Water Mixtures as Working Fluids in Organic Rankine Cycles †
Abstract
:1. Introduction
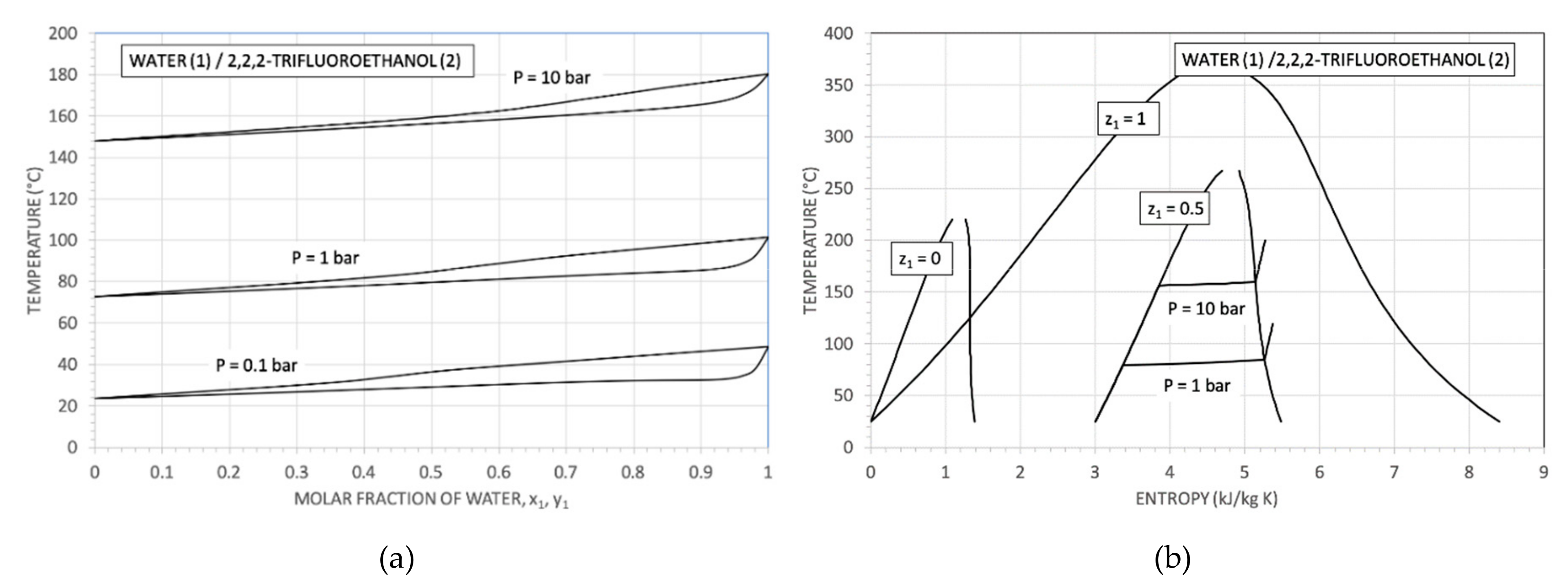
2. Water Mixtures Selection
3. Organic Rankine Cycle Performance Evaluation
3.1. Methodology
3.2. Results
4. Thermal Stability
5. Conclusions
- 1)
- mixture of organic fluids with water can drastically extend the working fluid selection with potential advantages in terms of safety concerns, thus reducing the flammability and toxicity;
- 2)
- thermodynamic properties can be tailored case-by-case to the heat source power and temperature level.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nomenclature
| A | Coefficient of vapour pressure equation, bar |
| B | Coefficient of vapour pressure equation, bar K |
| H | Specific enthalpy, kJ/kg |
| k* | Quasi-constant velocity of reaction, s−1 |
| MITA | Minimum temperature approach, °C |
| P | Pressure, bar |
| Pcr | Critical pressure, bar |
| Pv | Vapour pressure, bar |
| S | Specific entropy, kJ/kg K |
| T | Temperature, °C |
| Tb | Boiling temperature, °C |
| Tcr | Critical temperature, °C |
| Tr | Reduced temperature, - |
| x, y | Molar fractions in liquid and vapour phases - |
| Molar fraction of component 1 in a mixture, - | |
| Λ | Reaction degree, - |
| σ | Molecular complexity, - |
| Acronyms | |
| C | cold |
| F | NFPA flammability level |
| H | hot |
| L | liquid |
| MM | molar mass, kg/kmol |
| NRTL | Non-Random Two Liquid |
| NFPA | National Fire Protection Association |
| ORC | organic Rankine cycle |
| RKS | Redlich-Kwong-Soave |
| SP | size parameter () |
| V | vapour |
| VLE | Vapour Liquid Equilibrium |
References
- Invernizzi, C.M.; Iora, P.; Preißinger, M.; Manzolini, G. HFOs as substitute for R-134a as working fluids in ORC power plants: A thermodynamic assessment and thermal stability analysis. Appl. Therm. Eng. 2016, 103, 790–797. [Google Scholar] [CrossRef]
- Dai, X.; Shi, L.; An, Q.; Qian, W. Screening of working fluids and metal materials for high temperature organic Rankine cycles by compatibility. J. Renew. Sustain. Energy 2017, 9. [Google Scholar] [CrossRef]
- Tchanche, B.F.; Papadakis, G.; Lambrinos, G.; Frangoudakis, A. Fluid selection for a low-temperature solar organic Rankine cycle. Appl. Therm. Eng. 2009, 29, 2468–2476. [Google Scholar] [CrossRef] [Green Version]
- Macchi, E.; Astolfi, M. Organic Rankine Cycle (ORC) Power Systems: Technologies and Applications. Woodhead Publishing Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Astolfi, M.; Lasala, S.; Macchi, E. Selection Maps for ORC and CO2 Systems for Low-Medium Temperature Heat Sources. Energy Procedia 2017, 129, 971–978. [Google Scholar] [CrossRef]
- Sprouse, C.; Depcik, C. Review of organic Rankine cycles for internal combustion engine exhaust waste heat recovery. Appl. Therm. Eng. 2013, 51, 711–722. [Google Scholar] [CrossRef]
- Linke, P.; Papadopoulos, I.A.; Seferlis, P. Systematic Methods for Working Fluid Selection and the Design, Integration and Control of Organic Rankine Cycles—A Review. Energies 2015, 8, 4755–4801. [Google Scholar] [CrossRef]
- Blondel, Q.; Tauveron, N.; Caney, N.; Voeltzel, N. Experimental Study and Optimization of the Organic Rankine Cycle with Pure NovecTM649 and Zeotropic Mixture NovecTM649/HFE7000 as Working Fluid. Appl. Sci. 2019, 9, 1865. [Google Scholar] [CrossRef]
- Invernizzi, C.; Sheikh, N. High-efficiency small-scale combined heat and power organic binary Rankine cycles. Energies 2018, 11, 994. [Google Scholar] [CrossRef]
- Le, V.L.; Feidt, M.; Kheiri, A.; Pelloux-Prayer, S. Performance optimization of low-temperature power generation by supercritical ORCs (organic Rankine cycles) using low GWP (global warming potential) working fluids. Energy 2014, 67, 513–526. [Google Scholar] [CrossRef]
- Moloney, F.; Almatrafi, E.; Goswami, D.Y. Working fluid parametric analysis for regenerative supercritical organic Rankine cycles for medium geothermal reservoir temperatures. Energy Procedia 2017, 129, 599–606. [Google Scholar] [CrossRef]
- Scaccabarozzi, R.; Tavano, M.; Invernizzi, C.M.; Martelli, E. Comparison of working fluids and cycle optimization for heat recovery ORCs from large internal combustion engines. Energy 2018, 158, 396–416. [Google Scholar] [CrossRef]
- Keulen, L.; Gallarini, S.; Landolina, C.; Spinelli, A.; Iora, P.; Invernizzi, C.; Lietti, L.; Guardone, A. Thermal stability of hexamethyldisiloxane and octamethyltrisiloxane. Energy 2018, 165, 868–876. [Google Scholar] [CrossRef]
- Luo, X.; Liang, Z.; Guo, G.; Wang, C.; Chen, Y.; Ponce-Ortega, J.M.; El-Halwagi, M.M. Thermo-economic analysis and optimization of a zoetropic fluid organic Rankine cycle with liquid-vapor separation during condensation. Energy Convers. Manag. 2017, 148, 517–532. [Google Scholar] [CrossRef]
- Oyewunmi, O.A.; Kirmse, C.J.W.; Pantaleo, A.M.; Markides, C.N. Performance of working-fluid mixtures in ORC-CHP systems for different heat-demand segments and heat-recovery temperature levels. Energy Convers. Manag. 2017, 148, 1508–1524. [Google Scholar] [CrossRef]
- Di Battista, D.; Cipollone, R.; Villante, C.; Fornari, C.; Mauriello, M. The Potential of Mixtures of Pure Fluids in ORC-Based Power Units Fed by Exhaust Gases in Internal Combustion Engines. Energy Procedia 2016, 101, 1264–1271. [Google Scholar] [CrossRef]
- Andreasen, J.G.; Larsen, U.; Knudsen, T.; Pierobon, L.; Haglind, F. Selection and optimization of pure and mixed working fluids for low grade heat utilization using organic rankine cycles. Energy 2014, 73, 204–213. [Google Scholar] [CrossRef]
- Abadi, G.B.; Kim, K.C. Investigation of organic Rankine cycles with zeotropic mixtures as a working fluid: Advantages and issues. Renew. Sustain. Energy Rev. 2017, 73, 1000–1013. [Google Scholar] [CrossRef]
- Xu, W.; Deng, S.; Zhang, Y.; Zhao, D.; Zhao, L. How to give a full play to the advantages of zeotropic working fluids in organic Rankine cycle (ORC). Energy Procedia 2019, 158, 1591–1597. [Google Scholar] [CrossRef]
- Miao, Z.; Zhang, K.; Wang, M.; Xu, J. Thermodynamic selection criteria of zeotropic mixtures for subcritical organic Rankine cycle. Energy 2019, 167, 484–497. [Google Scholar] [CrossRef]
- Dong, B.; Xu, G.; Li, T.; Quan, Y.; Wen, J. Thermodynamic and economic analysis of zeotropic mixtures as working fluids in low temperature organic Rankine cycles. Appl. Therm. Eng. 2018, 132, 545–553. [Google Scholar] [CrossRef]
- Zhai, H.; An, Q.; Shi, L. Zeotropic mixture active design method for organic Rankine cycle. Appl. Therm. Eng. 2018, 129, 1171–1180. [Google Scholar] [CrossRef]
- Micheli, D.; Pinamonti, P.; Reini, M.; Taccani, R. Performance Analysis and Working Fluid Optimization of a Cogenerative Organic Rankine Cycle Plant. J. Energy Resour. Technol. 2013, 135, 21601–21611. [Google Scholar] [CrossRef]
- De Marchi Desenzani, P.; Gaia, M.; Invernizzi, C. Modification of working fluid in geothermal organic Rankine cycle engines. Int. Symp. Geotherm. Energy, Geotherm. Resour. Counc. 1985. Available online: https://www.geothermal-library.org/index.php?mode=pubs&action=view&record=1001478 (accessed on 6 July 2019).
- Collings, P.; Yu, Z.; Wang, E. A dynamic organic Rankine cycle using a zeotropic mixture as the working fluid with composition tuning to match changing ambient conditions. Appl. Energy 2016, 171, 581–591. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Gao, T. Off-design performance analysis of basic ORC, ORC using zeotropic mixtures and composition-adjustable ORC under optimal control strategy. Energy 2019, 171, 95–108. [Google Scholar] [CrossRef]
- Siemens. Siemens Technical Papers on Steam Turbines. 2019. Available online: www.energy.siemens.com/hq/en/energy-topics/publications/technical-papers/steam-turbines.htm (accessed on 11 May 2019 ).
- Rogdakis, E.D. Thermodynamic analysis, parametric study and optimum operation of the Kalina cycle. Int. J. Energy Res. 1996, 20, 359–370. [Google Scholar] [CrossRef]
- Somekh, G.S. Water-Pyridine Azeotrope is an Excellent Rankine Cycle Fluid. J. Eng. Power 1975, 97, 583–588. [Google Scholar] [CrossRef]
- Patel, P.; Doyle, E.F.; Raymond, R.J.; Sakhuia, R. Automotive Organic Rankine-Cycle Powerplant-Design and Performance Data; SAE Paper; SAE: Detroit, MI, USA, No. 740297.
- Victor, R.A.; Kim, J.-K.; Smith, R. Composition optimisation of working fluids for Organic Rankine Cycles and Kalina cycles. Energy 2013, 55, 114–126. [Google Scholar] [CrossRef]
- Ibrahim, O.M.; Klein, S.A. Absorption power cycles. Energy 1996, 21, 21–27. [Google Scholar] [CrossRef]
- Heberle, F.; Preißinger, M.; Brüggemann, D. Zeotropic mixtures as working fluids in Organic Rankine Cycles for low-enthalpy geothermal resources. Renew. Energy 2012, 37, 364–370. [Google Scholar] [CrossRef]
- Baik, Y.-J.; Kim, M.; Chang, K.-C.; Lee, Y.-S.; Yoon, H.-K. Power enhancement potential of a mixture transcritical cycle for a low-temperature geothermal power generation. Energy 2012, 47, 70–76. [Google Scholar] [CrossRef]
- Eller, T.; Heberle, F.; Brüggemann, D. Second law analysis of novel working fluid pairs for waste heat recovery by the Kalina cycle. Energy 2017, 119, 188–198. [Google Scholar] [CrossRef]
- Miller, D.R.; Null, H.R.; Thompson, Q.E. Optimum Working Fluids for Automotive Rankine Engines—Volume II; US Environmental Protection Agency: Washington, DC, USA, 1973. [Google Scholar]
- Teng, H.; Regner, G.; Cowland, C. Waste Heat Recovery of Heavy-Duty Diesel Engines by Organic Rankine Cycle Part I: Hybrid Energy System of Diesel and Rankine Engines; SAE Technical Paper; SAE: Detroit, MI, USA, No. 2007-01-0537; 2007. [Google Scholar]
- Teng, H.; Regner, G.; Cowland, C. Waste Heat Recovery of Heavy-Duty Diesel Engines by Organic Rankine Cycle Part II: Working Fluids for WHR-ORC; SAE Technical Paper; SAE: Detroit, MI, USA, 2007; No. 2007-01-0543. [Google Scholar]
- Katta, K.K.; Kim, M.; Taggett, M. Exhaust Heat Co-Generation System Using Phase Change Cooling for Heavy Duty Vehicles; SAE Technical Paper; SAE: Detroit, MI, USA, 2008; No. 2008-01-2450. [Google Scholar]
- Jain, M.L.; Demirgian, J.C.; Cole, R.L. Organic Rankine-Cycle Power Systems—Working Fluids Study: Topical Report No. 1—Fluorinol 85; Argonne National Laboratory: Lemont, IL, USA, 1986. [Google Scholar]
- Verneau, A.; Sulle, C. Recovery from exhaust gas on a diesel engine. In Proceedings of the 16th International Congress on Combustion Engines (CIMAC), Oslo, Norway, 3–7 June 1985. [Google Scholar]
- Absalam-Gadzhievich, D.T.; Ramazanovich, B.A. Research of Thermal Stability of Water Mixtures of Aliphatic Alcohols. J. Mater. Sci. Eng. A 2012, 2, 786–790. [Google Scholar]
- AspenTech, Aspen Plus. Available online: http://www.aspentech.com/products/aspen-plus.aspx (accessed on 11 May 2019).
- Renon, H.; Prausnitz, J.M. Local compositions in thermodynamic excess functions for liquid mixtures. AIChE J. 1968, 14, 135–144. [Google Scholar] [CrossRef]
- Sandler, S.I. Chemical, Biochemical, and Engineering Thermodynamics, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Invernizzi, C.; Iora, P.; Silva, P. Bottoming micro-Rankine cycles for micro-gas turbines. Appl. Therm. Eng. 2007, 27, 100–110. [Google Scholar] [CrossRef]
- U.S.-Based National Fire Protection Association, NFPA 704—Standard System for the Identification of the Hazards of Materials for Emergency Response. 2017. Available online: https://www.nfpa.org/codes-and-standards/all-codes-and-standards/list-of-codes-and-standards/detail?code=704 (accessed on 11 May 2019).
- Deiters, U.K.; Kraska, T. High-Pressure Fluid Phase Equilibria: Phenomenology and Computation. 2012. Available online: https://books.google.ru/books?hl=ru&lr=&id=28INNEzVnWsC&oi=fnd&pg=PP1&dq=Deiters+U.K.,+Kraska+Th.+High-Pressure+Fluid+Phase+Equilibria:+Phenomenology+and+Computation.+2012+Elsevier+B.V.,+Oxford,+Amsterdam.&ots=o0u6dVpxLG&sig=GvC4cfCQVe-NWYX2l1Dg_snso7k&re (accessed on 11 May 2019).
- Imre, A.R.; Quiñones-Cisneros, S.E.; Deiters, U.K. Adiabatic Processes in the Vapor-Liquid Two-Phase Region. 2. Binary Mixtures. Ind. Eng. Chem. Res. 2015, 54, 6559–6568. [Google Scholar] [CrossRef]
- Hesketh, J.A.; Walker, P.J. Effects of Wetness in Steam Turbines. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2005, 219, 1301–1314. [Google Scholar] [CrossRef]
- Petr, V.; Kolovratnik, M. Wet steam energy loss and related Baumann rule in low pressure steam turbines. Proc. Inst. Mech. Eng. Part A J. Power Energy 2013, 228, 206–215. [Google Scholar] [CrossRef]
- Liaw, H.J.; Chiu, Y.Y. The prediction of the flash point for binary aqueous-organic solutions. J. Hazard. Mater. 2003, 101, 83–106. [Google Scholar] [CrossRef]
- Jadot, R.; Fraiha, M. Isobaric vapor-liquid equilibrium of 2,2,2-Trifluoroethanol with water and 1-Propanol binary systems. J. Chem. Eng. Data 1988, 33, 237–240. [Google Scholar] [CrossRef]
- Phoon, L.Y.; Mustaffa, A.A.; Hashim, H.; Mat, R. A review of flash point prediction models for flammable liquid mixtures. Ind. Eng. Chem. Res. 2014, 53, 12553–12565. [Google Scholar] [CrossRef]
- Pasetti, M.; Invernizzi, C.M.; Iora, P. Thermal stability of working fluids for organic Rankine cycles: An improved survey method and experimental results for cyclopentane, isopentane and n-butane. Appl. Therm. Eng. 2014, 73, 762–772. [Google Scholar] [CrossRef]
- Invernizzi, C.M.; Iora, P.; Manzolini, G.; Lasala, S. Thermal stability of n-pentane, cyclo-pentane and toluene as working fluids in organic Rankine engines. Appl. Therm. Eng. 2017, 121, 172–179. [Google Scholar] [CrossRef]
- Invernizzi, C.M.; Iora, P.; Bonalumi, D.; Macchi, E.; Roberto, R.; Caldera, M. Titanium tetrachloride as novel working fluid for high temperature Rankine Cycles: Thermodynamic analysis and experimental assessment of the thermal stability. Appl. Therm. Eng. 2016, 107, 21–27. [Google Scholar] [CrossRef] [Green Version]
- Havens, V.N.; Ragaller, D.R.; Sibert, L.; Miller, D. Toluene stability space station Rankine power system. In Proceedings of the 22nd Intersociety Energy Conversion Engineering Conference, Philadelphia, PA, USA, 10–14 August 1987; pp. 121–126. [Google Scholar]












| Fluid | Tcr (°C) | pcr (bar) | MM (kg/kmol) | σ (-) | Tb (°C) |
|---|---|---|---|---|---|
| water | 373.95 | 220.64 | 18.02 | −10.38 | 100.00 |
| 2,2,2-trifluoroethanol | 225.42 | 48.09 | 100.04 | −2.76 | 73.80 |
| n-butanol | 289.95 | 44.14 | 74.12 | 4.24 | 118.75 |
| acetonitrile | 272.35 | 48.5 | 41.05 | −5.97 | 81.66 |
| 2-methylpyrazine | 354.85 | 51.17 | 94.12 | 8.17 | 130.30 |
| Fluid | Flash Temperature (°C) | Flammability Level | Health Hazard Level |
|---|---|---|---|
| 2,2,2-trifluoroethanol | 29 | 3 | 2 |
| n-butanol | 35 | 3 | 1 |
| acetonitrile | 2 | 3 | 2 |
| 2-methylpyrazine | 50 | 2 | 2 |
| Parameter | Units | Value |
|---|---|---|
| T Hot water (heat source) (TH,1) | °C | 100–150–200 |
| Minimum temperature approach in the evaporator, MITA(a)E | °C | 5 |
| Vapour quality at the evaporator outlet (x4) | % | 90 |
| T air at the condenser (TC,1) | °C | 15 |
| ΔT air at the condenser (ΔTC) | °C | 30 |
| Minimum temperature approach in the condenser, MITA(a)C | °C | 5 |
| Turbine isentropic efficiency (ηT) | % | 85 |
| Pump isentropic efficiency (ηP) | % | 70 |
| Fluid | Fluid Charge (g) | T (°C) | ΔTstep (°C) | Timestep (h) |
|---|---|---|---|---|
| 2,2,2-trifluoroethanol | 35 | 200–320 | 10 | 80 (60 at 310 °C) |
| n-butanol | 26 | 160–280 | 20 | 80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Invernizzi, C.; Binotti, M.; Bombarda, P.; Di Marcoberardino, G.; Iora, P.; Manzolini, G. Water Mixtures as Working Fluids in Organic Rankine Cycles. Energies 2019, 12, 2629. https://doi.org/10.3390/en12132629
Invernizzi C, Binotti M, Bombarda P, Di Marcoberardino G, Iora P, Manzolini G. Water Mixtures as Working Fluids in Organic Rankine Cycles. Energies. 2019; 12(13):2629. https://doi.org/10.3390/en12132629
Chicago/Turabian StyleInvernizzi, Costante, Marco Binotti, Paola Bombarda, Gioele Di Marcoberardino, Paolo Iora, and Giampaolo Manzolini. 2019. "Water Mixtures as Working Fluids in Organic Rankine Cycles" Energies 12, no. 13: 2629. https://doi.org/10.3390/en12132629
APA StyleInvernizzi, C., Binotti, M., Bombarda, P., Di Marcoberardino, G., Iora, P., & Manzolini, G. (2019). Water Mixtures as Working Fluids in Organic Rankine Cycles. Energies, 12(13), 2629. https://doi.org/10.3390/en12132629






