The Effect of Lignin Content in Birch and Beech Kraft Cellulosic Pulps on Simple Sugar Yields from the Enzymatic Hydrolysis of Cellulose
Abstract
:1. Introduction
2. Materials and Methods
2.1. Woodchips
2.2. Kraft Wood Pulps
2.3. Enzyme Activities
2.4. Enzymatic Hydrolysis
2.5. Analytics
2.6. Yield Calculations
3. Results and Discussion
3.1. Enzyme Preparation
3.2. Cellulosic Pulps Chemical Composition
3.3. Enzymatic Hydrolysis of Birch and Beech Woodchips
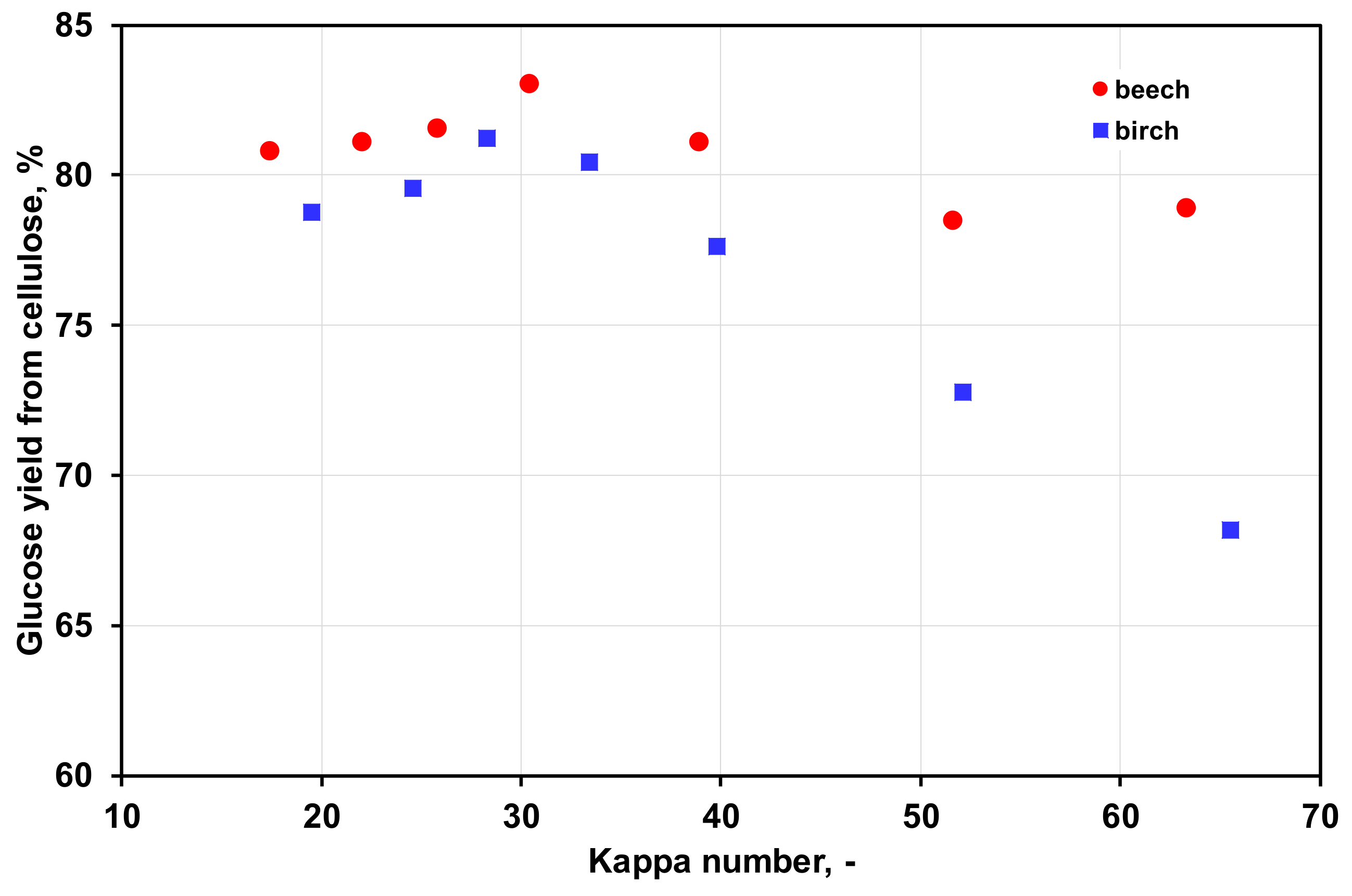
3.4. Enzymatic Hydrolysis of Birch and Beech Pulps
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kollman, F.F.P.; Côté, W.A., Jr. Principles of Wood Science and Technology: I Solid Wood, 1st ed.; Springer: Heidelberg, Germany, 1968. [Google Scholar] [CrossRef]
- Prosiński, S. Chemistry of Wood, 2nd ed.; PWRiL: Warsaw, Poland, 1984. [Google Scholar]
- Fengel, D.; Wegener, G. Wood Chemistry, Ultrastructure, Reactions, 1st ed.; Walter De Gruyter Inc.: Berlin, Germany, 1989. [Google Scholar]
- Kai, Y. Chemistry of Extractives. In Wood and Cellulosic Chemistry, 1st ed.; Hon, D.N.S., Shiraishi, N., Eds.; Marcel Dekker Inc.: New York, NY, USA, 1991; pp. 215–255. [Google Scholar]
- Sjöström, E. Wood Chemistry. Fundamentals and Applications, 2nd ed.; Academic Press Inc.: San Diego, CA, USA, 1993. [Google Scholar]
- Rowell, R.M.; Pettersen, R.; Tshabalala, M.A. Cell Wall Chemistry. In Handbook of Wood Chemistry and Wood Composites, 2nd ed.; Rowell, R.M., Ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 33–72. [Google Scholar] [CrossRef]
- Miazek, K.; Remacle, C.; Richel, A.; Goffin, D. Beech wood Fagus sylvatica dilute-acid hydrolysate as a feedstock to support Chlorella sorokiniana biomass, fatty acid and pigment production. Bioresour. Technol. 2017, 230, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Tippkotter, N.; Duwe, A.M.; Wiesen, S.; Sieker, T.; Ulber, R. Enzymatic hydrolysis of beech wood lignocellulose at high solids contents and its utilization as substrate for the production of biobutanol and dicarboxylic acids. Bioresour. Technol. 2014, 167, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Turley, D.B.; Chaudhry, Q.; Watkins, R.W.; Clark, J.H.; Deswarte, F.E.I. Chemical products from temperate forest tree species—Developing strategies for exploitation. Ind. Crops Prod. 2006, 24, 238–242. [Google Scholar] [CrossRef]
- Alvira, P.; Tomas-Pejo, E.; Ballesteros, M.; Negro, M.J. Pretreatment technologies for an efficient bioethanol production process based on enzymatic hydrolysis: A review. Bioresour. Technol. 2010, 101, 4851–4861. [Google Scholar] [CrossRef] [PubMed]
- Przybysz Buzała, K.; Przybysz, P.; Kalinowska, H.; Przybysz, K.; Kucner, M.; Dubowik, M. Evaluation of pine kraft cellulosic pulps and fines from papermaking as potential feedstocks for biofuel production. Cellulose 2016, 23, 649–659. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Hawkins, G.M.; Ghose, D.; Russel, J.; Doran-Peterson, J. Production of ethanol from high dry matter of pretreated loblolly pine by an evolved strain of Saccharomyces cerevisiae. J. Bioremediat. Biodegrad. 2013, 4. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Zhu, J.Y.; Ralph, S.A. Enzymatic hydrolysis of loblolly pine: Effects of cellulose crystallinity and delignification. Holzforschung 2013, 67, 371–377. [Google Scholar] [CrossRef]
- Modrzejewski, K.; Olszewski, J.; Rutkowski, J. Analyses in Papermaking Industry; Editorial Office of the Lodz University of Technology: Lodz, Poland, 1969; pp. 60–89, 206–250. [Google Scholar]
- TAPPI T203 cm-09. Alpha-, Beta- and Gamma-Cellulose in Pulp; Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2009. [Google Scholar]
- TAPPI T204 cm-07. Solvent Extractives of Wood and Pulp; Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2007. [Google Scholar]
- TAPPI T211 om-12. Ash in Wood, Pulp, Paper and Paperboard: Combustion at 525 Degrees C; Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2012. [Google Scholar]
- TAPPI T222 om-11. Acid Insoluble Lignin in Wood and Pulp; Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2011. [Google Scholar]
- TAPPI T249 cm-09. Carbohydrate Composition of Extractive-Free Wood and Wood Pulp by Gas-Liquid Chromatography; Technical Association of the Pulp and Paper Industry: Atlanta, GA, USA, 2009. [Google Scholar]
- Miller, G.L. Use of dinitrosalicylic reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Adney, B.; Baker, J. Measurement of Cellulase Activities; Technical Report NREL/TP-510-42628. Available online: https://www.nrel.gov/docs/gen/fy08/42628.pdf (accessed on 16 April 2019).
- Barham, D.; Trinder, P. An improved color reagent for the determination of blood glucose by the oxidase system. Analyst 1972, 97, 142–145. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Wyman, C.E. Effects of cellulase and xylanase enzymes on the deconstruction of solids from pretreatment of poplar by leading technologies. Biotechnol. Progr. 2009, 25, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Buzała, K.; Przybysz, P.; Rosicka-Kaczmarek, J.; Kalinowska, H. Production of glucose-rich enzymatic hydrolysates from cellulosic pulps. Cellulose 2015, 22, 663–674. [Google Scholar] [CrossRef]
- Van Dyk, J.S.; Pletschke, B.J. A review of lignocellulose bioconversion using enzymatic hydrolysis. Biotechnol. Adv. 2012, 30, 1458–1480. [Google Scholar] [CrossRef] [PubMed]
- Buzała, K.; Przybysz, P.; Rosicka-Kaczmarek, J.; Kalinowska, H. Comparison of digestibility of wood pulps produced by the sulfate and TMP methods and woodchips of various botanical origins and sizes. Cellulose 2015, 22, 2737–2747. [Google Scholar] [CrossRef] [Green Version]
- Goyal, G.C.; Lora, J.H.; Pye, E.K. Autocatalyzed organosolv pulping of hardwoods: Effect of pulping conditions on pulp properties and characteristics of soluble and residual lignin. Tappi J. 1992, 75, 110–116. [Google Scholar]
- Rodríguez, A.; Jiménez, L. Pulping with Organic Solvents other than Alcohols. Afinidad 2008, 65, 188–196. [Google Scholar]
- Brogdon, B.N.; Dimmel, D.R. Fundamental-study of relative delignification efficiencies (I): Conventional pulping systems. J. Wood Chem. Technol. 1996, 16, 261–283. [Google Scholar] [CrossRef]
- Brogdon, B.N.; Dimmel, D.R. Competing reactions affecting delignification in pulping systems. J. Wood Chem. Technol. 1996, 16, 405–419. [Google Scholar] [CrossRef]
- Wiedenhoeft, A.C.; Miller, R.B. Structure and Function of Wood. In Handbook of Wood Chemistry and Wood Composites, 1st ed.; Rowell, R.M., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 9–32. [Google Scholar] [CrossRef]
- Paszner, L.; Behera, N.C. Topochemistry of softwood delignification by alkali earth metal salt catalyzed organosolv pulping. Holzforschung 1989, 43, 159–168. [Google Scholar] [CrossRef]
- Mou, H.-Y.; Orblin, E.; Kruus, K.; Fardim, P. Topochemical pretreatment of wood biomass to enhance enzymatic hydrolysis of polysaccharides to sugars. Bioresour. Technol. 2013, 142, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.Y.; Pan, X.J.; Wang, G.S.; Gleisner, R. Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Bioresour. Technol. 2009, 100, 2411–2418. [Google Scholar] [CrossRef] [PubMed]


| Substrate | Kappa Number | Yield |
|---|---|---|
| [% DW] | ||
| Birch | 65.3 ± 0.4 | 44.15 ± 0.55 |
| 52.1 ± 0.5 | 48.20 ± 0.46 | |
| 39.8 ± 0.3 | 49.26 ± 0.34 | |
| 33.4 ± 0.4 | 51.75 ± 0.31 | |
| 28.3 ± 0.2 | 53.09 ± 0.18 | |
| 24.6 ± 0.1 | 52.13 ± 0.27 | |
| 19.5 ± 0.1 | 51.11 ± 0.12 | |
| Beech | 63.2 ± 0.3 | 44.22 ± 0.46 |
| 51.6 ± 0.2 | 45.01 ± 0.34 | |
| 38.9 ± 0.6 | 46.22 ± 0.32 | |
| 30.4 ± 0.2 | 48.55 ± 0.15 | |
| 25.3 ± 0.3 | 49.50 ± 0.08 | |
| 22.0 ± 0.2 | 48.61 ± 0.26 | |
| 17.4 ± 0.2 | 46.13 ± 0.06 |
| Substrate | Kappa Number | Cellulose | Hemicelluloses | Lignin | Extractives | Ash |
|---|---|---|---|---|---|---|
| [% DW] | ||||||
| Birch | 65.3 | 80.8 ± 0.6 | 8.7 | 9.8 ± 0.4 | 0.4 ± < 0.1 | 0.3 ± < 0.1 |
| 52.1 | 85.7 ± 0.6 | 5.9 | 7.8 ± 0.4 | 0.3 ± < 0.1 | 0.3 ± < 0.1 | |
| 39.8 | 88.8 ± 0.3 | 4.7 | 6.0 ± 0.3 | 0.3 ± < 0.1 | 0.2 ± < 0.1 | |
| 33.4 | 90.3 ± 0.8 | 4.3 | 5.0 ± 0.2 | 0.2 ± < 0.1 | 0.2 ± < 0.1 | |
| 28.3 | 92.3 ± 0.3 | 3.2 | 4.2 ± 0.3 | 0.1 ± < 0.1 | 0.2 ± < 0.1 | |
| 24.6 | 93.1 ± 0.5 | 2.8 | 3.7 ± 0.2 | 0.2 ± < 0.1 | 0.2 ± < 0.1 | |
| 19.5 | 94.6 ± 0.2 | 2.2 | 2.9 ± 0.3 | 0.1 ± < 0.1 | 0.2 ± < 0.1 | |
| woodchips (0.43–0.8 mm) | n/d * | 45.3 ± 0.9 | 25.1 | 26.4 ± 0.3 | 2.6 ± 0.2 | 0.6 ± < 0.1 |
| Beech | 63.2 | 76.7 ± 0.8 | 13.1 | 9.5 ± 0.5 | 0.5 ± < 0.1 | 0.2 ± < 0.1 |
| 51.6 | 82.7 ± 0.7 | 9.1 | 7.7 ± 0.3 | 0.3 ± < 0.1 | 0.2 ± < 0.1 | |
| 38.9 | 86.6 ± 0.4 | 7.3 | 5.8 ± 0.4 | 0.2 ± < 0.1 | 0.1 ± < 0.1 | |
| 30.4 | 89.3 ± 0.6 | 6.0 | 4.6 ± 0.3 | 0.0 ± < 0.1 | 0.1 ± < 0.1 | |
| 25.3 | 91.3 ± 0.3 | 4.8 | 3.9 ± 0.3 | 0.0 ± < 0.1 | 0.1 ± < 0.1 | |
| 22.0 | 92.4 ± 0.4 | 4.1 | 3.3 ± 0.2 | 0.1 ± < 0.1 | 0.1 ± < 0.1 | |
| 17.4 | 94.0 ± 0.2 | 3.3 | 2.6 ± 0.2 | 0.0 ± < 0.1 | 0.1 ± < 0.1 | |
| woodchips (0.43–0.8 mm) | n/d * | 41.9 ± 0.3 | 31.2 | 22.7 ± 0.6 | 3.1 ± 0.2 | 1.1 ± < 0.1 |
| Substrate | Kappa Number | Glucose Concentration | Mean Glucose Yield | Total Reducing Sugars Concentration | Mean Reducing Sugars Yield | ||
|---|---|---|---|---|---|---|---|
| [mg/ml] | [% DW pulp] | [% DW wood] | [mg/ml] | [% DW pulp] | [% DW wood] | ||
| Birch cellulosic pulp | 65.3 | 24.26 ± 0.21 | 55.07 | 23.76 | 29.14 ± 0.18 | 72.03 | 31.08 |
| 52.1 | 27.47 ± 0.15 | 62.36 | 29.43 | 31.25 ± 0.18 | 77.25 | 36.46 | |
| 39.8 | 30.36 ± 0.06 | 68.92 | 34.43 | 36.12 ± 0.08 | 89.29 | 44.61 | |
| 33.4 | 31.99 ± 0.08 | 72.62 | 38.31 | 38.22 ± 0.09 | 94.48 | 49.84 | |
| 28.3 | 30.33 ± 0.02 | 74.98 | 39.81 | 39.68 ± 0.02 | 98.09 | 52.08 | |
| 24.6 | 32.63 ± 0.03 | 74.07 | 38.61 | 39.72 ± 0.02 | 98.19 | 51.19 | |
| 19.5 | 32.82 ± 0.01 | 74.50 | 38.08 | 39.75 ± 0.02 | 98.26 | 50.22 | |
| woodchips (0.43–0.8 mm) | n/d ** | 1.84 ± 0.01 | n/d ** | 10.00 | 4.48 ± 0.09 | n/d ** | 26.19 |
| Beech cellulosic pulp | 63.2 | 24.49 ± 0.33 | 60.53 | 25.56 | 30.81 ± 0.26 | 76.15 | 32.15 |
| 51.6 | 26.26 ± 0.25 | 64.90 | 29.21 | 32.11 ± 0.13 | 79.37 | 35.72 | |
| 38.9 | 28.42 ± 0.07 | 70.24 | 32.47 | 37.52 ± 0.08 | 92.74 | 42.86 | |
| 30.4 | 30.01 ± 0.12 | 74.17 | 36.01 | 38.89 ± 0.19 | 96.12 | 46.67 | |
| 25.3 | 30.13 ± 0.16 | 74.47 | 36.86 | 39.39 + 0.05 | 97.36 | 48.19 | |
| 22.0 | 30.33 ± 0.12 | 74.96 | 36.44 | 39.45 ± 0.09 | 97.51 | 47.40 | |
| 17.4 | 30.74 ± 0.09 | 75.98 | 35.05 | 39.89 ± 0.05 | 98.60 | 45.48 | |
| woodchips (0.43–0.8 mm) | n/d ** | 2.09 ± 0.09 | n/d ** | 11.42 | 4.63 ± 0.02 | n/d ** | 26.63 |
| Substrate | Kappa Number | Glucose | Cellobiose | Xylose | Arabinose | Rhamnose | Mannose | Galactose |
|---|---|---|---|---|---|---|---|---|
| [% DW] | ||||||||
| Birch cellulosic pulp | 65.3 | 68.21 | 5.02 | 24.74 | 0.36 | n/d * | 0.46 | 1.21 |
| 52.1 | 70.56 | 5.12 | 22.67 | 0.27 | n/d * | 0.31 | 1.07 | |
| 39.8 | 73.48 | 4.83 | 20.12 | 0.32 | n/d * | 0.41 | 0.84 | |
| 33.4 | 75.98 | 4.26 | 18.31 | 0.21 | n/d * | 0.33 | 0.91 | |
| 28.3 | 76.43 | 4.00 | 18.01 | 0.28 | n/d * | 0.36 | 0.92 | |
| 24.6 | 77.06 | 4.02 | 17.68 | 0.11 | n/d * | 0.25 | 0.88 | |
| 19.5 | 77.11 | 3.69 | 17.81 | 0.17 | n/d * | 0.18 | 1.04 | |
| woodchips (0.43–0.8 mm) | - | 41.06 | 32.08 | 3.65 | n/d * | n/d * | 23.21 | n/d * |
| Beech cellulosic pulp | 63.2 | 66.76 | 5.09 | 26.33 | 0.42 | 0.38 | n/d * | 1.02 |
| 51.6 | 71.39 | 4.84 | 21.95 | 0.39 | 0.35 | n/d * | 1.08 | |
| 38.9 | 74.58 | 4.69 | 18.93 | 0.34 | 0.39 | n/d * | 1.07 | |
| 30.4 | 75.44 | 4.02 | 18.98 | 0.31 | 0.36 | n/d * | 0.89 | |
| 25.3 | 76.49 | 3.94 | 17.96 | 0.35 | 0.31 | n/d * | 0.95 | |
| 22.0 | 76.89 | 3.66 | 17.87 | 0.28 | 0.32 | n/d * | 0.98 | |
| 17.4 | 77.05 | 3.51 | 17.92 | 0.26 | 0.30 | n/d * | 0.96 | |
| woodchips (0.43–0.8 mm) | 45.09 | 25.6 | 0.51 | n/d * | n/d * | 28.80 | n/d * | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybysz Buzała, K.; Kalinowska, H.; Małachowska, E.; Boruszewski, P.; Krajewski, K.; Przybysz, P. The Effect of Lignin Content in Birch and Beech Kraft Cellulosic Pulps on Simple Sugar Yields from the Enzymatic Hydrolysis of Cellulose. Energies 2019, 12, 2952. https://doi.org/10.3390/en12152952
Przybysz Buzała K, Kalinowska H, Małachowska E, Boruszewski P, Krajewski K, Przybysz P. The Effect of Lignin Content in Birch and Beech Kraft Cellulosic Pulps on Simple Sugar Yields from the Enzymatic Hydrolysis of Cellulose. Energies. 2019; 12(15):2952. https://doi.org/10.3390/en12152952
Chicago/Turabian StylePrzybysz Buzała, Kamila, Halina Kalinowska, Edyta Małachowska, Piotr Boruszewski, Krzysztof Krajewski, and Piotr Przybysz. 2019. "The Effect of Lignin Content in Birch and Beech Kraft Cellulosic Pulps on Simple Sugar Yields from the Enzymatic Hydrolysis of Cellulose" Energies 12, no. 15: 2952. https://doi.org/10.3390/en12152952
APA StylePrzybysz Buzała, K., Kalinowska, H., Małachowska, E., Boruszewski, P., Krajewski, K., & Przybysz, P. (2019). The Effect of Lignin Content in Birch and Beech Kraft Cellulosic Pulps on Simple Sugar Yields from the Enzymatic Hydrolysis of Cellulose. Energies, 12(15), 2952. https://doi.org/10.3390/en12152952





