; Wang, Z.P. Fouling corrosion in aluminum heat exchangers. Chin. J. Aeronaut. 2015, 28, 954–960. [Google Scholar] [CrossRef] [Green Version]
- Kleiner, M.B.; Kühn, S.A.; Haberger, K. High Performance Forced Air Cooling Scheme Employing Microchannel Heat Exchangers. IEEE Trans. Compon. Pack. Manuf. 1995, 18, 795–804. [Google Scholar] [CrossRef]
- Gurrappa, I. Cathodic protection of cooling water systems and selection of appropriate materials. J. Mater. Process. Tech. 2005, 166, 256–267. [Google Scholar] [CrossRef]
- Tian, J.; Kim, T.; Lu, T.J.; Hodson, H.P.; Queheillalt, D.T.; Sypeck, D.J.; Wadley, H.N.G. The effects of topology upon fluid-flow and heat-transfer within cellular copper structures. Int. J. Heat Mass Transf. 2004, 47, 3171–3186. [Google Scholar] [CrossRef]
- Ranjbar, K. Effect of flow induced corrosion and erosion on failure of a tubular heat exchanger. Mater. Des. 2010, 31, 613–619. [Google Scholar] [CrossRef]
- Damon, G.H. Acid corrosion of steel: Effect of carbon content on the corrodibility of steel in sulfuric acid. Ind. Eng. Chem. 1941, 33, 67–69. [Google Scholar] [CrossRef]
- Wang, Y.G.; Zhao, Q.X.; Zhang, Z.X.; Zhang, Z.C.; Tao, W.Q. Mechanism research on coupling effect between dew point corrosion and ash deposition. Appl. Therm. Eng. 2013, 54, 102–110. [Google Scholar] [CrossRef]
- Zucchi, F.; Trabanelli, G.; Monticelli, C. The inhibition of copper corrosion in 0.1M NaCl under heat exchange conditions. Corros. Sci. 1996, 38, 147–154. [Google Scholar] [CrossRef]
- Brady, M.P.; Banta, K.; Mizia, J.; Lorenz, N.; Leonard, D.N.; Yamamoto, Y.; Defoort, M.; Keiser, J.R. Alloy corrosion considerations in low-cost, clean biomass cookstoves for the developing world. Energy Sustain. Dev. 2017, 37, 20–32. [Google Scholar] [CrossRef]
- Hwanga, K.; Song, C.H.; Saito, K.; Kawai, S. Experimental study on titanium heat exchanger used in a gas fired water heater for latent heat recovery. Appl. Therm. Eng. 2010, 30, 2730–2737. [Google Scholar] [CrossRef]
- Gruber, T.; Schulze, K.; Scharler, R.; Obernberger, I. Investigation of the corrosion behaviour of 13CrMo4-5 for biomass fired boilers with coupled online corrosion and deposit probe measurements. Fuel 2015, 144, 15–24. [Google Scholar] [CrossRef]
- Jeong, K.; Levy, E.K. Theoretical prediction of sulfuric acid condensation rates in boiler flue gas. Int. J. Heat Mass Trans. 2012, 55, 8010–8019. [Google Scholar] [CrossRef]
- Blanco, J.M.; Peña, F. Increase in the boiler’s performance in terms of the acid dew point temperature: Environmental advantages of replacing fuels. Appl. Therm. Eng. 2008, 28, 777–784. [Google Scholar] [CrossRef]
- Guo, J.Z.; Liu, X.W.; Wang, H.; Zhang, P.H.; Xu, Y.S.; Chen, Z.G.; Xu, M.H. Effect of HCl and CO on sulfur trioxide formation mechanisms during oxy fuel combustion. Fuel Process. Technol. 2018, 174, 95–103. [Google Scholar] [CrossRef]
- Zheng, Y.X.; Zhao, H.Y.; Ye, Y.Z.; Zhong, J.S.; Xia, Z.Z.; Wu, G.F. Experimental study on corrosion prevention of low-temperature section of heat exchanger in condensing gas water heater. Gas. Heat 2007, 27, 35–41. (In Chinese) [Google Scholar]
- Jia, M.S.; Ling, C.M. Factors of affecting the flue gas acid dew point temperature and its way of calculation. Ind. Boiler 2013, 6, 31–35. (In Chinese) [Google Scholar]
- Haar, L.; Gallagher, J.S.; Kell, G.S. NBS/NRC Steam Tables: Thermodynamic and Transport Properties and Computer Programs for Vapor and Liquid States of Water; Hemisphere Publishing Company: Washington, DC, USA, 1984. [Google Scholar]
- Bahadori, A. Estimation of combustion flue gas acid dew point during heat recovery and efficiency gain. Appl. Therm. Eng. 2011, 31, 1457–1462. [Google Scholar] [CrossRef]
- International Organization for Standardization. A Guide to Expression of Uncertainty in Measurement (ISO /1995); International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]
- Bürger, S.; Essex, R.M.; Mathew, K.J.; Richter, S.; Thomas, R.B. Implementation of Guide to the expression of Uncertainty in Measurement (GUM) to multi-collector TIMS uranium isotope ratio metrology. Int. J. Mass Spectrom. 2010, 294, 65–76. [Google Scholar] [CrossRef]
- He, Y.L.; Walsh, D.; Shi, C. Fluoropolymer composite coating for condensing heat exchangers: Characterization of the mechanical, tribological and thermal properties. Appl. Therm. Eng. 2015, 91, 387–398. [Google Scholar] [CrossRef]
- Wang, Y.C.; Tang, G.H. Prediction of sulfuric acid dew point temperature on heat transfer fin surface. Appl. Therm. Eng. 2016, 98, 492–501. [Google Scholar] [CrossRef]
- Chen, J.; Ninomiya, Y.; Naganuma, H.; Sasaki, Y.; Noguchi, M.; Cho, H.; Ueki, Y.; Yoshiie, R.; Naruse, I. Development of thermal spraying materials through several corrosion tests for heat exchanger tube of incinerators. Fuel Process. Technol. 2016, 141, 216–224. [Google Scholar] [CrossRef]








| Energy Efficiency Rate | Minimum Thermal Efficiency (%) | |
|---|---|---|
| Rating Heat Input | ≤50% Rating Heat Input | |
| 1 | 96 | 94 |
| 2 | 88 | 84 |
| 3 | 84 | - |
| Country | Year | Annual Corrosion Cost | The Proportion of Corrosion Cost in GDP/GNP |
|---|---|---|---|
| China | 2014 | More than 2.1 trillion yuan | 3.34% [13] |
| U.S. | 1998 | $276 billion (direct) | 3.1% (directly) Around 6% (direct and indirect) [11] |
| Japan | 1997 | 3938 billion yen | 0.77% [12] |
| India | 2004 | $364 billion (direct) | 3.1% (direct and indirect) 1 |
| Britain | 1971 | 1.365 billion pounds | 3.5% [13] |
| Partial Vapor Pressure (kPa) | Water Dew Point (°C) |
|---|---|
| 5.5 | 34.589 |
| 6 | 36.167 |
| 6.5 | 37.635 |
| 7 | 39.008 |
| 7.5 | 40.299 |
| 8 | 41.518 |
| 8.5 | 42.673 |
| 9 | 43.771 |
| 9.5 | 44.817 |
| 10 | 45.817 |
| Number of Line | Formula | Coefficient Formula |
|---|---|---|
| 1 | ln(F1) = a + b/S + c/S2 + d/S3 | a = A1 + B1/ξ + C1/ξ2 + D1/ξ3 b = A2 + B2/ξ + C2/ξ2 + D2/ξ3 c = A3 + B3/ξ + C3/ξ2 + D3/ξ3 d = A4 + B4/ξ + C4/ξ2 + D4/ξ3 |
| 2 | ln(F2) = a + b/(F1) + c/(F1)2 + d/(F1)3 | a = A1 + B1/α + C1/α2 + D1/α3 b = A2 + B2/α + C2/α2 + D2/α3 c = A3 + B3/α + C3/α2 + D3/α3 d = A4 + B4/α + C4/α2 + D4/α3 |
| 3 | ln(T) = a + b(F2) + c(F2)2 + d(F2)3 | a = A1 + B1/ξ + C1/ξ2 + D1/ξ3 b = A2 + B2/ξ + C2/ξ2 + D2/ξ3 c = A3 + B3/ξ + C3/ξ2 + D3/ξ3 d = A4 + B4/ξ + C4/ξ2 + D4/ξ3 |
| Coefficient | Tuned Coefficient Values for Equations in Line 1 of Table 4 | Tuned Coefficient Values for Equations in Line 2 of Table 4 | Tuned Coefficient Values for Equations in Line 3 of Table 4 |
|---|---|---|---|
| A1 | 1.6169452391 | 0 | 5.9522580890 |
| B1 | 6.1776693132 × 10−1 | 1.2168193901 | 3.7435527319 × 10−2 |
| C1 | −3.9653315194 × 10−1 | −1.2791492533 × 10−1 | −2.9745433707 × 10−2 |
| D1 | 8.9951003043 × 10−2 | 3.7468691518 × 10−3 | 8.0120588749 × 10−3 |
| A2 | −7.3040211893 × 10−3 | 0 | 1.4895974061 × 10−2 |
| B2 | 6.9240838238 × 10−3 | −3.5038003546 | −2.5447171264 × 10−2 |
| C2 | −5.8556137034 × 10−3 | 3.5352583979 × 10−1 | 2.8643928069 × 10−2 |
| D2 | 1.5959152735 × 10−3 | −1.0220477526 × 10−2 | −8.5637681681 × 10−3 |
| A3 | 1.0870177961 × 10−5 | 0 | 6.4518970719 × 10−5 |
| B3 | −8.5890701138 × 10−6 | 4.2457067212 | 9.7931326979 × 10−3 |
| C3 | 8.1950413835 × 10−6 | −4.2048282453 × 10−1 | −1.1108881728 × 10−2 |
| D3 | −2.4343154548 × 10−6 | 1.2049410160 × 10−2 | 3.3304384324 × 10−3 |
| A4 | −6.1128433684 × 10−9 | 0 | 5.0957445440 × 10−5 |
| B4 | 4.6119957003 × 10−9 | −1.9582714148 | −9.3033575126 × 10−4 |
| C4 | 4.4972769065 × 10−9 | 1.8294597537 × 10−1 | 1.0575132247 × 10−3 |
| D4 | 1.3588028938 × 10−9 | −5.1643724550 × 10−3 | −3.1726950227 × 10−4 |
| Test Prototype | Thermal Load (kW) | Coating on the Heat Exchanger |
|---|---|---|
| A | 28 | None |
| B | 28 | Lead |
| C | 32 | None |
| D | 32 | Lead |
| E | 40 | None |
| Components | Materials | Specifications (mm) |
|---|---|---|
| Water inlet | Copper (C1220T) | Φ12 × 0.6 (diameter × thickness) |
| Water outlet | Copper (C1220T) | Φ12 × 0.6 |
| Connecting pipe | Copper (C1220T) | Φ12 × 0.7 |
| Tube | Copper (C1220T) | Φ16 × 0.8 |
| Tube pitch | - | 32 |
| Coil out of the shell | Copper (C1220T) | Φ12 × 0.6 |
| Shell | Copper (C1220P-1/4H) | 0.4 (thickness) |
| Fins | Copper (C1220P-1/4H) | 0.25 (thickness) |
| Fin pitch | - | 2.55 |
| Fin height | - | 68 |
| Coating of B and D | More than 95% lead | 0.2–0.3 (thickness) |
| Test Content | Units | Results |
|---|---|---|
| CH4 | % | 95.02 |
| C2H6 | 1.719 | |
| C3H8 | 0.287 | |
| i-C4H10 | 0.0526 | |
| n-C4H10 | 0.0526 | |
| i-C5H12 | 0.0193 | |
| n-C5H12 | 0.0116 | |
| C6+ | 0.0152 | |
| O2 | 0.28 | |
| N2 | 1.34 | |
| CO2 | 1.2 | |
| Higher heating value (20 °C) | MJ/m3 | 39.46 |
| Lower heating value (20 °C) | MJ/m3 | 35.46 |
| H2S | mg/m3 | 12.29 |
| Name | Model | |
|---|---|---|
| 1 | Flue gas analyzer | ecom-J2KN |
| 2 | Gas chromatograph | GC-1690 |
| 3 | Gas flowmeter | DC-2 (10L/R) |
| 4 | Gas thermometer | Mercury thermometer (0–100 °C) |
| 5 | Gas pressure gauge | U pressure gauge (0–12 kPa) |
| 6 | Inlet and outlet thermometer | Mercury thermometer (0–100 °C) |
| 7 | Flue gas thermometer | Mercury thermometer (0–200 °C) |
| 8 | Scale | KW-30B (0–30 kg) |
| Test Prototype | A | B | C | D | E |
|---|---|---|---|---|---|
| Weight before tests (g) | 3698 | 3960 | 3712 | 3946 | 3675 |
| Weight after tests (g) | 3580 | 3740 | 3595 | 3825 | 3595 |
| Loss (g) | 118 | 220 | 117 | 121 | 80 |
| Rate of corrosion (g/h) | 0.0590 | 0.110 | 0.0585 | 0.0605 | 0.0400 |
| Standard uncertainty (g/h) [46] | 0.00182 | 0.00193 | 0.00183 | 0.00194 | 0.00182 |
| Cycles | Test Prototype | uc (%) | U (%) | Final Results of the Thermal Efficiency (%) |
|---|---|---|---|---|
| 1000 | A | 0.26 | 0.53 | 86.40 ± 0.53 |
| B | 0.28 | 0.55 | 88.47 ± 0.55 | |
| C | 0.31 | 0.62 | 88.90 ± 0.62 | |
| D | 0.26 | 0.52 | 88.39 ± 0.52 | |
| E | 0.31 | 0.61 | 87.00 ± 0.61 | |
| 1600 | A | 0.26 | 0.51 | 84.22 ± 0.51 |
| B | 0.29 | 0.58 | 89.97 ± 0.58 | |
| C | 0.30 | 0.61 | 87.38 ± 0.61 | |
| D | 0.25 | 0.50 | 86.59 ± 0.50 | |
| E | 0.26 | 0.51 | 83.53 ± 0.51 | |
| 2000 | A | 0.33 | 0.66 | 84.07 ± 0.66 |
| B | 0.28 | 0.56 | 87.01 ± 0.56 | |
| C | 0.29 | 0.58 | 83.22 ± 0.58 | |
| D | 0.26 | 0.51 | 86.22 ± 0.51 | |
| E | 0.25 | 0.50 | 83.40 ± 0.50 |
| Cycles | Test Prototype | uc (%) | U (%) | Final Results of the Thermal Efficiency (%) |
|---|---|---|---|---|
| 1000 | A | 0.56 | 1.11 | 94.07 ± 1.11 |
| B | 0.45 | 0.91 | 95.56 ± 0.91 | |
| C | 0.53 | 1.06 | 90.69 ± 1.06 | |
| D | 0.53 | 1.06 | 94.69 ± 1.06 | |
| E | 0.65 | 1.30 | 92.06 ± 1.30 | |
| 1600 | A | 0.51 | 1.02 | 91.93 ± 1.02 |
| B | 0.43 | 0.86 | 90.90 ± 0.86 | |
| C | 0.53 | 1.07 | 88.42 ± 1.07 | |
| D | 0.49 | 0.98 | 93.47 ± 0.98 | |
| E | 0.56 | 1.12 | 88.25 ± 1.12 | |
| 2000 | A | 0.63 | 1.27 | 90.98 ± 1.27 |
| B | 0.47 | 0.93 | 89.59 ± 0.93 | |
| C | 0.52 | 1.04 | 87.22 ± 1.04 | |
| D | 0.50 | 0.99 | 92.27 ± 0.99 | |
| E | 0.58 | 1.17 | 91.64 ± 1.17 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Sun, M.; Kang, Y. Fireside Corrosion on Heat Exchanger Surfaces and Its Effect on the Performance of Gas-Fired Instantaneous Water Heaters. Energies 2019, 12, 2583. https://doi.org/10.3390/en12132583
Huang X, Sun M, Kang Y. Fireside Corrosion on Heat Exchanger Surfaces and Its Effect on the Performance of Gas-Fired Instantaneous Water Heaters. Energies. 2019; 12(13):2583. https://doi.org/10.3390/en12132583
Chicago/Turabian StyleHuang, Xiaomei, Mengxiao Sun, and Yinhu Kang. 2019. "Fireside Corrosion on Heat Exchanger Surfaces and Its Effect on the Performance of Gas-Fired Instantaneous Water Heaters" Energies 12, no. 13: 2583. https://doi.org/10.3390/en12132583





