Effects of Two-Stage Operation on Stability and Efficiency in Co-Digestion of Food Waste and Waste Activated Sludge
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Sludge
2.2. Feedstock
2.3. Experimental Design and Operation
2.4. Chemical Analysis
2.5. Microbial Analysis
2.6. Statistical Analysis
3. Results
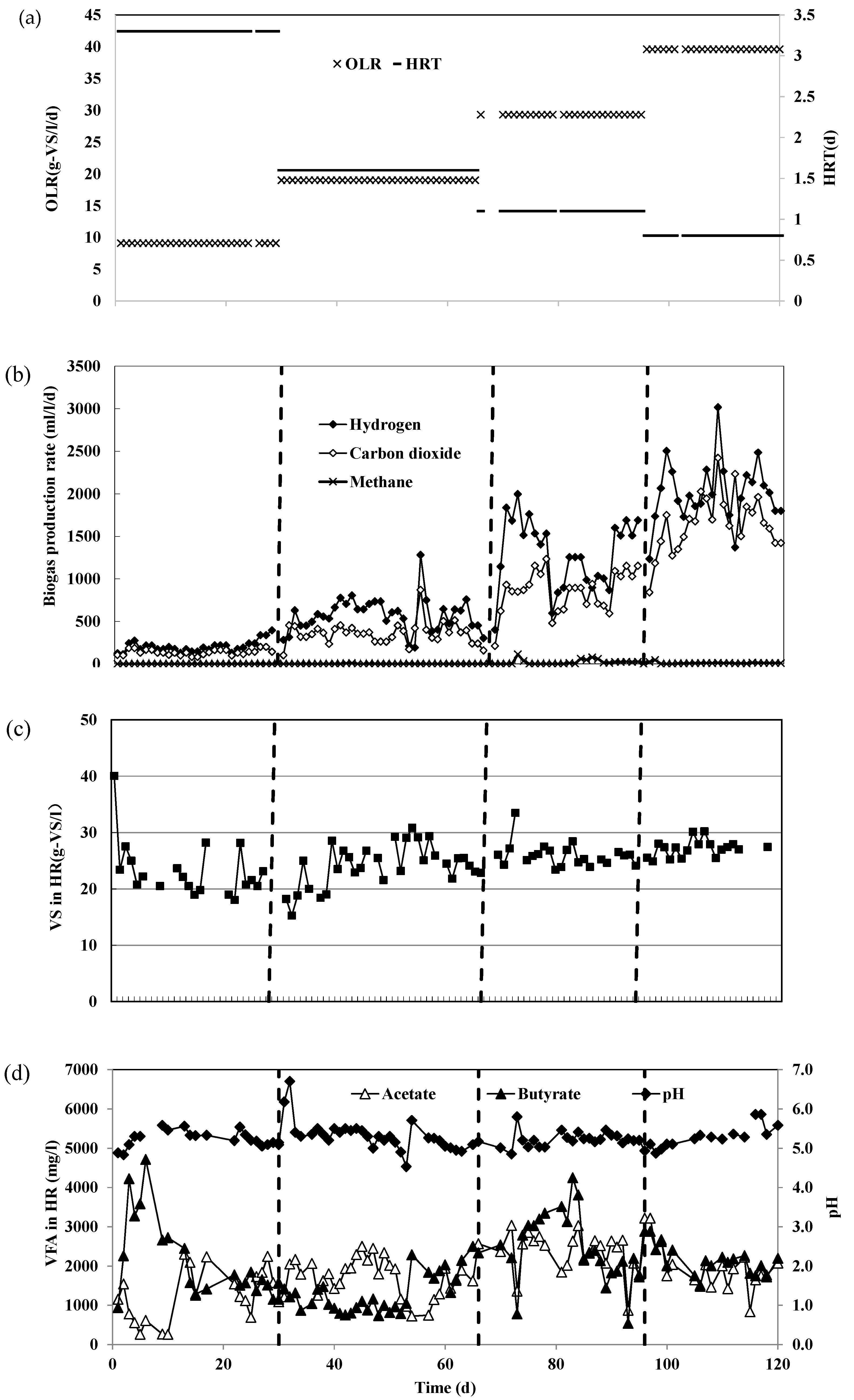
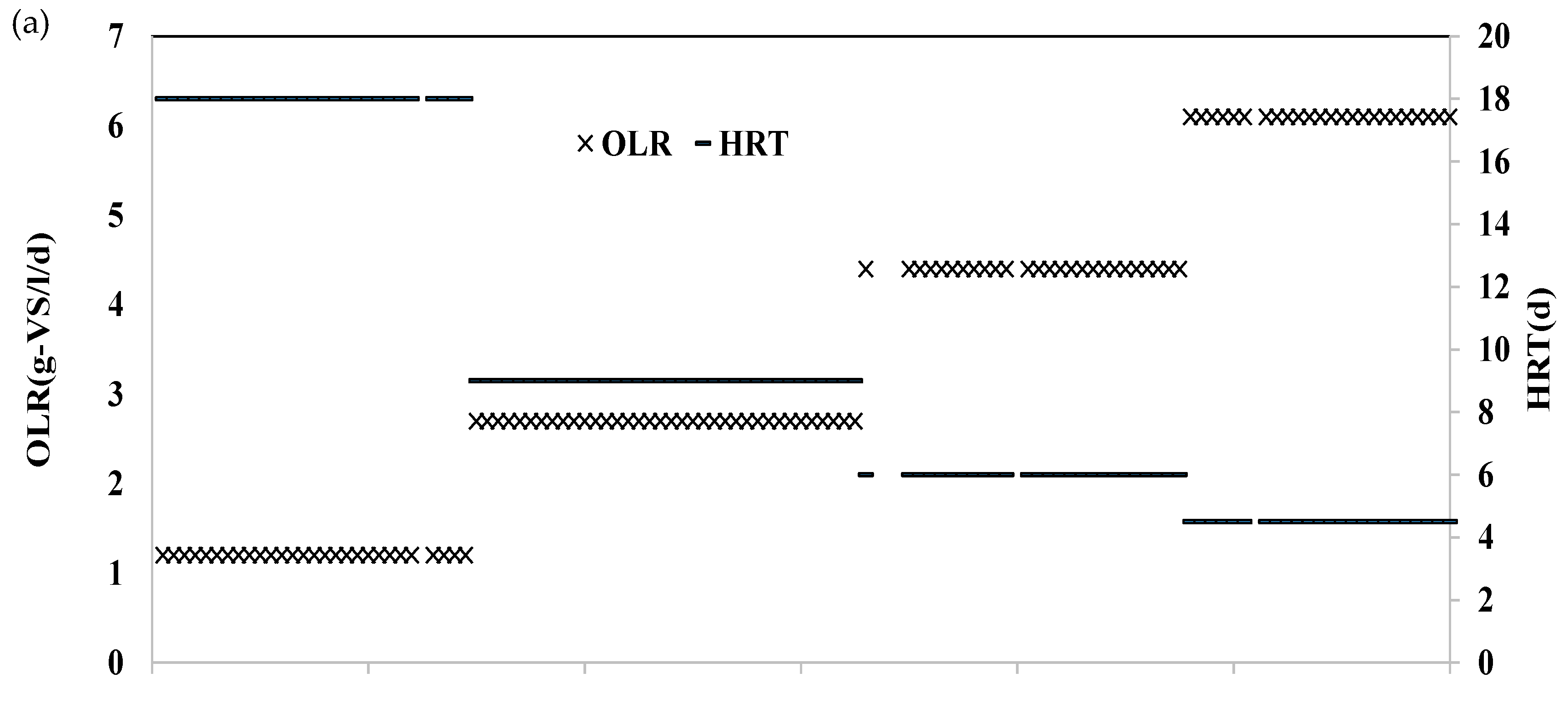
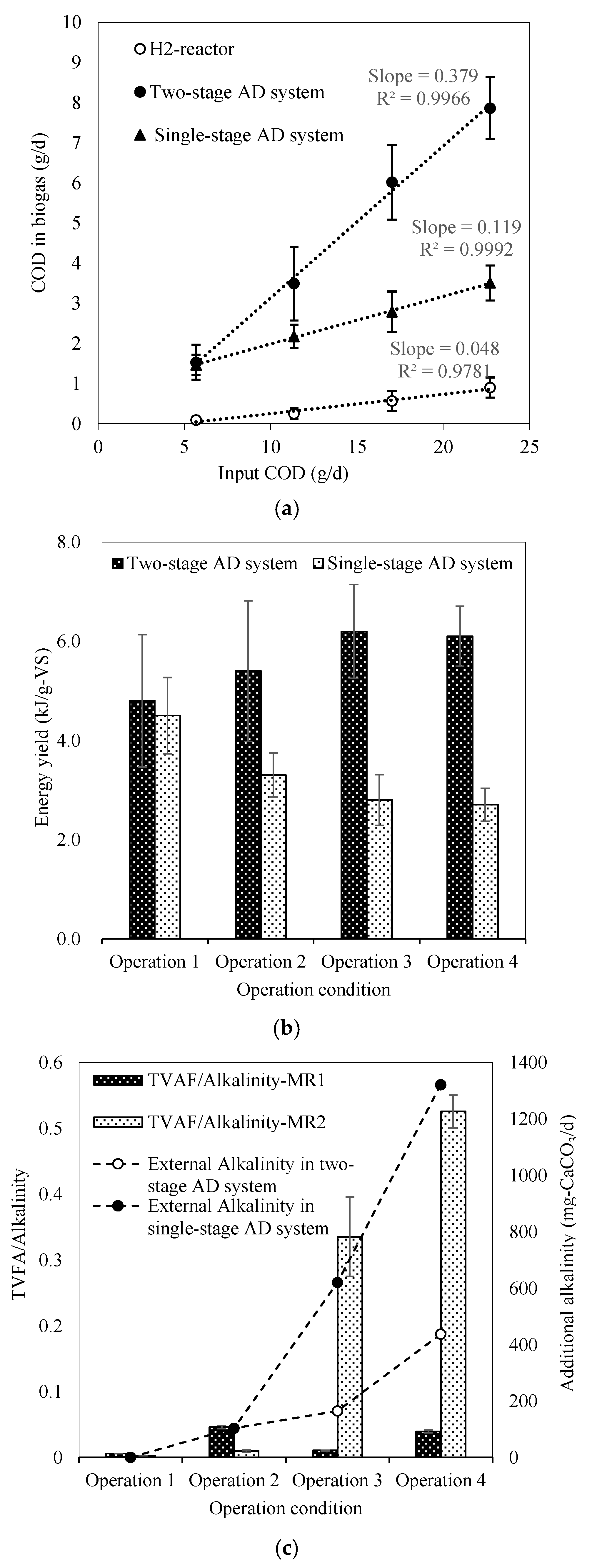
3.1. Operational Performance in HR at Various HRT
3.2. Comparison of Operational Performance between MR1 and MR2 at Various HRTs
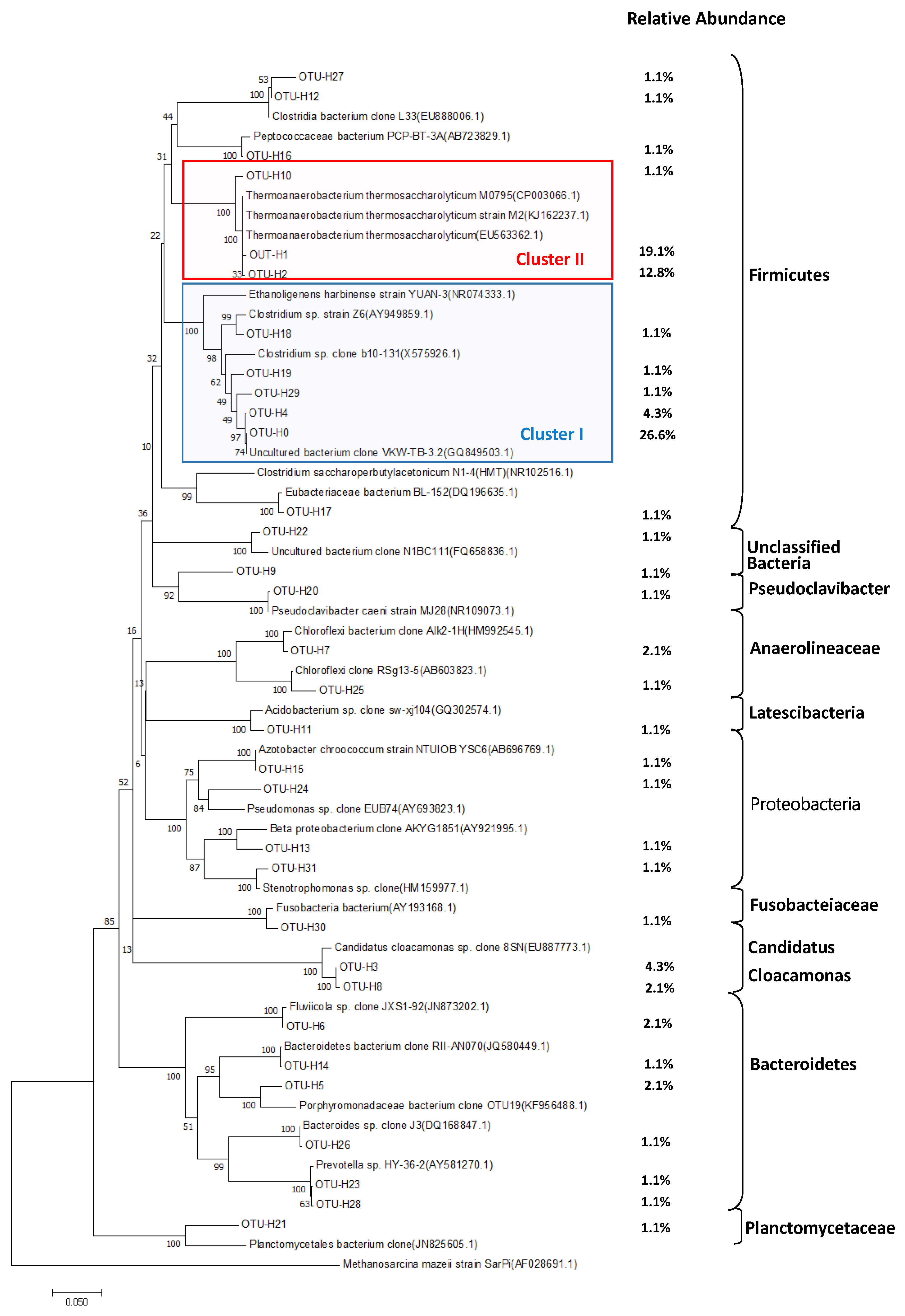
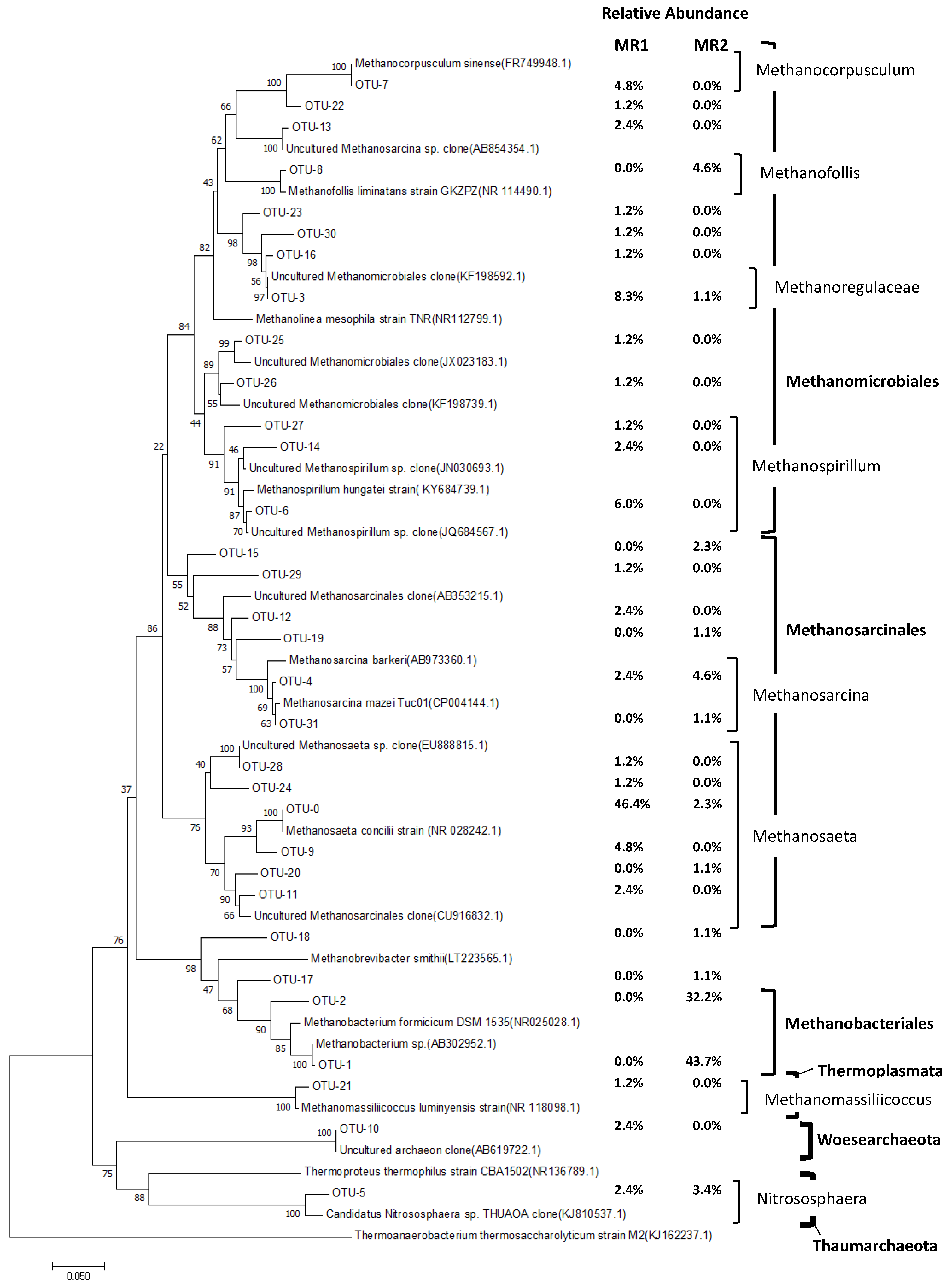
3.3. Microbial Communities in Each Reactor
3.3.1. Bacterial Community Dynamics in Hydrogen Reactor
3.3.2. Comparison of Archaeal Community Dynamics in MR1 and MR2
4. Discussion
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Guimarães, C.; Maia, D.; Serra, E. Construction of Biodigesters to Optimize the Production of Biogas from Anaerobic Co-Digestion of Food Waste and Sewage. Energies 2018, 11, 870. [Google Scholar] [CrossRef]
- Liu, X.; Li, R.; Ji, M.; Han, L. Hydrogen and methane production by co-digestion of waste activated sludge and food waste in the two-stage fermentation process: Substrate conversion and energy yield. Bioresour. Technol. 2013, 146, 317–323. [Google Scholar] [CrossRef]
- Gou, C.; Yang, Z.; Huang, J.; Wang, H.; Xu, H.; Wang, L. Effects of temperature and organic loading rate on the performance and microbial community of anaerobic co-digestion of waste activated sludge and food waste. Chemosphere 2014, 105, 146–151. [Google Scholar] [CrossRef]
- Fonoll, X.; Astals, S.; Dosta, J.; Mata-Alvarez, J. Anaerobic co-digestion of sewage sludge and fruit wastes: Evaluation of the transitory states when the co-substrate is changed. Chem. Eng. J. 2015, 262, 1268–1274. [Google Scholar] [CrossRef]
- Li, L.; Peng, X.; Wang, X.; Wu, D. Anaerobic digestion of food waste: A review focusing on process stability. Bioresour. Technol. 2018, 248, 20–28. [Google Scholar] [CrossRef]
- Ratanatamskul, C.; Wattanayommanaporn, O.; Yamamoto, K. An on-site prototype two-stage anaerobic digester for co-digestion of food waste and sewage sludge for biogas production from high-rise building. Int. Biodeterior. Biodegrad. 2015, 102, 143–148. [Google Scholar] [CrossRef]
- Cabbai, V.; De Bortoli, N.; Goi, D. Pilot plant experience on anaerobic codigestion of source selected OFMSW and sewage sludge. Waste Manag. 2016, 49, 47–54. [Google Scholar] [CrossRef]
- Guven, H.; Ersahin, M.E.; Dereli, R.K.; Ozgun, H.; Isik, I.; Ozturk, I. Energy recovery potential of anaerobic digestion of excess sludge from high-rate activated sludge systems co-treating municipal wastewater and food waste. Energy 2019, 172, 1027–1036. [Google Scholar] [CrossRef]
- Siddiqui, Z.; Horan, N.J.; Salter, M. Energy optimisation from co-digested waste using a two-phase process to generate hydrogen and methane. Int. J. Hydrogen Energy 2011, 36, 4792–4799. [Google Scholar] [CrossRef]
- Zhu, H.G.; Parker, W.; Conidi, D.; Basnar, R.; Seto, P. Eliminating methanogenic activity in hydrogen reactor to improve biogas production in a two-stage anaerobic digestion process co-digesting municipal food waste and sewage sludge. Bioresour. Technol. 2011, 102, 7086–7092. [Google Scholar] [CrossRef]
- Wu, L.-J.; Kobayashi, T.; Li, Y.-Y.; Xu, K.-Q. Comparison of single-stage and temperature-phased two-stage anaerobic digestion of oily food waste. Energy Convers. Manag. 2015, 106, 1174–1182. [Google Scholar] [CrossRef]
- Nasr, N.; Elbeshbishy, E.; Hafez, H.; Nakhla, G.; El Naggar, M.H. Comparative assessment of single-stage and two-stage anaerobic digestion for the treatment of thin stillage. Bioresour. Technol. 2012, 111, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Nathao, C.; Sirisukpoka, U.; Pisutpaisal, N. Production of hydrogen and methane by one and two stage fermentation of food waste. Int. J. Hydrogen Energy 2013, 38, 15764–15769. [Google Scholar] [CrossRef]
- Schievano, A.; Alberto, T.; Scaglia, B.; Merlino, G.; Rizzi, A.; Daffonchio, D.; Oberti, R.; Adani, F. Two-stage vs single-stage thermophilic anaerobic digestion comparison of energy production and biodegradation efficiencies. Environ. Sci. Technol. 2012, 46, 8502–8510. [Google Scholar] [CrossRef] [PubMed]
- Sreela-or, C.; Plangklang, P.; Imai, T.; Reungsang, A. Co-digestion of food waste and sludge for hydrogen production by anaerobic mixed cultures: Statistical key factors optimization. Int. J. Hydrogen Energy 2011, 36, 14227–14237. [Google Scholar] [CrossRef]
- Zhang, T.; Mao, C.; Zhai, N.; Wang, X.; Yang, G. Influence of initial pH on thermophilic anaerobic co-digestion of swine manure and maize stalk. Waste Manag. 2015, 35, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.B.; Liu, X.Y.; Stabnikova, O.; Wang, J.Y. Effect of protein on biohydrogen production from starch of food waste. Water Sci. Technol. 2008, 57, 1031–1036. [Google Scholar] [CrossRef]
- Kobayashi, T.; Xu, K.Q.; Li, Y.Y.; Inamori, Y. Effect of sludge recirculation on characteristics of hydrogen production in a two-stage hydrogen–methane fermentation process treating food wastes. Int. J. Hydrogen Energy 2012, 37, 5602–5611. [Google Scholar] [CrossRef]
- Algapani, D.E.; Qiao, W.; Ricci, M.; Bianchi, D.; Wandera, S.M.; Adani, F.; Dong, R. Bio-hydrogen and bio-methane production from food waste in a two-stage anaerobic digestion process with digestate recirculation. Renew. Energy 2019, 130, 1108–1115. [Google Scholar] [CrossRef]
- Zhu, H.; Parker, W.; Basnar, R.; Proracki, A.; Falletta, P.; Beland, M.; Seto, P. Biohydrogen production by anaerobic co-digestion of municipal food waste and sewage sludges. Int. J. Hydrogen Energy 2008, 33, 3651–3659. [Google Scholar] [CrossRef]
- Baldi, F.; Pecorini, I.; Iannelli, R. Comparison of single-stage and two-stage anaerobic co-digestion of food waste and activated sludge for hydrogen and methane production. Renew. Energy 2019, 143, 1755–1765. [Google Scholar] [CrossRef]
- Ren, N.Q.; Guo, W.Q.; Wang, X.J.; Xiang, W.S.; Liu, B.F.; Wang, X.Z.; Ding, J.; Chen, Z.B. Effects of different pretreatment methods on fermentation types and dominant bacteria for hydrogen production. Int. J. Hydrogen Energy 2008, 33, 4318–4324. [Google Scholar] [CrossRef]
- Gaudy, A.F. Colorimetric determination of protein and carbohydrate. Ind. Water Wastes 1962, 7, 17–22. [Google Scholar]
- Lowery, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; APHA: Washington, DC, USA, 2005. [Google Scholar]
- Ritalahti, K.M.; Amos, B.K.; Sung, Y.; Wu, Q.Z.; Koenigsberg, S.S.; Loffler, F.E. Quantitative PCR targeting 16S rRNA and reductive dehalogenase genes simultaneously monitors multiple Dehalococcoides strains. Appl. Environ. Microb. 2006, 72, 2765–2774. [Google Scholar] [CrossRef]
- DeLong, E.F. Archaea in coastal marine environments. Proc. Natl. Acad. Sci. USA 1992, 89, 5685–5689. [Google Scholar] [CrossRef]
- Merlino, G.; Rizzi, A.; Schievano, A.; Tenca, A.; Scaglia, B.; Oberti, R.; Adani, F.; Daffonchio, D. Microbial community structure and dynamics in two-stage vs single-stage thermophilic anaerobic digestion of mixed swine slurry and market bio-waste. Water Res. 2013, 47, 1983–1995. [Google Scholar] [CrossRef]
- Ueno, Y.; Fukui, H.; Goto, M. Operation of a Two-Stage Fermentation Process Producing Hydrogen and Methane from Organic Waste. Environ. Sci. Technol. 2007, 41, 1413–1419. [Google Scholar] [CrossRef]
- Buitrón, G.; Carvajal, C. Biohydrogen production from tequila vinasses in an anaerobic sequencing batch reactor: Effect of initial substrate concentration, temperature and hydraulic retention time. Bioresour. Technol. 2010, 101, 9071–9077. [Google Scholar] [CrossRef]
- Llirós, M.; Trias, R.; Borrego, C.; Bañeras, L. Specific Archaeal communities are selected on the root surfaces of Ruppia spp. and Phragmites australis. Wetlands 2013, 34, 403–411. [Google Scholar] [CrossRef]
- Lay, J. Influence of chemical nature of organic wastes on their conversion to hydrogen by heat-shock digested sludge. Int. J. Hydrogen Energy 2003, 28, 1361–1367. [Google Scholar] [CrossRef]
- Chu, C.F.; Li, Y.Y.; Xu, K.Q.; Ebie, Y.; Inamori, Y.; Kong, H.N. A PH-and temperature-phased two-stage process for hydrogen and methane production from food waste. Int. J. Hydrogen Energy 2008, 33, 4739–4746. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Zhang, Y.L.; Wang, J.B.; Meng, L. Effect of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenerg. 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Massanet-Nicolau, J.; Dinsdale, R.; Guwy, A.; Shipley, G. Utilising biohydrogen to increase methane production, energy yields and process efficiency via two stage anaerobic digestion of grass. Bioresour. Technol. 2015, 189, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Nam, J.Y.; Shin, H.S. A comparison study on the high-rate co-digestion of sewage sludge and food waste using a temperature-phased anaerobic sequencing batch reactor system. Bioresour. Technol. 2011, 102, 7272–7279. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Lee, S.H.; Yoon, J.J.; Kim, S.H.; Park, H.D. Predominance of cluster I Clostridium in hydrogen fermentation of galactose seeded with various heat-treated anaerobic sludges. Bioresour. Technol. 2014, 157, 98–106. [Google Scholar] [CrossRef]
- Lee, Z.-K.; Li, S.-L.; Kuo, P.-C.; Chen, I.C.; Tien, Y.-M.; Huang, Y.-J.; Chuang, C.-P.; Wong, S.-C.; Cheng, S.-S. Thermophilic bio-energy process study on hydrogen fermentation with vegetable kitchen waste. Int. J. Hydrogen Energy 2010, 35, 13458–13466. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Xu, K.-Q.; Kobayashi, T.; Li, Y.-Y.; Inamori, Y. Effect of organic loading rate on continuous hydrogen production from food waste in submerged anaerobic membrane bioreactor. Int. J. Hydrogen Energy 2014, 39, 16863–16871. [Google Scholar] [CrossRef]
- Rotaru, A.-E.; Shrestha, P.M.; Liu, F.; Shrestha, M.; Shrestha, D.; Embree, M.; Zengler, K.; Wardman, C.; Nevin, K.P.; Lovley, D.R. A new model for electron flow during anaerobic digestion: Direct interspecies electron transfer to Methanosaeta for the reduction of carbon dioxide to methane. Energy Environ. Sci. 2014, 7, 408–415. [Google Scholar] [CrossRef]
- Watanabe, K.; Koyama, M.; Ueda, J.; Ban, S.; Kurosawa, N.; Toda, T. Effect of operating temperature on anaerobic digestion of the Brazilian waterweed Egeria densa and its microbial community. Anaerobe 2017, 47, 8–17. [Google Scholar] [CrossRef]
- Demirel, B.; Scherer, P. The roles of acetotrophic and hydrogenotrophic methanogens during anaerobic conversion of biomass to methane: A review. Rev. Environ. Sci. BioTechnol. 2008, 7, 173–190. [Google Scholar] [CrossRef]
- Zellner, G.; Boone, D.R. Methanofollis. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley and Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Kerou, M.; Schleper, C. Nitrososphaera. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley and Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Ren, Y.; Yu, M.; Wu, C.; Wang, Q.; Gao, M.; Huang, Q.; Liu, Y. A comprehensive review on food waste anaerobic digestion: Research updates and tendencies. Bioresour. Technol. 2018, 247, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Kongjan, P.; O-Thong, S.; Angelidaki, I. Performance and microbial community analysis of two-stage process with extreme thermophilic hydrogen and thermophilic methane production from hydrolysate in UASB reactors. Bioresour. Technol. 2011, 102, 4028–4035. [Google Scholar] [CrossRef] [PubMed]
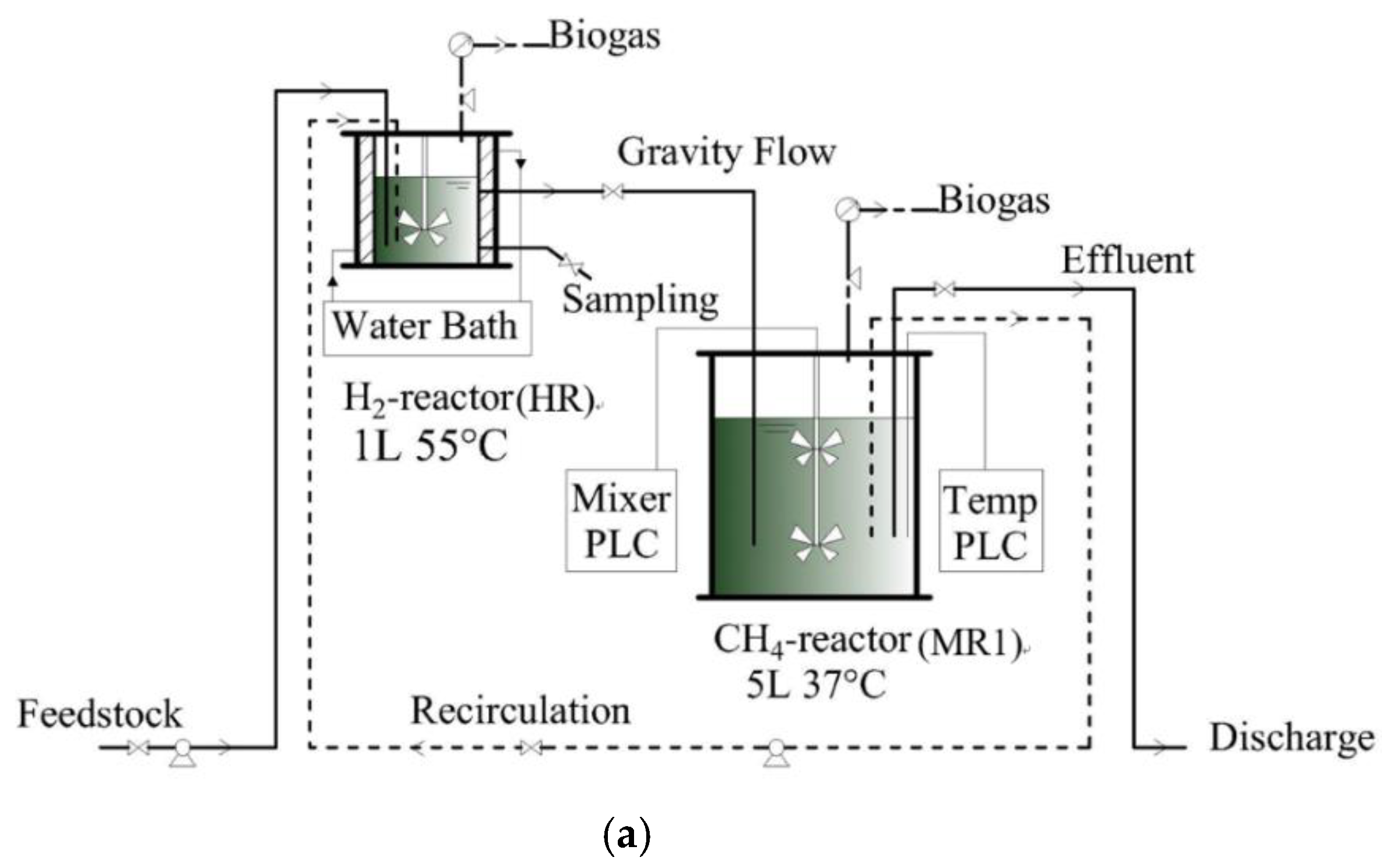
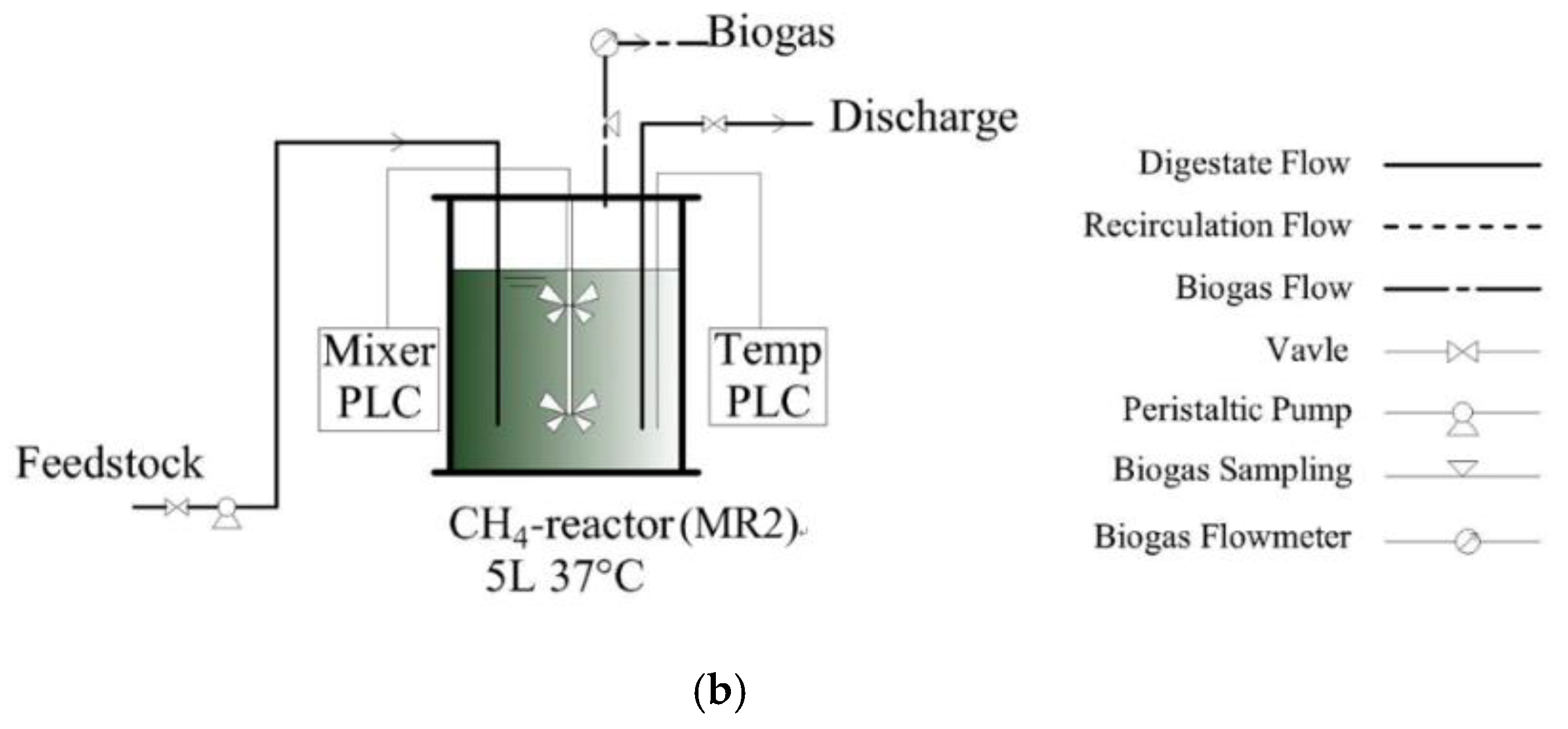


| Parameters | Waste Activated Sludge | Food Waste | Feedstock |
|---|---|---|---|
| pH | 7.1 (0.3) | 5.7 (0.8) | 6.7 (0.5) |
| VS/TS (%) | 69.6 (1.5) | 97.4 (1.8) | 82.3 (1.2) |
| NH4+-N (mg/g-VS) | 0.1 (0.2) | 0.9 (0.6) | 0.6 (0.3) |
| Soluble COD (mg/g-VS) | 9.6 (11.4) | 549.3 (95.7) | 310.0 (51.9) |
| Soluble Protein (mg/g-VS) | 0.2 (0.4) | 18.8 (6.5) | 10.2 (3.5) |
| Soluble Carbohydrate (mg/g-VS) | 0.3 (0.2) | 287.2 (45.6) | 155.3 (24.6) |
| Total COD (mg/g-VS) | 1514.9 (153.1) | 1129.9 (78.0) | 1307.0 (82.0) |
| Total Carbohydrate (mg/g-VS) | 138.2 (18.3) | 647.6 (79.7) | 413.3 (43.9) |
| Alkalinity (mg-CaCO3/g-VS) | 8.6 (2.1) | 0.2 (0.1) | 4.1 (1.0) |
| H2-Reactor | Two-Stage CH4-Reactor | Single-Stage CH4-Reactor | Running Time (d) | ||||
|---|---|---|---|---|---|---|---|
| HRT (d) | OLR (g-VS/L/d) | HRT (d) | OLR (g-VS/L/d) | HRT (d) | OLR (g-VS/L/d) | ||
| Operation 1 | 3.3 | 9.1 | 18 | 1.2 | 36 | 1.2 | 1~30 |
| Operation 2 | 1.6 | 19.0 | 9 | 2.7 | 18 | 2.4 | 31~66 |
| Operation 3 | 1.1 | 29.3 | 6 | 4.4 | 12 | 3.6 | 67~96 |
| Operation 4 | 0.8 | 39.6 | 4.5 | 6.1 | 9 | 4.5 | 97~120 |
| Items | Unit | Operation 1 | Operation 2 | Operation 3 | Operation 4 | |
|---|---|---|---|---|---|---|
| Biogas | H2 production rate | mL-H2/L/d | 211.6 (66.5) | 574.8 (198.8) | 1294.8 (396.8) | 2057.1 (349.8) |
| H2 yield | mL/g-VS | 31.6 (2.3) | 42.9 (1.7) | 64.5 (1.5) | 76.8 (0.7) | |
| H2 content | % | 60.0% (5.0%) | 60.9% (7.8%) | 60.0% (4.3%) | 54.5% (5.6%) | |
| CH4 content | % | a | 0.09% (0.2%) | 1.0% (1.3%) | 0.3% (0.4%) | |
| CO2 content | % | 40.0% (5.0%) | 39.4% (7.9%) | 49.9% (4.4%) | 35.5% (5.0%) | |
| Digestate | pH | 5.24 (0.21) | 5.29 (0.39) | 5.21 (0.19) | 5.19 (0.15) | |
| TVFA | mg/L | 3988.1 (893.2) | 3642.5 (556.2) | 5326.7 (1384.1) | 4858 (965.8) | |
| NH4+-N | mg-N/L | 208.5 (29.8) | 147.0 (23.2) | 115.1 (44.0) | 80.9 (15.3) | |
| Alkalinity | g-CaCO3/d | 1.44 (0.27) | 1.45 (0.22) | 2.18 (0.37) | 2.28 (0.56) | |
| VS | g/L | 22.29 (3.06) | 24.14 (3.77) | 25.94 (2.08) | 27.39 (1.45) | |
| Total COD | g/L | 34.7 (4.5) | 38.7 (6.3) | 44.9 (2.9) | 47.0 (4.4) | |
| Soluble COD | g/L | 9.2 (3.2) | 9.6 (3.1) | 12.5 (1.7) | 13.4 (5.7) | |
| Total carbohydrate | g/L | 2.2 (0.4) | 2.6 (0.7) | 2.8 (1.5) | 4.0 (0.7) | |
| Soluble carbohydrate | g/L | 0.16 (0.04) | 0.17 (0.07) | 0.22 (0.5) | 0.41 (0.05) | |
| Soluble protein | g/L | 0.31 (0.07) | 0.39 (0.1) | 0.53 (0.1) | 0.53 (0.1) | |
| Items | Unit | Operation 1 | Operation 2 | Operation 3 | Operation 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| MR1 | MR2 | MR1 | MR2 | MR1 | MR2 | MR1 | MR2 | ||
| Biogas | |||||||||
| CH4 production rate | mL-CH4/L/d | 149.43 (38.6) I | 150.3 (25.2) I | 332.4 (84.7) S | 222.9 (29.9) S | 558.5 (100.9) S | 286.2 (138.6) S | 713.6 (73.9) S | 357.8 (62.2) |
| CH4 yield | mL/g-VS | 122.1 (2.1) I | 122.0 (4.8) I | 137.6 (1.1) S | 92.2 (0.8) S | 154.1 (0.6) S | 79.9 (2.9) S | 147.6 (0.2) S | 74.4 (0.7) |
| CH4 content | % | 81.3% (3.8%) S | 64.1% (3.3%) S | 76.6% (5.1%) S | 56.3% (3.4%) S | 80.6% (3.0%) S | 57.4% (6.6%) S | 71.5% (5.3%) S | 53.4% (4.1%) S |
| CO2 content | % | 18.7% (4.1%) S | 35.9% (3.3%) S | 23.4% (5.3%) S | 43.7% (3.4%) S | 19.4% (3.1%) S | 42.6% (6.9%) S | 28.5% (5.9%) S | 46.6% (4.3%) S |
| Digestate | |||||||||
| pH | 7.23 (0.16) S | 7.05 (0.12) S | 7.40 (0.23) S | 7.11 (0.24) S | 7.08 (0.19) S | 6.79 (0.22) S | 7.02 (0.08) S | 6.76 (0.14) S | |
| TVFA | mg/L | 16.9 (58.7) I | 5.4 (6.4) I | 113.0 (222.5) S | 16.1 (24.9) S | 40.8 (48.0) S | 1387.9 (676.6) S | 156.5 (118.7) S | 1981 (525.9) S |
| NH4+-N | mg-N/L | 462.8 (37.4) S | 250.6 (22.1) S | 300.4 (69.7) S | 87.8 (61.4) S | 269.0 (33.0) S | 13.0 (18.3) S | 224.3 (26.7) S | a |
| Alkalinity | g-CaCO3/d | 2.83 (0.28) S | 2.06 (0.37) S | 2.44 (0.41) S | 1.66 (0.49) S | 3.84 (0.51) S | 4.14 (0.60) S | 3.96 (0.47) I | 3.77 (0.75) I |
| VS | g/L | 15.74 (0.54) S | 17.42 (0.39) S | 18.42 (1.27) S | 21.18 (1.49) S | 19.89 (0.60) S | 24.08 (1.21) S | 20.63 (0.77) S | 25.01 (0.93) S |
| Total COD | g/L | 22.9 (2.2) I | 26.8 (3.0) I | 27.9 (2.2) I | 30.1 (5.0) I | 31.4 (4.0) S | 41.2 (6.0) S | 34.7 (2.5) S | 42.5 (1.5) S |
| Soluble COD | g/L | 1.3 (1.6) I | 1.6 (0.9) I | 1.8 (0.7) S | 3.2 (1.1) S | 1.7 (0.7) S | 6.1 (2.3) S | 2.7 (0.6) S | 7.4 (0.7) S |
| Total carbohydrate | g/L | 1.9 (0.2) I | 2.1 (0.1) I | 2.4 (0.3) S | 2.5 (0.5) S | 2.5 (0.4) S | 2.6 (0.5) S | 3.4 (0.5) S | 3.8 (0.5) S |
| Soluble carbohydrate | g/L | 0.06 (0.01) S | 0.09 (0.01) S | 0.14 (0.2) I | 0.27 (0.2) I | 0.1 (0.04) I | 0.2 (0.1) I | 0.14 (0.04) I | 0.37 (0.1) I |
| Soluble protein | g/L | 0.23 (0.05) I | 0.29 (0.1) I | 0.28 (0.1) S | 0.62 (0.1) S | 0.34 (0.1) I | 0.84 (0.5) I | 0.53 (0.2) S | 1.57 (0.3) S |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Li, R.; Ji, M. Effects of Two-Stage Operation on Stability and Efficiency in Co-Digestion of Food Waste and Waste Activated Sludge. Energies 2019, 12, 2748. https://doi.org/10.3390/en12142748
Liu X, Li R, Ji M. Effects of Two-Stage Operation on Stability and Efficiency in Co-Digestion of Food Waste and Waste Activated Sludge. Energies. 2019; 12(14):2748. https://doi.org/10.3390/en12142748
Chicago/Turabian StyleLiu, Xinyuan, Ruying Li, and Min Ji. 2019. "Effects of Two-Stage Operation on Stability and Efficiency in Co-Digestion of Food Waste and Waste Activated Sludge" Energies 12, no. 14: 2748. https://doi.org/10.3390/en12142748
APA StyleLiu, X., Li, R., & Ji, M. (2019). Effects of Two-Stage Operation on Stability and Efficiency in Co-Digestion of Food Waste and Waste Activated Sludge. Energies, 12(14), 2748. https://doi.org/10.3390/en12142748






