Continuous Lithium Extraction from Aqueous Solution Using Flow-Electrode Capacitive Deionization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. FCDI Cell Configuration
2.3. Assessment of FCDI Desalination Performance
3. Results and Discussions
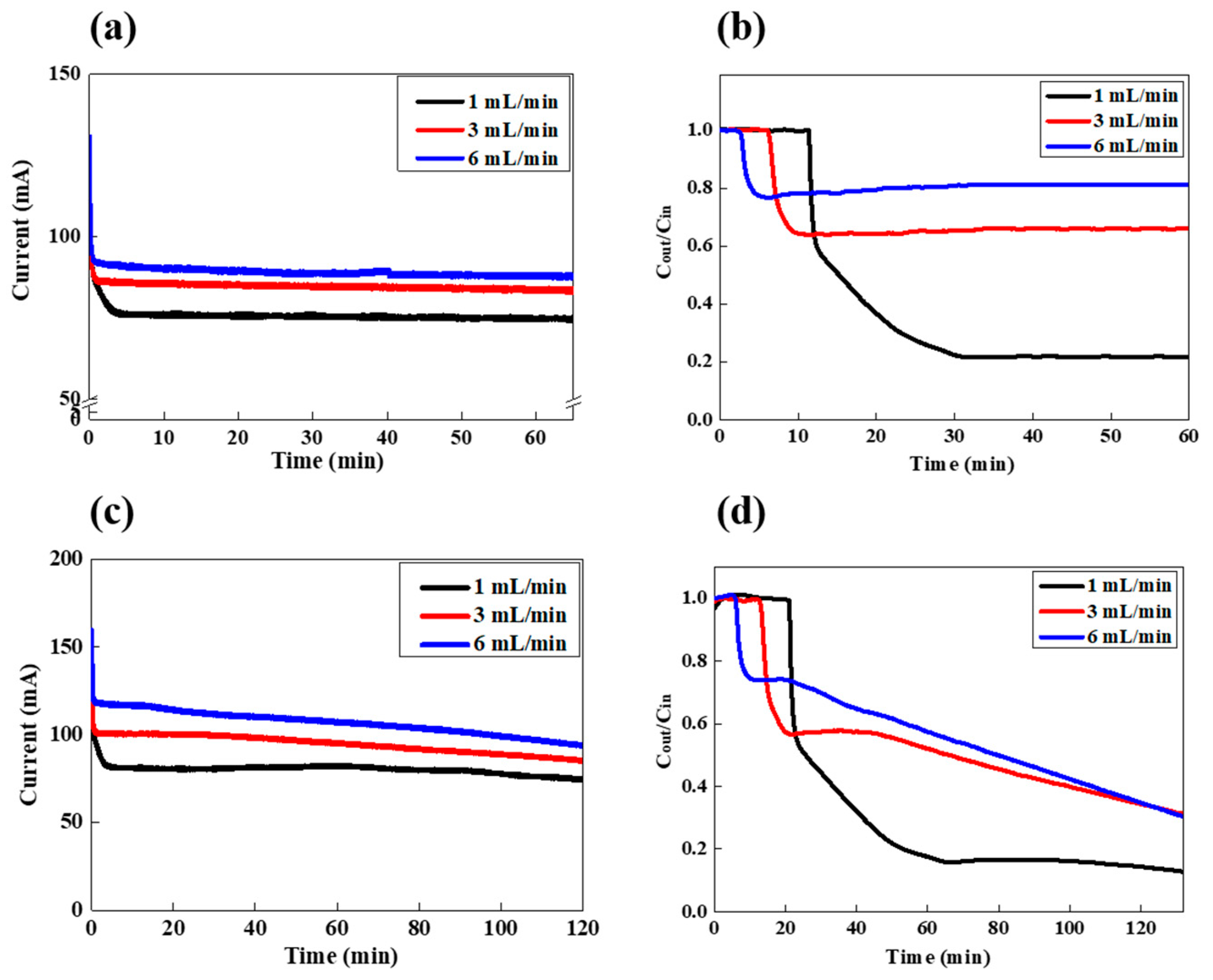
3.1. FCDI LiCl Desalination in a Continuous Mode
3.2. FCDI LiCl Desalination in a Batch Mode
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Swain, B. Recovery and recycling of lithium: A review. Sep. Purif. Technol. 2017, 172, 388–403. [Google Scholar] [CrossRef]
- Ebensperger, A.; Maxwell, P.; Moscoso, C. The lithium industry: Its recent evolution and future prospects. Resour. Policy 2005, 30, 218–231. [Google Scholar] [CrossRef]
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium market research–global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Vikström, H.; Davidsson, S.; Höök, M. Lithium availability and future production outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Liu, X.; Ye, C.; Cao, X.; Gao, C.; Shen, J. Efficient lithium extraction by membrane capacitive deionization incorporated with monovalent selective cation exchange membrane. Sep. Purif. Technol. 2019, 210, 885–890. [Google Scholar] [CrossRef]
- Ryu, T.; Lee, D.H.; Ryu, J.C.; Shin, J.; Chung, K.S.; Kim, Y.H. Lithium recovery system using electrostatic field assistance. Hydrometallurgy 2015, 151, 78–83. [Google Scholar] [CrossRef]
- Bryjak, M.; Siekierka, A.; Kujawski, J.; Smolińska-Kempisty, K.; Kujawski, W. Capacitive Deionization for Selective Extraction of Lithium from Aqueous Solutions. J. Membr. Sep. Technol. 2015, 4, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.Y.; Ji, Z.Y.; Chen, Q.B.; Liu, J.; Zhao, Y.Y.; Li, F.; Liu, Z.Y.; Yuan, J.S. Prefractionation of LiCl from concentrated seawater/salt lake brines by electrodialysis with monovalent selective ion exchange membranes. J. Clean. Prod. 2018, 193, 338–350. [Google Scholar] [CrossRef]
- Tsuchiya, S.; Nakatani, Y.; Ibrahim, R.; Ogawa, S. Highly Efficient Separation of Lithium Chloride from Seawater. J. Am. Chem. Soc. 2002, 124, 4936–4937. [Google Scholar] [CrossRef]
- Romero, V.C.E.; Tagliazucchi, M.; Flexer, V.; Calvo, E.J. Sustainable Electrochemical Extraction of Lithium from Natural Brine for Renewable Energy Storage. J. Electrochem. Soc. 2018, 165, A2294–A2302. [Google Scholar] [CrossRef]
- Hoshino, T. Innovative lithium recovery technique from seawater by using world-first dialysis with a lithium ionic superconductor. Desalination 2015, 359, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Trócoli, R.; Erinmwingbovo, C.; La Mantia, F. Optimized lithium recovery from brines by using an electrochemical ion-pumping process based on λ-mno2 and nickel hexacyanoferrate. Chem Electro Chem 2017, 4, 143–149. [Google Scholar] [CrossRef]
- Palagonia, M.S.; Brogioli, D.; La Mantia, F. Influence of Hydrodynamics on the Lithium Recovery Efficiency in an Electrochemical Ion Pumping Separation Process. J. Electrochem. Soc. 2017, 164, E586–E595. [Google Scholar] [CrossRef]
- Sun, Y.; Guo, X.; Hu, S.; Xiang, X. Highly efficient extraction of lithium from salt lake brine by LiAl-layered double hydroxides as lithium-ion-selective capturing material. J. Energy Chem. 2019, 34, 80–87. [Google Scholar] [CrossRef]
- Park, J.; Sato, H.; Nishihama, S.; Yoshizuka, K. Lithium Recovery from Geothermal Water by Combined Adsorption Methods. Solvent Extr. Ion. Exch. 2012, 30, 398–404. [Google Scholar] [CrossRef]
- Zhao, R.; Biesheuvel, P.M.; Van Der Wal, A. Energy consumption and constant current operation in membrane capacitive deionization. Energy Environ. Sci. 2012, 5, 9520. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Kim, C.; Yoon, J. Hybrid capacitive deionization to enhance the desalination performance of capacitive techniques. Energy Environ. Sci. 2014, 7, 3683–3689. [Google Scholar] [CrossRef]
- Suss, M.E.; Porada, S.; Sun, X.; Biesheuvel, P.M.; Yoon, J.; Presser, V. Water desalination via capacitive deionization: What is it and what can we expect from it? Energy Environ. Sci. 2015, 8, 2296–2319. [Google Scholar] [CrossRef]
- Ramachandran, A.; Oyarzun, D.I.; Hawks, S.A.; Stadermann, M.; Santiago, J.G. High water recovery and improved thermodynamic efficiency for capacitive deionization using variable flowrate operation. Water Res. 2019, 155, 76–85. [Google Scholar] [CrossRef]
- Zhang, C.; He, D.; Ma, J.; Tang, W.; Waite, T.D. Faradaic reactions in capacitive deionization (CDI)–problems and possibilities: A review. Water Res. 2018, 128, 314–330. [Google Scholar] [CrossRef]
- Cho, Y.; Yoo, C.Y.; Lee, S.W.; Yoon, H.; Lee, K.S.; Yang, S.; Kim, D.K. Flow-electrode capacitive deionization with highly enhanced salt removal performance utilizing high-aspect ratio functionalized carbon nanotubes. Water Res. 2019, 151, 252–259. [Google Scholar] [CrossRef]
- Hand, S.; Shang, X.; Guest, J.S.; Smith, K.C.; Cusick, R.D. Global Sensitivity Analysis to Characterize Operational Limits and Prioritize Performance Goals of Capacitive Deionization Technologies. Environ. Sci. Technol. 2019, 53, 3748–3756. [Google Scholar] [CrossRef]
- Santangelo, S.; Pantò, F.; Triolo, C.; Stelitano, S.; Frontera, P.; Fernández-Carretero, F.; Rincon, I.; Azpiroz, P.; García-Luis, A.; Belaustegui, Y. Evaluation of the electrochemical performance of electrospun transition metal oxide-based electrode nanomaterials for water CDI applications. Electrochim. Acta 2019, 309, 125–139. [Google Scholar] [CrossRef]
- Wang, K.; Liu, Y.; Ding, Z.; Li, Y.; Lu, T.; Pan, L. Metal–organic-frameworks-derived NaTi2(PO4)3/carbon composites for efficient hybrid capacitive deionization. J. Mater. Chem. A 2019, 7, 12126–12133. [Google Scholar] [CrossRef]
- Yasin, A.S.; Mohamed, A.Y.; Mohamed, I.M.A.; Cho, D.Y.; Park, C.H.; Kim, C.S. Theoretical insight into the structure-property relationship of mixed transition metal oxides nanofibers doped in activated carbon and 3d graphene for capacitive deionization. Chem. Eng. J. 2019, 371, 166–181. [Google Scholar] [CrossRef]
- Zhang, C.; He, D.; Ma, J.; Tang, W.; Waite, T.D. Comparison of faradaic reactions in flow-through and flow-by capacitive deionization (CDI) systems. Electrochim. Acta 2019, 299, 727–735. [Google Scholar] [CrossRef]
- Suss, M.E.; Biesheuvel, P.; Baumann, T.F.; Stadermann, M.; Santiago, J.G. In Situ Spatially and Temporally Resolved Measurements of Salt Concentration between Charging Porous Electrodes for Desalination by Capacitive Deionization. Environ. Sci. Technol. 2014, 48, 2008–2015. [Google Scholar] [CrossRef]
- Porada, S.; Zhao, R.; Van Der Wal, A.; Presser, V.; Biesheuvel, P. Review on the science and technology of water desalination by capacitive deionization. Prog. Mater. Sci. 2013, 58, 1388–1442. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Yoon, H.; Shin, D.; Lee, J.; Yoon, J. Electrochemical selective ion separation in capacitive deionization with sodium manganese oxide. J. Colloid Interface Sci. 2017, 506, 644–648. [Google Scholar] [CrossRef]
- Lee, D.-H.; Ryu, T.; Shin, J.; Ryu, J.C.; Chung, K.S.; Kim, Y.H. Selective lithium recovery from aqueous solution using a modified membrane capacitive deionization system. Hydrometallurgy 2017, 173, 283–288. [Google Scholar] [CrossRef]
- Kim, J.; Jain, A.; Zuo, K.; Verduzco, R.; Walker, S.; Elimelech, M.; Zhang, Z.; Zhang, X.; Li, Q. Removal of calcium ions from water by selective electrosorption using target-ion specific nanocomposite electrode. Water Res. 2019, 160, 445–453. [Google Scholar] [CrossRef]
- Jeon, S.I.; Park, H.R.; Yeo, J.G.; Yang, S.; Cho, C.H.; Han, M.H.; Kim, D.K. Desalination via a new membrane capacitive deionization process utilizing flow-electrodes. Energy Environ. Sci. 2013, 6, 1471. [Google Scholar] [CrossRef]
- Cho, Y.; Lee, K.S.; Yang, S.; Choi, J.; Park, H.R.; Kim, D.K. A novel three-dimensional desalination system utilizing honeycomb-shaped lattice structures for flow-electrode capacitive deionization. Energy Environ. Sci. 2017, 10, 1746–1750. [Google Scholar] [CrossRef]
- Lee, K.S.; Cho, Y.; Choo, K.Y.; Yang, S.; Han, M.H.; Kim, D.K. Membrane-spacer assembly for flow-electrode capacitive deionization. Appl. Surf. Sci. 2018, 433, 437–442. [Google Scholar] [CrossRef]
- Yang, S.; Choi, J.; Yeo, J.G.; Jeon, S.I.; Park, H.R.; Kim, D.K. Flow-Electrode Capacitive Deionization Using an Aqueous Electrolyte with a High Salt Concentration. Environ. Sci. Technol. 2016, 50, 5892–5899. [Google Scholar] [CrossRef]




| Feed-flow Mode | Feed Rate (mL/min) | Amount of Salt Removed for 2 h (g) | Salt Removal Efficiency (%) |
|---|---|---|---|
| Batch | 1 | 0.428 | 85.7 |
| 3 | 0.328 | 65.6 | |
| 6 | 0.326 | 65.2 | |
| Continuous | 1 | 0.705 | 78.2 |
| 3 | 0.751 | 33.8 | |
| 6 | 0.749 | 18.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ha, Y.; Jung, H.B.; Lim, H.; Jo, P.S.; Yoon, H.; Yoo, C.-Y.; Pham, T.K.; Ahn, W.; Cho, Y. Continuous Lithium Extraction from Aqueous Solution Using Flow-Electrode Capacitive Deionization. Energies 2019, 12, 2913. https://doi.org/10.3390/en12152913
Ha Y, Jung HB, Lim H, Jo PS, Yoon H, Yoo C-Y, Pham TK, Ahn W, Cho Y. Continuous Lithium Extraction from Aqueous Solution Using Flow-Electrode Capacitive Deionization. Energies. 2019; 12(15):2913. https://doi.org/10.3390/en12152913
Chicago/Turabian StyleHa, Yuncheol, Hye Bin Jung, Hyunseung Lim, Pil Sung Jo, Hana Yoon, Chung-Yul Yoo, Tuan Kiet Pham, Wook Ahn, and Younghyun Cho. 2019. "Continuous Lithium Extraction from Aqueous Solution Using Flow-Electrode Capacitive Deionization" Energies 12, no. 15: 2913. https://doi.org/10.3390/en12152913
APA StyleHa, Y., Jung, H. B., Lim, H., Jo, P. S., Yoon, H., Yoo, C.-Y., Pham, T. K., Ahn, W., & Cho, Y. (2019). Continuous Lithium Extraction from Aqueous Solution Using Flow-Electrode Capacitive Deionization. Energies, 12(15), 2913. https://doi.org/10.3390/en12152913






