Produce Low Aromatic Contents with Enhanced Cold Properties of Hydrotreated Renewable Diesel Using Pt/Alumina-Beta-Zeolite: Reaction Path Studied via Monoaromatic Model Compound
Abstract
1. Introduction
2. Materials and Methods
2.1. Feed Material
2.2. Zeolite Precursor
2.3. Zeolite Extrusion
2.4. Experimental Procedure
2.5. Analytical Study
3. Results and Discussion
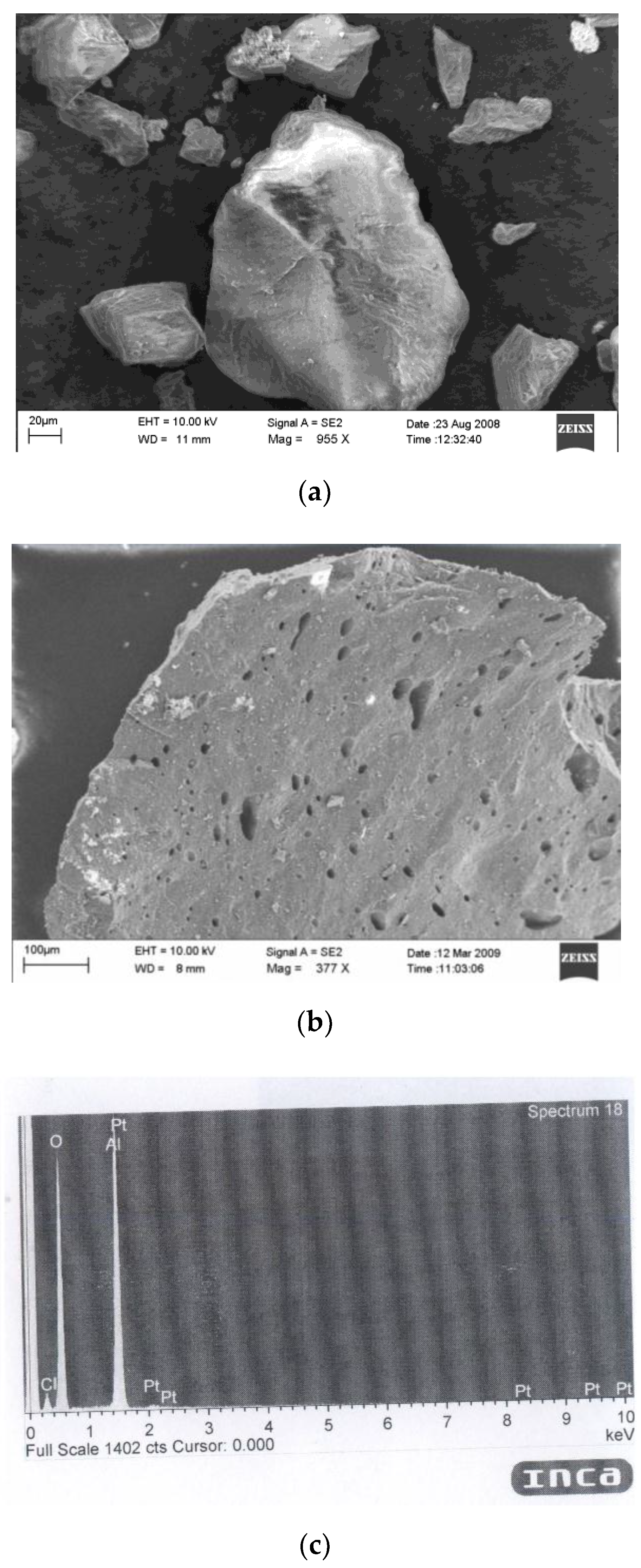
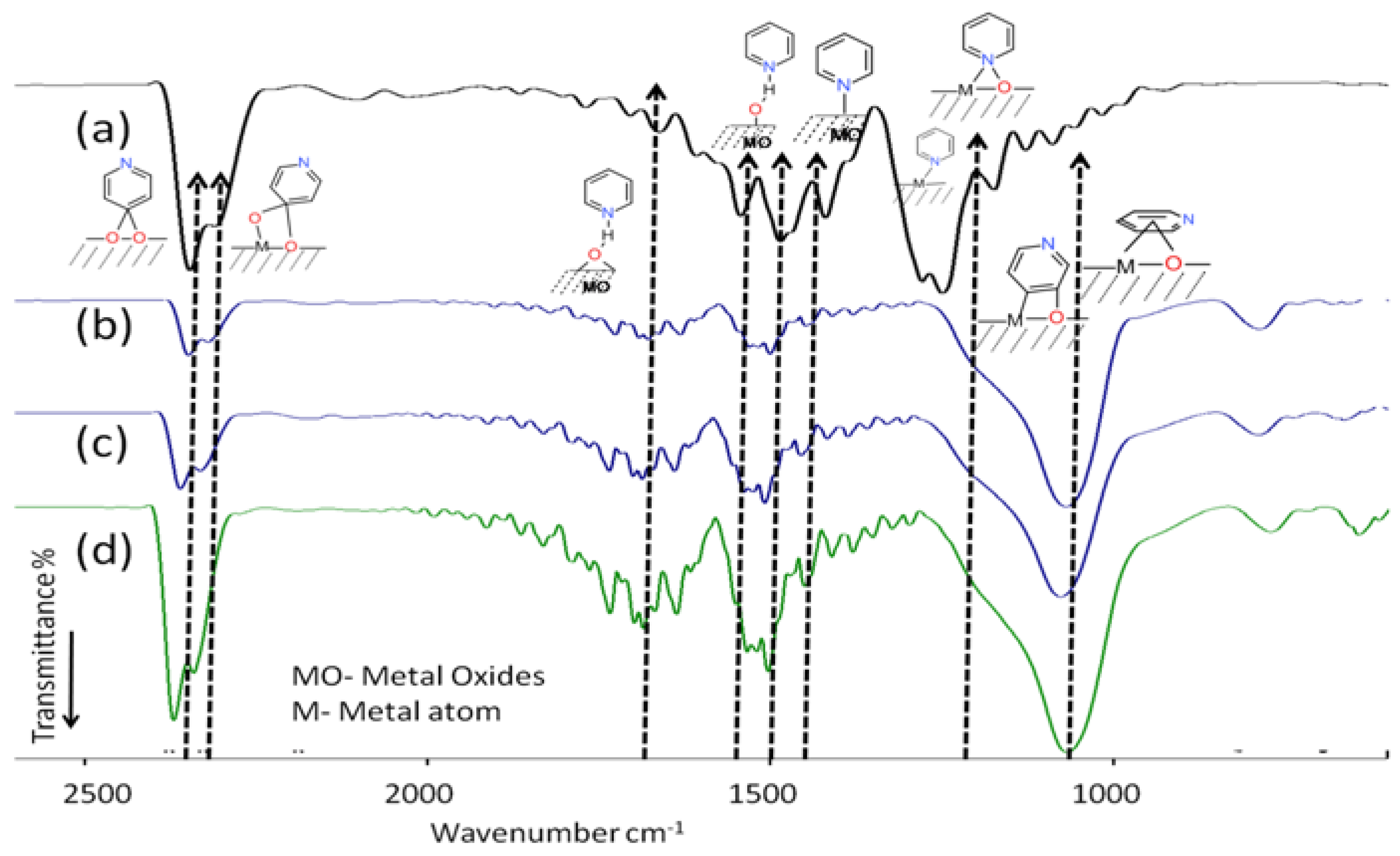
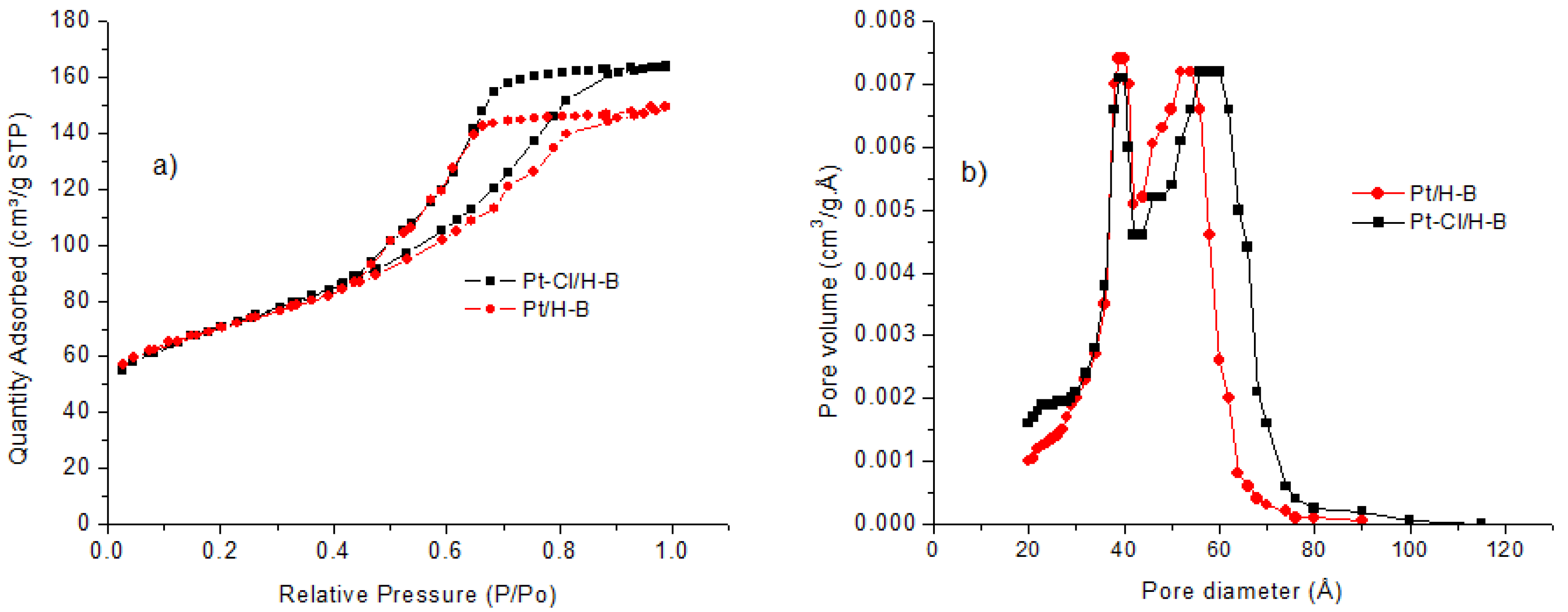
3.1. Catalyst Characterization
3.2. Hydro-Isomerisation of n-Hexadecane in ULSD
3.3. n-Hexadecane Isomerisation
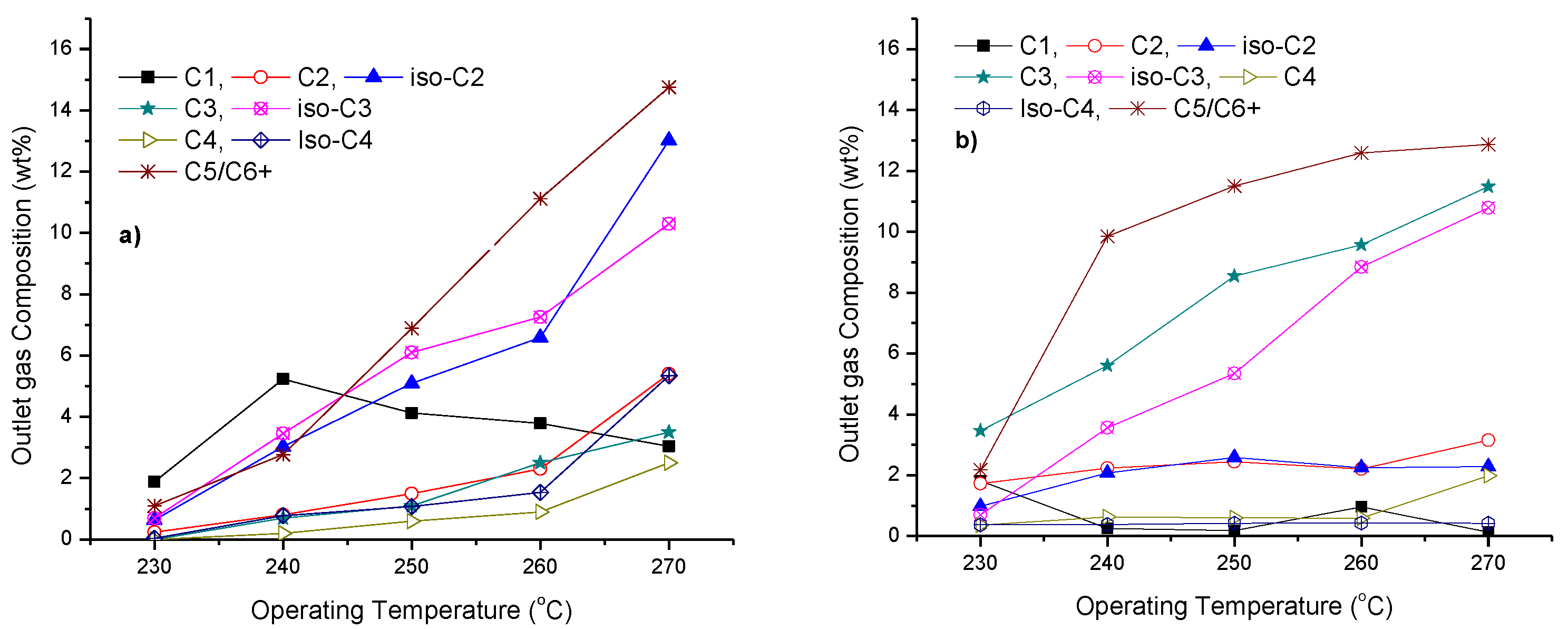
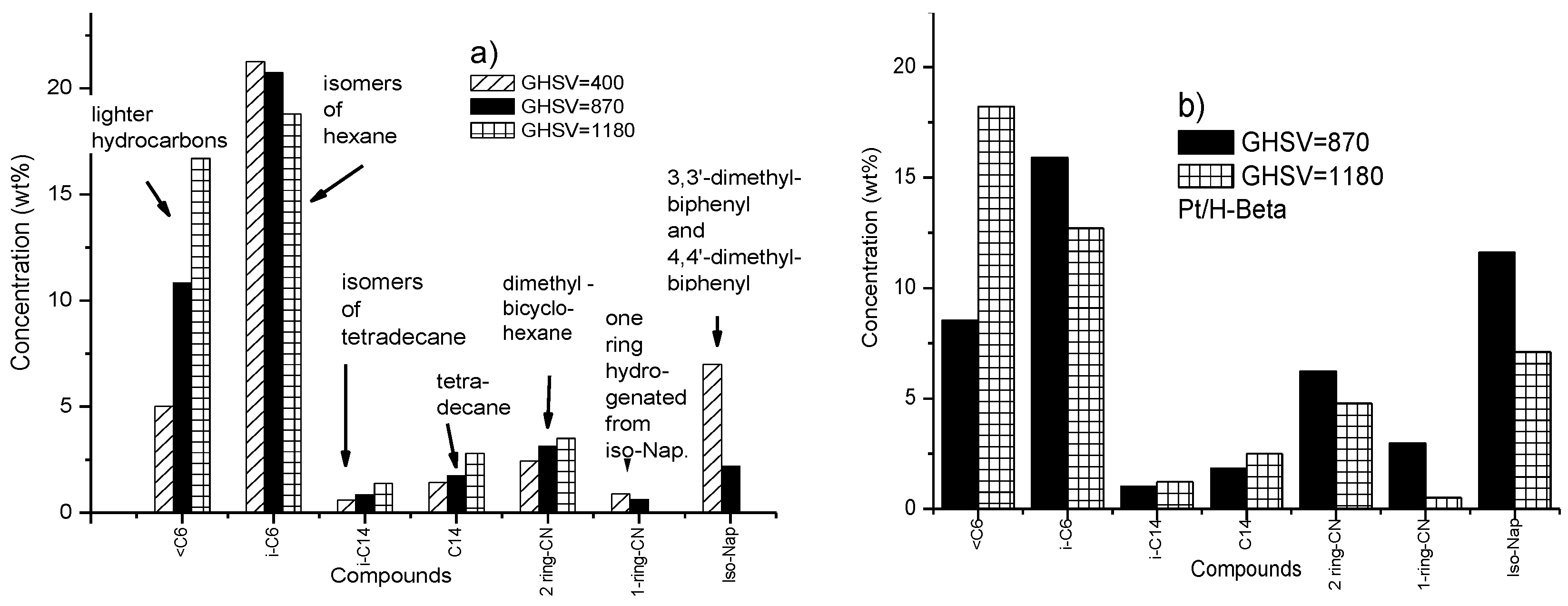
3.4. Toluene Dearomatisation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reaume, S.J.; Ellis, N. Use of isomerization and hydroisomerization reactions to improve the cold flow properties of vegetable oil-based biodiesel. Energies 2013, 6, 619–633. [Google Scholar] [CrossRef]
- Palanisamy, S.; Gevert, B.S. Hydroprocessing of fatty acid methylester containing resin acids blended with gas oil. Fuel Process. Technol. 2014, 126, 435–440. [Google Scholar] [CrossRef]
- Liu, C.Y.; Hao, Y.A.N.G.; Jing, Z.Y.; Xi, K.Z.; Qiao, C.Z. Hydrodeoxygenation of fatty acid methyl esters and isomerization of products over NiP/SAPO-11 catalysts. J. Fuel Chem. Technol. 2016, 44, 1211–1216. [Google Scholar] [CrossRef]
- Labeckas, G.; Slavinskas, S. Combustion phenomenon, performance and emissions of a diesel engine with aviation turbine JP-8 fuel and rapeseed biodiesel blends. Energy Convers. Manag. 2015, 105, 216–229. [Google Scholar] [CrossRef]
- Kassem, Y.; Camur, H. A laboratory study of the effects of wide range temperature on the properties of biodiesel produced from various waste vegetable oils. Waste Biomass Valoriz. 2017, 8, 1995–2007. [Google Scholar] [CrossRef][Green Version]
- Radlik, M.; Małolepszy, A.; Matus, K. Alkane isomerization on highly reduced Pd/Al2O3 catalysts. The crucial role of Pd-Al species. Catal. Commun. 2019, 123, 17–22. [Google Scholar] [CrossRef]
- Hancsok, J.; Kovacs, S.; Polczmann, G.; Kasz, T. Investigation the effect of oxygenic compounds on the isomerization of bioparaffins over Pt/SAPO-11. Top. Catal. 2011, 54, 1094–1101. [Google Scholar] [CrossRef]
- Zhang, Z.; Pittman, C.U.; Sui, J.S.; Sunand, J.; Wang, Q. Catalytic upgrading of bio-oil by reacting with olefins and alcohols over solid acids: Reaction paths via model compound studies. Energies 2013, 6, 1568–1589. [Google Scholar] [CrossRef]
- Gomes, L.C.; Rosas, D.D.O.; Chistone, R.C.; Zotin, F.M.Z.; de Araujo, L.R.R.; Zotin, J.L. Hydroisomerization of n-hexadecane using Pt/alumina-Beta zeolite catalysts for producing renewable diesel with low pour point. Fuel 2017, 209, 521–528. [Google Scholar] [CrossRef]
- Gläser, R.; Gomm, S.; Weitkamp, J. In situ investigation of cumene synthesis over dealuminated zeolite catalysts by means of a tapered element oscillating microbalance. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2004; Volume 154, pp. 2125–2132. [Google Scholar]
- Aboul-Gheit, A.K.; Awadallah, A.E.; Aboul-Gheit, N.A.K.; Solyman, E.A.; Abdel-Aaty, M.A. Effect of hydrochlorination and hydrofluorination of Pt/H-ZSM-5 and Pt–Ir/H-ZSM-5 catalysts for n-hexane hydroconversion. Appl. Catal. A Gen. 2008, 334, 304–310. [Google Scholar] [CrossRef]
- Aboul-Gheit, N.A.K. Effect of hydrohalogenation of metal/zeolite catalysts for cyclohexene hydroconversion- Part 3- Pd/H-ZSM-5 catalysts. J. Chin. Chem. Soc. 2007, 54, 1211–1222. [Google Scholar] [CrossRef]
- Sidhpuria, K.B.; Parikh, P.A.; Bahadur, P.; Jasra, R.V. Rhodium supported Hβ zeolite for the hydrogenation of toluene. Ind. Eng. Chem. Res. 2008, 47, 4034–4042. [Google Scholar] [CrossRef]
- Cui, S.; Wang, G.; Yang, Y.; Liu, B. Influence of Si/Al molar ratio on the hydrogenation, isomerization and ring opening of naphthalene over silica-alumina supported Ni2P catalyst. Fuel 2018, 225, 10–17. [Google Scholar] [CrossRef]
- Fan, Y.; Bao, X.; Shi, G.; Wei, W.; Xu, J. Olefin reduction of FCC gasoline via hydroisomerization aromatisation over modified HMOR/HZSM-5/H-beta composite carriers. Appl. Catal. A Gen. 2004, 275, 61–71. [Google Scholar] [CrossRef]
- Tian, S.; Chen, J. Hydroisomerization of n-dodecane on a new kind of bifunctional catalyst: Nickel phosphide supported on SAPO-11 molecular sieve. Fuel Process. Technol. 2014, 122, 120–128. [Google Scholar] [CrossRef]
- Park, K.C.; Ihm, S.K. Hydroisomerization of n-tetradecane over Pt/SAPO-11 catalyst. Appl. Catal. A Gen. 2000, 203, 201–209. [Google Scholar] [CrossRef]
- Geng, C.; Zhang, F.; Gao, Z.; Zhao, L.; Zhou, J. Hydroisomerization of n-tetradecane over Pt/SAPO-11 catalyst. Catal. Today 2014, 93–95, 485–491. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Rooney, D.W.; Thompson, J.; Halawy, S.A.; Mohamed, M.A. Surface hydrophobicity and acidity effect on alumina catalyst in catalytic methanol dehydration reaction. J. Chem. Technol. Biotechnol. 2017, 92, 2952–2962. [Google Scholar] [CrossRef]
- Kubicka, D.; Kikhtyanin, O. Opportunities for zeolites in biomass upgrading—Lessons from the refining and petrochemical industry. Catal. Today 2015, 243, 10–22. [Google Scholar] [CrossRef]
- Osman, A.I.; Abu-Dahrieh, J.K.; Abdelkader, A.; Hassan, N.M.; Laffir, F.; McLaren, M.; Rooney, D.W. Silver-Modified η-Al2O3 Catalyst for DME Production. J. Phys. Chem. C 2017, 121, 25018–25032. [Google Scholar] [CrossRef]
- Li, X.; Shen, B.; Guo, Q.; Gao, J. Effects of large pore zeolite additions in the catalytic pyrolysis catalyst on the light olefins production. Catal. Today 2007, 125, 270–277. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, Z.; Wu, B.; Xu, J.; Huo, C.; Li, K.; Chen, H.; Yang, Y.; Li, Y. Effect of metal precursors on the performance of Pt/ZSM-22 catalysts for n-hexadecane hydroisomerization. J. Catal. 2015, 22, 1–13. [Google Scholar] [CrossRef]
- Palanisamy, S.; Gevert, B.S. Hydrodeoxygenation of fatty acid methyl ester in gas oil blend-NiMoS/Alumina. Green Process. Synth. 2017, 7, 260–267. [Google Scholar] [CrossRef]
- Palanisamy, S.; Gevert, B.S. Study of non-catalytic thermal decomposition of triglyceride at hydroprocessing condition. Appl. Therm. Eng. 2016, 107, 301–310. [Google Scholar] [CrossRef]
- Song, Y.; Lin, W.; Guo, X.; Dong, L.; Mu, X.; Tian, H.; Wang, L. Aromatization and isomerization of methylcyclohexane over Ni catalysts supported on different supports. Green Energy Environ. 2018, 4, 75–82. [Google Scholar] [CrossRef]
- Ramos, M.J.; Lucas, A.D.; Jiménez, V.; Sánchez, P.; Valverde, J.L. Hydro-isomerization of different refinery naphtha streams by using a beta zeolite catalyst. Fuel Process. Technol. 2008, 89, 721–727. [Google Scholar] [CrossRef]
- Buzetzki, E.; Sidorová, K.; Cvengrošová, Z.; Kaszonyi, A.; Cvengroš, J. The influence of zeolite catalysts on the products of rapeseed oil cracking. Fuel Process. Technol. 2011, 92, 1623–1631. [Google Scholar] [CrossRef]
- Ono, Y.A. Survey of the mechanism in the catalytic isomerisation of alkanes. Catal. Today 2003, 81, 3–16. [Google Scholar] [CrossRef]
- Soualah, A.; Lemberton, J.; Pinard, L.; Chater, M.; Magnoux, P.; Moljord, K. Hydroconversion of n-decane on Pt/HZSM-5 bifunctional catalysts: Effect of the Si/Al ratio of the zeolite on selectivities. React. Kinet. Mech. Catal. 2010, 101, 209–219. [Google Scholar] [CrossRef]
- Saxena, S.K.; Viswanadham, N.; Garg, M.O. Cracking and isomerisation functionalities of bi-metallic zeolites for naphtha value upgradation. Fuel 2013, 107, 432–438. [Google Scholar] [CrossRef]
- Jaroszewska, K.; Masalska, A.; Czycz, D.; Grzechowiak, J. Activity of shaped Pt/AlSBA-15 catalysts in n-hexadecane hydroisomerization. Fuel Process. Technol. 2017, 167, 1–10. [Google Scholar] [CrossRef]
- Ye, G.; Sun, Y.; Guo, Z.; Zhu, K.; Liu, H.; Zhou, X.; Coppens, M.O. Effects of zeolite particle size and internal grain boundaries on Pt/Beta catalyzed isomerization of n-pentane. J. Catal. 2018, 360, 152–159. [Google Scholar] [CrossRef]
- Degnan, J.T.F. Applications of zeolites in petroleum refining. Top. Catal. 2000, 13, 349–356. [Google Scholar] [CrossRef]
- Claude, M.C.; Martens, J.A. Monomethyl-Branching of Long n-Alkanes in the Range from Decane to Tetracosane on Pt/H-ZSM-22 Bi-functional Catalyst. J. Catal. 2000, 190, 39–48. [Google Scholar] [CrossRef]
- Shi, Q.; Gonçalves, J.C.; Ferreira, A.F.P.; Plaza, M.G.; Rodrigues, A.E. Xylene isomerization side reactions over Beta zeolite: Disproportionation and transalkylation of C8 aromatics and toluene. Appl. Catal. A Gen. 2018, 562, 198–205. [Google Scholar] [CrossRef]
- Wang, J.J.; Chuang, Y.Y.; Hsu, H.Y.; Tsai, T.C. Toward industrial catalysis of zeolite for linear alkylbenzene synthesis: A mini-review. Catal. Today 2017, 298, 109–116. [Google Scholar] [CrossRef]


| Parameters | Hydrotreated FAME in Gas Oil (ULSD) | Conventional Diesel (LGO) | Reference Test Method * |
|---|---|---|---|
| Compositions | |||
| Aromatic (mono-) (% v/v) | 9.9 | 5.0 | IP 391 |
| Aromatic (di-) (% v/v) | 0.2 | 0.1 | IP 391 |
| PAH (tri+) (% v/v)# | <0.02 | <0.02 | IP 391 |
| Liquid Paraffins (wt%) | |||
| Gasoline fraction (<195 °C) | 0.6 | 0 | |
| Diesel fraction (195-300 °C) | 93.2 | 98 | |
| Heavy Fraction (>300 °C) | 6.2 | 2.1 | |
| Distillation temperature v/v rec. (°C)(SIM-DIST) | |||
| IBP | 176 | 196 | ASTM D86 |
| Dist: Temp. at 10% v/v | 195 | 216 | ASTM D86 |
| Dist: Temp. at 50% v/v | 202 | 237 | ASTM D86 |
| Dist: Temp. at 65% v/v | 231 | 245 | ASTM D86 |
| Dist: Temp. at 90% v/v | 259 | 272 | ASTM D86 |
| Dist: Temp. at 95% v/v | 289 | 384 | ASTM D86 |
| FBP | 303 | 295 | ASTM D86 |
| Other Properties | |||
| Cloud point (°C) | −32 | −34 | EN ISO 23015:1994 |
| Viscosity at 40 °C (mm2/s) | 2.083 | 1.909 | EN ISO 3104 |
| Density at 15 °C (kg/m3) | 820.6 | 821.2 | ASTM D4052-09 |
| Cetane Index | 51.86 | 51 | ASTM D 4737 |
| Nitrogen content (mg/kg) | <1 | <1 | ASTM D 4629 |
| Sulphur content (mg/kg) | <1 | <1 | EN ISO 8754 |
| Material | Surface Area (m²/g) | Micropore Area (m²/g) | Total Pore Volume (cm³/g) | Micropore Volume (cm³/g) | Avg. Pore Width (Å) | Adsorption Avg. Pore Diameter 1 (Å) | Desorption Avg. Pore Diameter 2 (Å) | Coke db 3 (ASTM D5373) |
|---|---|---|---|---|---|---|---|---|
| H-β # | 461.4 | 295.8 | 0.36 | 0.13 | 31.5 | 58.2 | 54.1 | ** |
| Pt/H-β | 244.2 | 105.0 | 0.21 | 0.05 | 41.2 | 49.9 | 43.3 | 2.4@ |
| Pt-Cl/H-β | 248.4 | 87.5 | 0.23 | 0.04 | 40.8 | 51.4 | 46.3 | 0.1 |
| Spent Pt-Cl/H-β | 186.3 | 83.1 | 0.16 | 0.023 | 26.4 | 44.6 | 41.1 | 2.0@ |
| Spectrum Identity (wt%) | H-β Extruder | Pt-Cl/H-β | Pt/H-β | |
|---|---|---|---|---|
| Top View | Top View | Segmented View | Top View | |
| O | 52.83 | 47.32 | 49.31 | 52.97 |
| Al | 32.67 | 31.74 | 30.76 | 32.69 |
| Si | 12.83 | 18.24 | 17.24 | 13.29 |
| Cl | 1.67 | 0.67 | 0.58 | ** |
| Pt | ** | 2.03 | 2.11 | 2.05 |
| SiO2/Al2O3 (mol%)# | 0.5 | 0.73 | 0.71 | 0.52 |
| Parameters | 10 H-ULSD | P (MPa) at T (°C) = 240 °C and LHSV = 1.0 h−1 | T (°C) at LHSV = 0.5 h−1 and P = 5 Mpa | ||||||||
| 4.0 | 4.5 | 5.0 | 5.5 | 6.5 | 230 | 240 | 250 | 260 | 270 | ||
| Aromatic (mono-) (% v/v) | 8.8 | ** | 4.5 | 3.8 | ** | 3.3 | 5.6 | 4.9 | 2.2 | <0.1 | <0.1 |
| Aromatic (di-) (% v/v) | 0.2 | ** | 0.1 | 0.1 | ** | 0.1 | 0.2 | 0.1 | 0.1 | <0.1 | <0.1 |
| PAH (tri+) (% v/v)# | <0.02 | ** | <0.02 | <0.02 | ** | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 | <0.02 |
| Liquid Products in Boiling Point Range (Temperature in °C) (wt%) | |||||||||||
| Gasoline fraction (<195) | 11.1 | 12.7 | 14.8 | 15.1 | 15.8 | 17.3 | 11.8 | 16.4 | 26 | 38.7 | 47.6 |
| Diesel fraction (195–300) | 85.8 | 85.6 | 83.3 | 83.2 | 82.5 | 81.4 | 83 | 82.7 | 74 | 61.4 | 52.4 |
| Heavy Fraction (>300) | 3.1 | 1.8 | 1.8 | 1.7 | 1.7 | 1.3 | 5.2 | 0.9 | 0 | 0 | 0 |
| Distillation Temperature v/v Recovery (°C) (SIM-DIST) | |||||||||||
| IBP | 182 | 183 | ** | 180 | 189 | 189 | 179 | 178 | 177 | 175 | 175 |
| 10% v/v | 191 | 188 | ** | 194 | 196 | 199 | 187 | 187 | 186 | 185 | 185 |
| 50% v/v | 216 | 233 | ** | 234 | 237 | 238 | 231 | 231 | 233 | 235 | 235 |
| 65% v/v | 237 | 249 | ** | 251 | 261 | 270 | 248 | 248 | 247 | 248 | 265 |
| 90% v/v | 245 | 276 | ** | 275 | 280 | 283 | 276 | 276 | 274 | 274 | 273 |
| 95% v/v | 289 | 283 | ** | 287 | 285 | 288 | 285 | 289 | 285 | 282 | 280 |
| FBP | 309 | 306 | ** | 301 | 295 | 290 | 302 | 299 | 296 | 291 | 290 |
| Other Properties for Distilled Product | |||||||||||
| Cloud point (°C) | 1 | −24 | −21 | −20 | −20 | −22 | −20 | −25 | −32 | −34 | −35 |
| Viscosity at 40 °C (mm2/s) | 2.1 | 2.0 | 2.0 | 1.9 | 1.9 | 1.9 | 2.0 | 2.0 | 1.9 | 1.9 | 1.9 |
| Density at 15 °C (kg/m3) | 823.7 | 819.5 | 819.4 | 819.3 | 819 | 818.6 | 821.3 | 820.3 | 812.5 | 804 | 798 |
| Parameters | 10 H-ULSD | 10 H-ULSD | 10 H-ULSD | 10 H-ULSD +T * |
| LHSV (h−1) | - | 0.5 | 1 | 1 |
| Aromatic (mono-) (% v/v) | 8.8 | 7.5 | 7.4 | 10.4 |
| Aromatic (di-) (% v/v) | 0.2 | 3.2 | 0.3 | 0.4 |
| PAH (tri+) (% v/v)# | <0.02 | 0.08 | 0.03 | 0.02 |
| Liquid Products in Boiling Point Range (Temperature in °C) (wt%) | ||||
| <125 | 0.0 | 5.9 | 2.9 | 6.2 |
| 125–195 | 5.2 | 14.4 | 17 | 9.3 |
| 196–215 | 9.4 | 9.8 | 9.7 | 9.6 |
| 216–250 | 36.0 | 32.4 | 30.9 | 32.3 |
| 251–265 | 19.7 | 16.3 | 17.4 | 19.2 |
| 265–300 | 23.5 | 19.3 | 20 | 21.3 |
| Heavy Fraction | 6.2 | 1.9 | 2.1 | 2.1 |
| Other Properties for Distilled Product | ||||
| Cloud point (°C) | 1 | −21 | −14 | −20 |
| Viscosity at 40 °C (mm2/s) | 2.1 | 1.9 | 2 | 1.9 |
| Density at 15 °C (kg/m3) | 823.7 | 820.6 | 819.5 | 819.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palanisamy, S.; Gevert, B.S.; Sankaran, P.; Kandasamy, K. Produce Low Aromatic Contents with Enhanced Cold Properties of Hydrotreated Renewable Diesel Using Pt/Alumina-Beta-Zeolite: Reaction Path Studied via Monoaromatic Model Compound. Energies 2019, 12, 2853. https://doi.org/10.3390/en12152853
Palanisamy S, Gevert BS, Sankaran P, Kandasamy K. Produce Low Aromatic Contents with Enhanced Cold Properties of Hydrotreated Renewable Diesel Using Pt/Alumina-Beta-Zeolite: Reaction Path Studied via Monoaromatic Model Compound. Energies. 2019; 12(15):2853. https://doi.org/10.3390/en12152853
Chicago/Turabian StylePalanisamy, Shanmugam, Börje Sten Gevert, Pranav Sankaran, and Kannan Kandasamy. 2019. "Produce Low Aromatic Contents with Enhanced Cold Properties of Hydrotreated Renewable Diesel Using Pt/Alumina-Beta-Zeolite: Reaction Path Studied via Monoaromatic Model Compound" Energies 12, no. 15: 2853. https://doi.org/10.3390/en12152853
APA StylePalanisamy, S., Gevert, B. S., Sankaran, P., & Kandasamy, K. (2019). Produce Low Aromatic Contents with Enhanced Cold Properties of Hydrotreated Renewable Diesel Using Pt/Alumina-Beta-Zeolite: Reaction Path Studied via Monoaromatic Model Compound. Energies, 12(15), 2853. https://doi.org/10.3390/en12152853






