Quantitative Analysis of CO2 Uptake and Mechanical Properties of Air Lime-Based Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Test Methods
2.3.1. Particle Size of Raw Materials
2.3.2. Mechanical Properties
2.3.3. Weight Change
2.3.4. Carbonation Depth
2.3.5. Pore Size Distribution
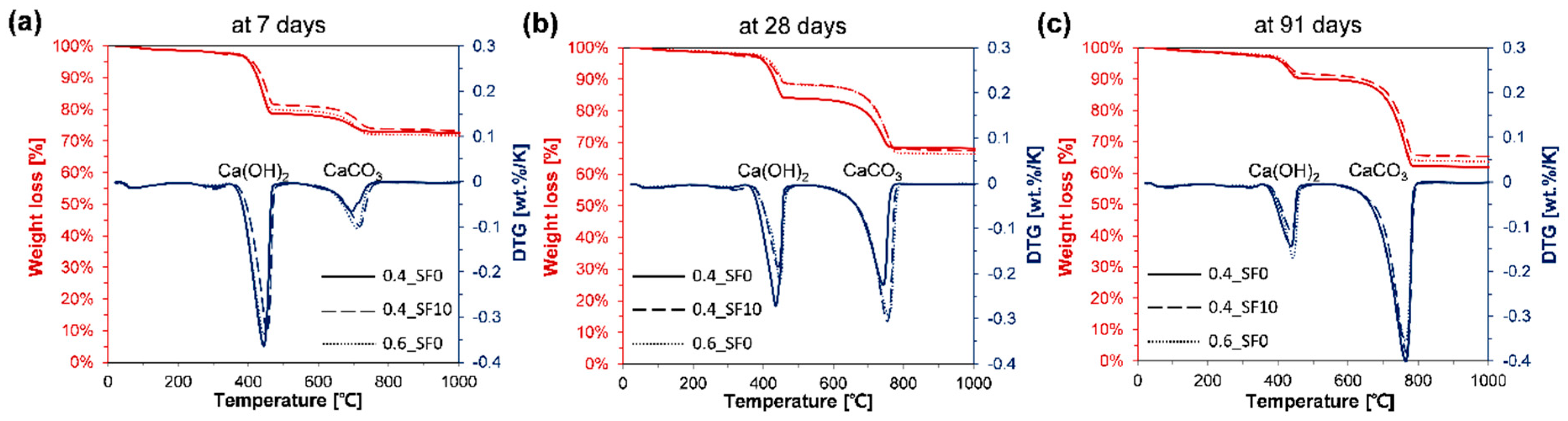
2.3.6. Thermogravimetry-Based Quantitative Analysis
3. Results and Discussion
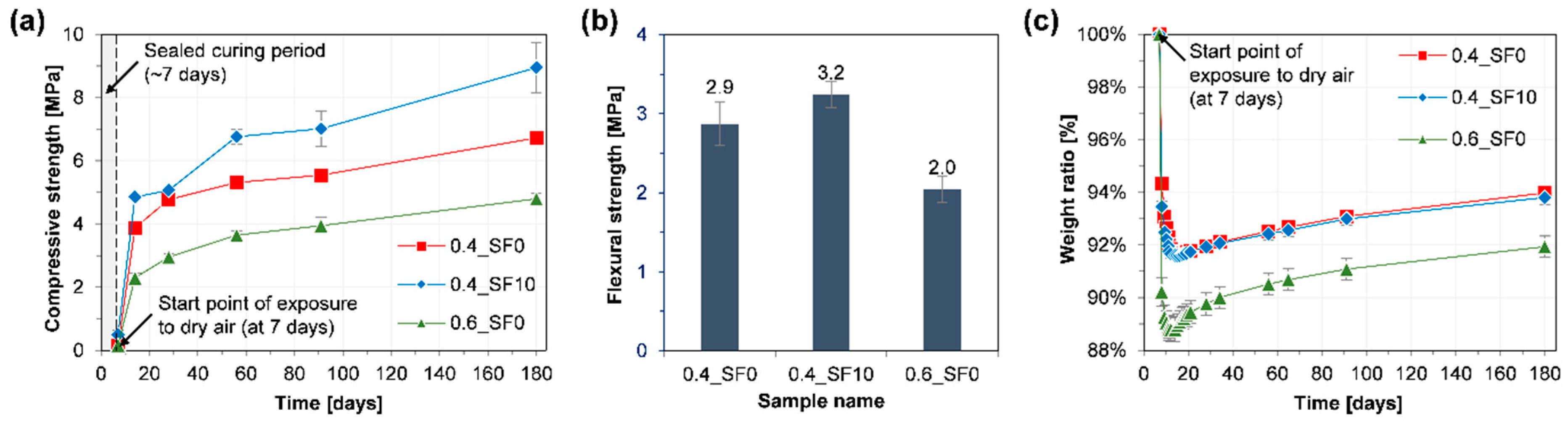
3.1. Mechanical Properties and Weight Change
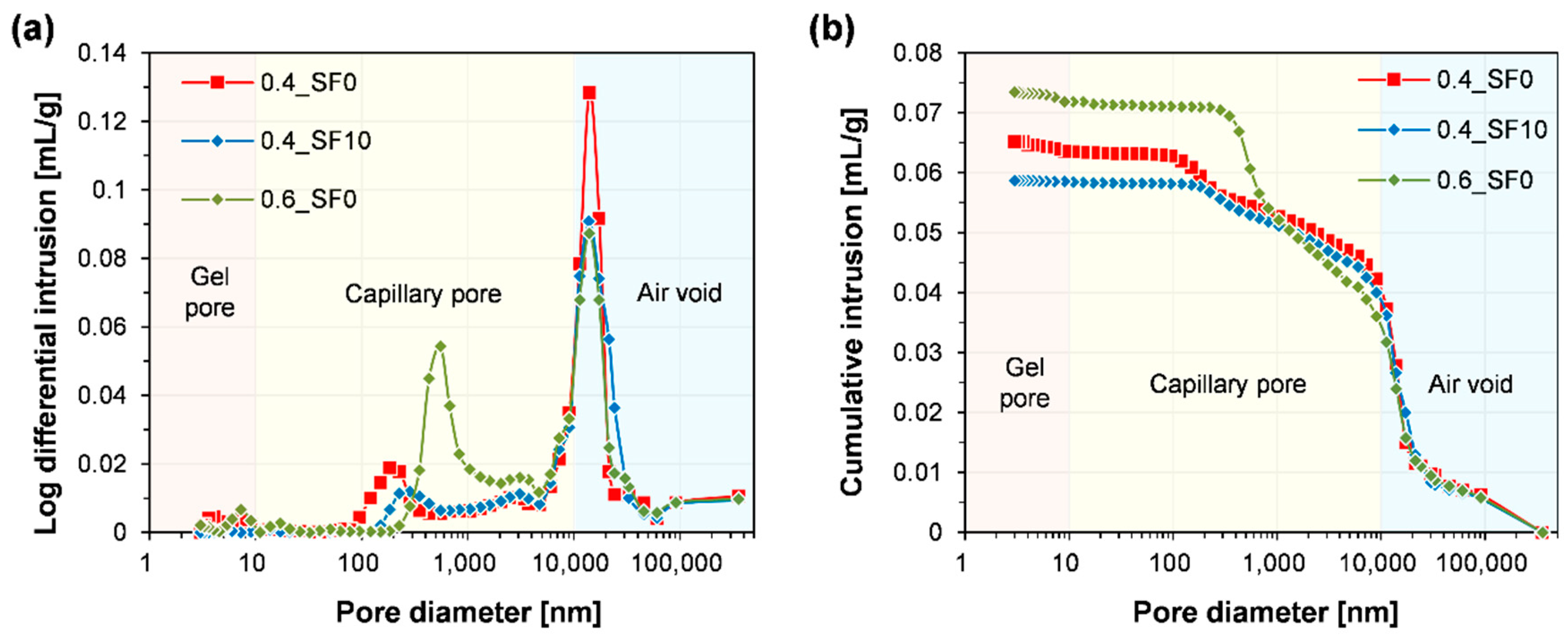
3.2. Carbonation Depth and Pore Structure
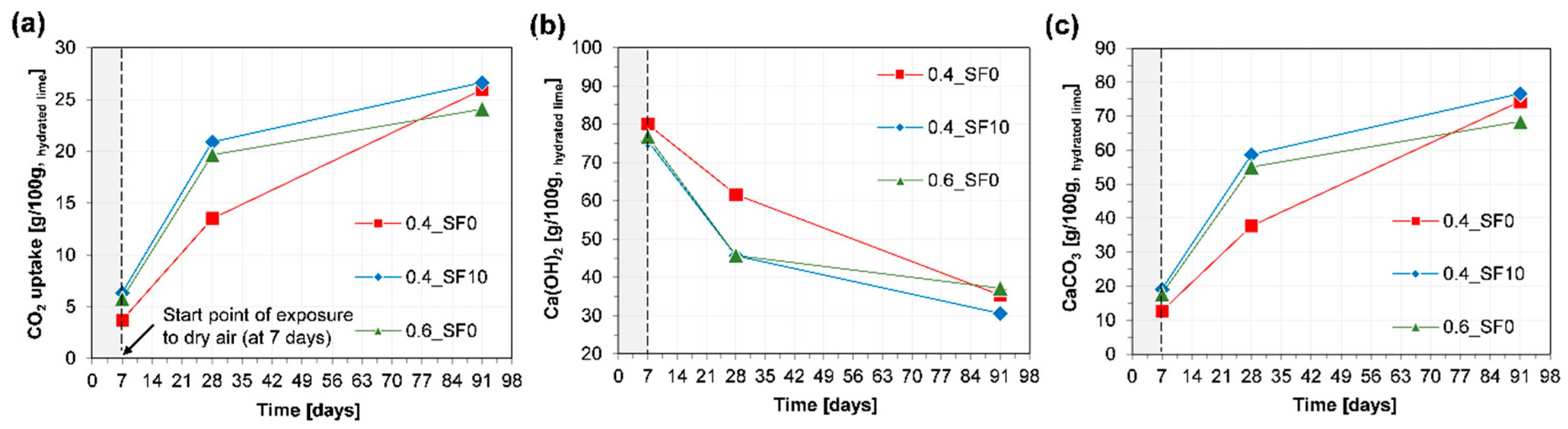
3.3. Component Change and CO2 Uptake Rate
4. Conclusions
- The setting and hardening of the mortars were made possible by absorbing CO2 from the atmosphere.
- Within three months, the air lime-based binder recovered more than half of the CO2 released during the manufacturing process of the raw material.
- The weight and compressive strength of the mortar steadily increased due to CO2 uptake and carbonation reaction for 180 days.
- When 10% of hydrated lime was replaced with silica fume, the strength of the mortar significantly improved without noticeable changes in carbonation depth and the amount of CO2 uptake. On the other hand, when the w/b of the mortar was increased from 0.4 to 0.6, the strength and carbonation depth were significantly decreased and increased, respectively.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kang, S.H.; Kwon, Y.H.; Hong, S.G.; Chun, S.; Moon, J. Hydrated lime activation on byproducts for eco-friendly production of structural mortars. J. Clean. Prod. 2019, 231, 1389–1398. [Google Scholar] [CrossRef]
- Monteiro, P.J.M.; Miller, S.A.; Horvath, A. Towards sustainable concrete. Nat. Mater. 2017, 16, 698. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Zhang, Y.; Jia, Y.; She, W.; Liu, G.; Yang, Z.; Zhang, Y.; Zhang, W.; Sun, W. Effects of sodium sulfate on the hydration and properties of lime-based low carbon cementitious materials. J. Clean. Prod. 2019, 220, 677–687. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change (IPCC). Special Report on Global Warming of 1.5 °C: Summary for Policymakers; Intergovernmental Panel on Climate Change (IPCC): Incheon, Korea, 2018; pp. 1–33. [Google Scholar]
- Kang, S.H.; Jeong, Y.; Tan, K.H.; Moon, J. High-volume use of limestone in ultra-high performance fiber-reinforced concrete for reducing cement content and autogenous shrinkage. Constr. Build. Mater. 2019, 213, 292–305. [Google Scholar] [CrossRef]
- Mo, L.; Hao, Y.; Liu, Y.; Wang, F.; Deng, M. Preparation of calcium carbonate binders via CO2 activation of magnesium slag. Cem. Concr. Res. 2019, 121, 81–90. [Google Scholar] [CrossRef]
- Kang, S.H.; Jeong, Y.; Kim, M.O.; Moon, J. Pozzolanic reaction on alkali-activated Class F fly ash for ambient condition curable structural materials. Constr. Build. Mater. 2019, 218, 235–244. [Google Scholar] [CrossRef]
- Bontempi, E. A new approach for evaluating the sustainability of raw materials substitution based on embodied energy and the CO2 footprint. J. Clean. Prod. 2017, 162, 162–169. [Google Scholar] [CrossRef]
- Bontempi, E. A new approach to evaluate the sustainability of raw materials substitution. In Raw Materials Substitution Sustainability; Springer International Publishing: Cham, Korea, 2017; pp. 79–101. [Google Scholar]
- Quina, M.J.; Bontempi, E.; Bogush, A.; Schlumberger, S.; Weibel, G.; Braga, R.; Funari, V.; Hyks, J.; Rasmussen, E.; Lederer, J. Technologies for the management of MSW incineration ashes from gas cleaning: New perspectives on recovery of secondary raw materials and circular economy. Sci. Total Environ. 2018, 635, 526–542. [Google Scholar] [CrossRef]
- Bosio, A.; Zacco, A.; Borgese, L.; Rodella, N.; Colombi, P.; Benassi, L.; Depero, L.E.; Bontempi, E. A sustainable technology for Pb and Zn stabilization based on the use of only waste materials: A green chemistry approach to avoid chemicals and promote CO2 sequestration. Chem. Eng. J. 2014, 253, 377–384. [Google Scholar] [CrossRef]
- Bernal, S.A.; van Deventer, J.S.J.; Provis, J.L. Natural and accelerated carbonation rates of alkali-activated slag/fly ash blended concretes. In Proceedings of the International Conference on Sustainable Materials, Systems and Structures (SMSS2019): Durability, Monitoring and Repair of Structures, Rovinj, Croatia, 20–22 March 2019; pp. 357–364. [Google Scholar]
- Van den Heede, P.; De Belie, N. Difference in carbonation behavior at 0.04%, 1% and 10% CO2 for High-Volume Fly Ash (HVFA) mortar: Effect on internal humidity and resistivity. In Proceedings of the International Conference on Sustainable Materials, Systems and Structures (SMSS2019): Durability, Monitoring and Repair of Structures, Rovinj, Croatia, 20–22 March 2019; pp. 317–324. [Google Scholar]
- Rahman, A.; Rasul, M.G.; Khan, M.M.K.; Sharma, S.C. Assessment of energy performance and emission control using alternative fuels in cement industry through a process model. Energies 2017, 10, 1996. [Google Scholar] [CrossRef]
- Shao, M.; Li, L.; Chen, W.; Liu, J. Investigation and modification of two kinds of Chinese traditional lime in cultural building relics. J. Cult. Herit. 2019, 36, 118–127. [Google Scholar] [CrossRef]
- Carran, D.; Hughes, J.; Leslie, A.; Kennedy, C. A short history of the use of lime as a building material beyond Europe and North America. Int. J. Archit. Herit. 2012, 6, 117–146. [Google Scholar] [CrossRef]
- Veiga, R. Air lime mortars: What else do we need to know to apply them in conservation and rehabilitation interventions? A review. Constr. Build. Mater. 2017, 157, 132–140. [Google Scholar] [CrossRef]
- Nogueira, R.; Ferreira Pinto, A.P.; Gomes, A. Design and behavior of traditional lime-based plasters and renders. Review and critical appraisal of strengths and weaknesses. Cem. Concr. Compos. 2018, 89, 192–204. [Google Scholar] [CrossRef]
- Zhang, D.; Zhao, J.; Wang, D.; Xu, C.; Zhai, M.; Ma, X. Comparative study on the properties of three hydraulic lime mortar systems: Natural hydraulic lime mortar, cement-aerial lime-based mortar and slag-aerial lime-based mortar. Constr. Build. Mater. 2018, 186, 42–52. [Google Scholar] [CrossRef]
- Cazalla, O.; Rodriguez-Navarro, C.; Sebastian, E.; Cultrone, G.; De la Torre, M.J. Aging of lime putty: Effects on traditional lime mortar carbonation. J. Am. Ceram. Soc. 2000, 83, 1070–1076. [Google Scholar] [CrossRef]
- Lawrence, R.M.; Mays, T.J.; Rigby, S.P.; Walker, P.; D’Ayala, D. Effects of carbonation on the pore structure of non-hydraulic lime mortars. Cem. Concr. Res. 2007, 37, 1059–1069. [Google Scholar] [CrossRef]
- Borges, C.; Santos Silva, A.; Veiga, R. Durability of ancient lime mortars in humid environment. Constr. Build. Mater. 2014, 66, 606–620. [Google Scholar] [CrossRef]
- Martínez-García, C.; González-Fonteboa, B.; Carro-López, D.; Martínez-Abella, F. Impact of mussel shell aggregates on air lime mortars. Pore structure and carbonation. J. Clean Prod. 2019, 215, 650–668. [Google Scholar] [CrossRef]
- Izaguirre, A.; Lanas, J.; Álvarez, J.I. Ageing of lime mortars with admixtures: Durability and strength assessment. Cem. Concr. Res. 2010, 40, 1081–1095. [Google Scholar] [CrossRef]
- Izaguirre, A.; Lanas, J.; Álvarez, J.I. Characterization of aerial lime-based mortars modified by the addition of two different water-retaining agents. Cem. Concr. Compos. 2011, 33, 309–318. [Google Scholar] [CrossRef]
- Alvarez, J.I.; Fernández, J.M.; Navarro-Blasco, I.; Duran, A.; Sirera, R. Microstructural consequences of nanosilica addition on aerial lime binding materials: Influence of different drying conditions. Mater. Charact. 2013, 80, 36–49. [Google Scholar] [CrossRef]
- Silva, B.A.; Ferreira Pinto, A.P.; Gomes, A. Influence of natural hydraulic lime content on the properties of aerial lime-based mortars. Constr. Build. Mater. 2014, 72, 208–218. [Google Scholar] [CrossRef]
- Silva, B.A.; Ferreira Pinto, A.P.; Gomes, A. Natural hydraulic lime versus cement for blended lime mortars for restoration works. Constr. Build. Mater. 2015, 94, 346–360. [Google Scholar] [CrossRef]
- Gulbe, L.; Vitina, I.; Setina, J. The influence of cement on properties of lime mortars. Procedia Eng. 2017, 172, 325–332. [Google Scholar] [CrossRef]
- Fernández, J.M.; Duran, A.; Navarro-Blasco, I.; Lanas, J.; Sirera, R.; Alvarez, J.I. Influence of nanosilica and a polycarboxylate ether superplasticizer on the performance of lime mortars. Cem. Concr. Res. 2013, 43, 12–24. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 679, Cement–Test Methods–Determination of Strength; International Organization for Standardization: Geneva, Switzerland, 2009; p. 29. [Google Scholar]
- ASTM International. ASTM C230/C230M-14, Standard Specification for Flow Table for Use in Tests of Hydraulic Cement; ASTM International: West Conshohocken, PA, USA, 2014; p. 6. [Google Scholar]
- British Standards Institution (BSI). BS EN 459-2: 2010. In Building Lime Test Methods; BSI Standards Publication: London, UK, 2010. [Google Scholar]
- Kwon, Y.H.; Kang, S.H.; Hong, S.G.; Moon, J. Intensified pozzolanic reaction on kaolinite clay-based mortar. Appl. Sci. 2017, 7, 522. [Google Scholar] [CrossRef]
- Cizer, Ö.; Van Balen, K.; Elsen, J.; Van Gemert, D. Real-time investigation of reaction rate and mineral phase modifications of lime carbonation. Constr. Build. Mater. 2012, 35, 741–751. [Google Scholar] [CrossRef]
- Kang, S.H.; Jeong, Y.; Tan, K.H.; Moon, J. The use of limestone to replace physical filler of quartz powder in UHPFRC. Cem. Concr. Compos. 2018, 94, 238–247. [Google Scholar] [CrossRef]
- ASTM International. ASTM D4284-12, Standard Test Method for Determining Pore Volume Distribution of Catalysts and Catalyst Carriers by Mercury Intrusion Porosimetry; ASTM International: West Conshohocken, PA, USA, 2012; p. 7. [Google Scholar]
- Scrivener, K.; Snellings, R.; Lothenbach, B. A Practical Guide to Microstructural Analysis of Cementitious Materials; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Snellings, R.; Chwast, J.; Cizer, Ö.; De Belie, N.; Dhandapani, Y.; Durdzinski, P.; Elsen, J.; Haufe, J.; Hooton, D.; Patapy, C.; et al. Report of TC 238-SCM: Hydration stoppage methods for phase assemblage studies of blended cements—results of a round robin test. Mater. Struct. 2018, 51, 111. [Google Scholar] [CrossRef]
- Kang, S.H.; Lee, J.H.; Hong, S.G.; Moon, J. Microstructural investigation of heat-treated ultra-high performance concrete for optimum production. Materials 2017, 10, 1106. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Kang, S.H.; Hong, S.G.; Moon, J. Enhancement of material properties of lime-activated slag mortar from intensified Pozzolanic reaction and pore filling effect. Sustainability 2018, 10, 4290. [Google Scholar] [CrossRef]
- Letelier, V.; Henríquez-Jara, B.I.; Manosalva, M.; Parodi, C.; Ortega, J.M. Use of waste glass as a replacement for raw materials in mortars with a lower environmental impact. Energies 2019, 12, 1974. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Kang, S.H.; Hong, S.G.; Moon, J. Acceleration of intended pozzolanic reaction under initial thermal treatment for developing cementless fly ash based mortar. Materials 2017, 10, 225. [Google Scholar] [CrossRef]
- Kang, S.H.; Hong, S.G.; Moon, J. The use of rice husk ash as reactive filler in ultra-high performance concrete. Cem. Concr. Res. 2019, 115, 389–400. [Google Scholar] [CrossRef]
- Kang, S.H.; Hong, S.G.; Moon, J. The effect of superabsorbent polymer on various scale of pore structure in ultra-high performance concrete. Constr. Build. Mater. 2018, 172, 29–40. [Google Scholar] [CrossRef]
- Mindess, S.; Young, J.F.; Darwin, D. Concrete, 2nd ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2003. [Google Scholar]
- Aligizaki, K.K. Pore Structure of Cement-Based Materials: Testing, Interpretation and Requirements; Taylor & Francis: Abingdon, Oxfordshire, UK, 2006; p. 388. [Google Scholar]
- Librandi, P.; Nielsen, P.; Costa, G.; Snellings, R.; Quaghebeur, M.; Baciocchi, R. Mechanical and environmental properties of carbonated steel slag compacts as a function of mineralogy and CO2 uptake. J. CO2 Util. 2019, 33, 201–214. [Google Scholar] [CrossRef]
- Xi, F.; Davis, S.J.; Ciais, P.; Crawford-Brown, D.; Guan, D.; Pade, C.; Shi, T.; Syddall, M.; Lv, J.; Ji, L.; et al. Substantial global carbon uptake by cement carbonation. Nat. Geosci 2016, 9, 880. [Google Scholar] [CrossRef]
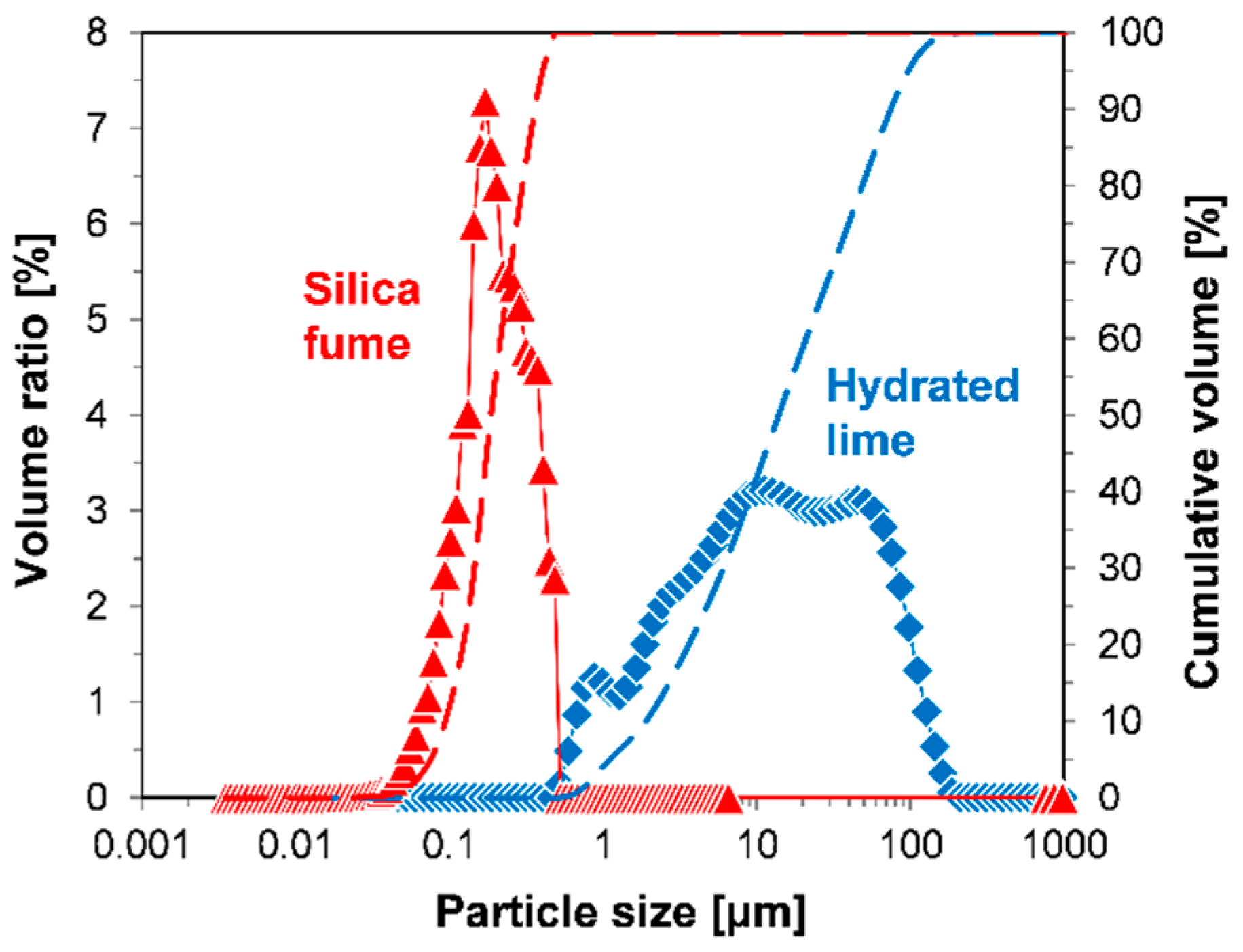

| Materials | CaO | SiO2 | MgO | Al2O3 | Fe2O3 | SO3 | K2O | Na2O | P2O5 | Cl | LOI 1 | Total |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hydrated lime | 70.6 | 1.7 | 1.6 | 0.8 | 0.3 | 0.6 | 0.1 | - | - | - | 24.1 | 99.9 |
| Silica fume | 0.3 | 97.0 | 0.4 | 0.7 | 0.1 | 0.2 | 0.8 | 0.3 | 0.1 | 0.1 | - | 100.0 |
| Sample Name | Hydrated Lime | Silica Fume | Water | Super-Plasticizer 1 | Fine Aggregate | Curing Condition |
|---|---|---|---|---|---|---|
| 0.4_SF0 | 1 | 0 | 0.4 | 0.06 | 3 | Sealed curing (20 °C) for 7 days followed by air dry curing (20 °C, Relative humidity 60% and CO2 concentration 0.04%) |
| 0.4_SF10 | 0.9 | 0.1 | ||||
| 0.6_SF0 | 1 | 0 | 0.6 | 0.03 |
| Method | Effectiveness 1 | |||
|---|---|---|---|---|
| Compressive Strength | Flexural Strength | Carbonation Depth | CO2 Uptake | |
| Silica fume addition instead of 10% hydrated lime | +27% | +13% | −1% | −7% |
| Increase of the w/b from 0.4 to 0.6 | −29% | −29% | +49% | +3% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.-H.; Kwon, Y.-H.; Moon, J. Quantitative Analysis of CO2 Uptake and Mechanical Properties of Air Lime-Based Materials. Energies 2019, 12, 2903. https://doi.org/10.3390/en12152903
Kang S-H, Kwon Y-H, Moon J. Quantitative Analysis of CO2 Uptake and Mechanical Properties of Air Lime-Based Materials. Energies. 2019; 12(15):2903. https://doi.org/10.3390/en12152903
Chicago/Turabian StyleKang, Sung-Hoon, Yang-Hee Kwon, and Juhyuk Moon. 2019. "Quantitative Analysis of CO2 Uptake and Mechanical Properties of Air Lime-Based Materials" Energies 12, no. 15: 2903. https://doi.org/10.3390/en12152903
APA StyleKang, S.-H., Kwon, Y.-H., & Moon, J. (2019). Quantitative Analysis of CO2 Uptake and Mechanical Properties of Air Lime-Based Materials. Energies, 12(15), 2903. https://doi.org/10.3390/en12152903





