Optimization of Bio-Hydrogenated Kerosene from Refined Palm Oil by Catalytic Hydrocracking
Abstract
:1. Introduction
2. Materials and Methods
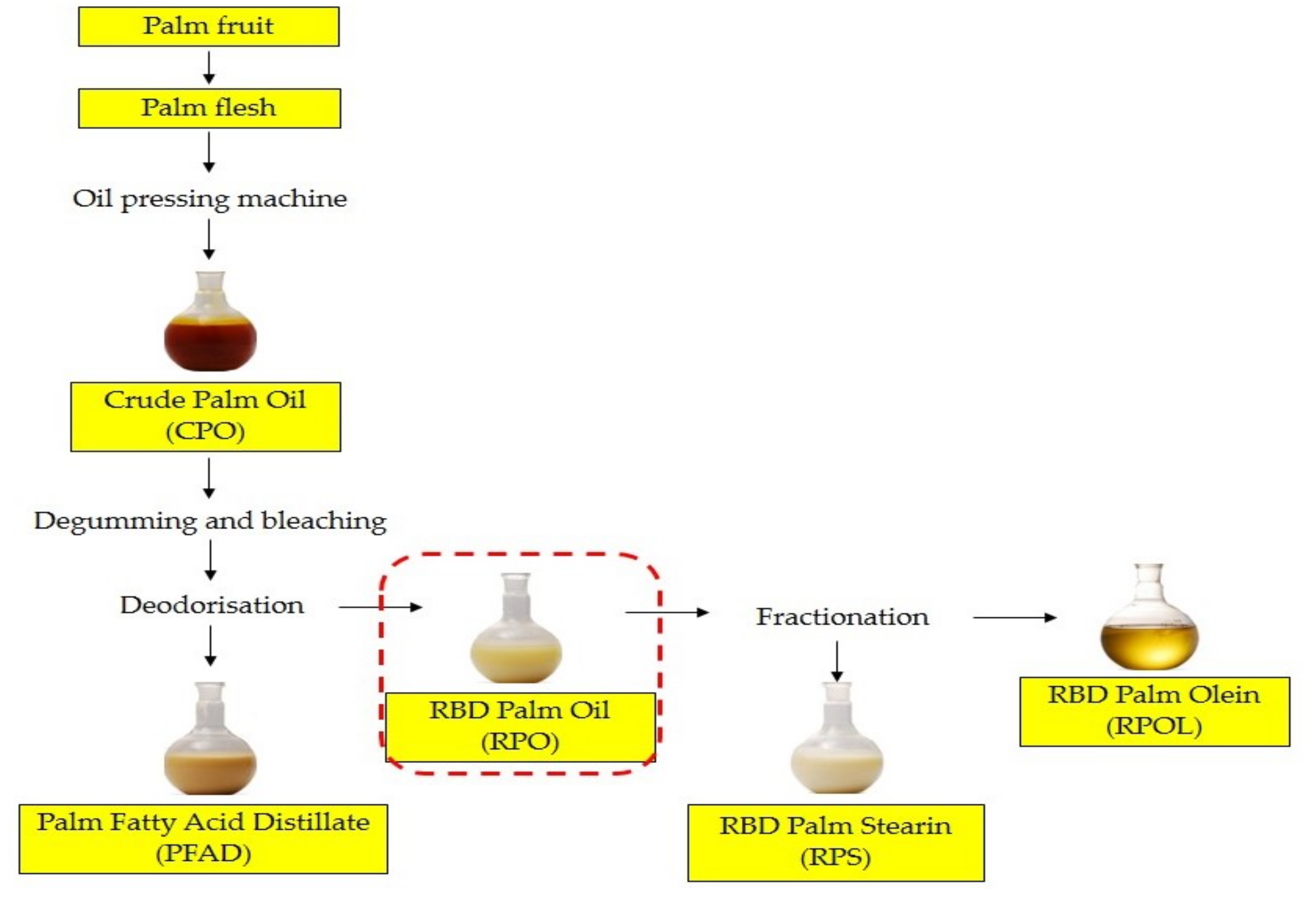
2.1. Materials
2.2. Catalyst Characterization
2.3. Reaction and DOE
2.4. Bio-Hydrogenated Kerosene Properties
3. Results and Discussion
3.1. Catalyst Characterization
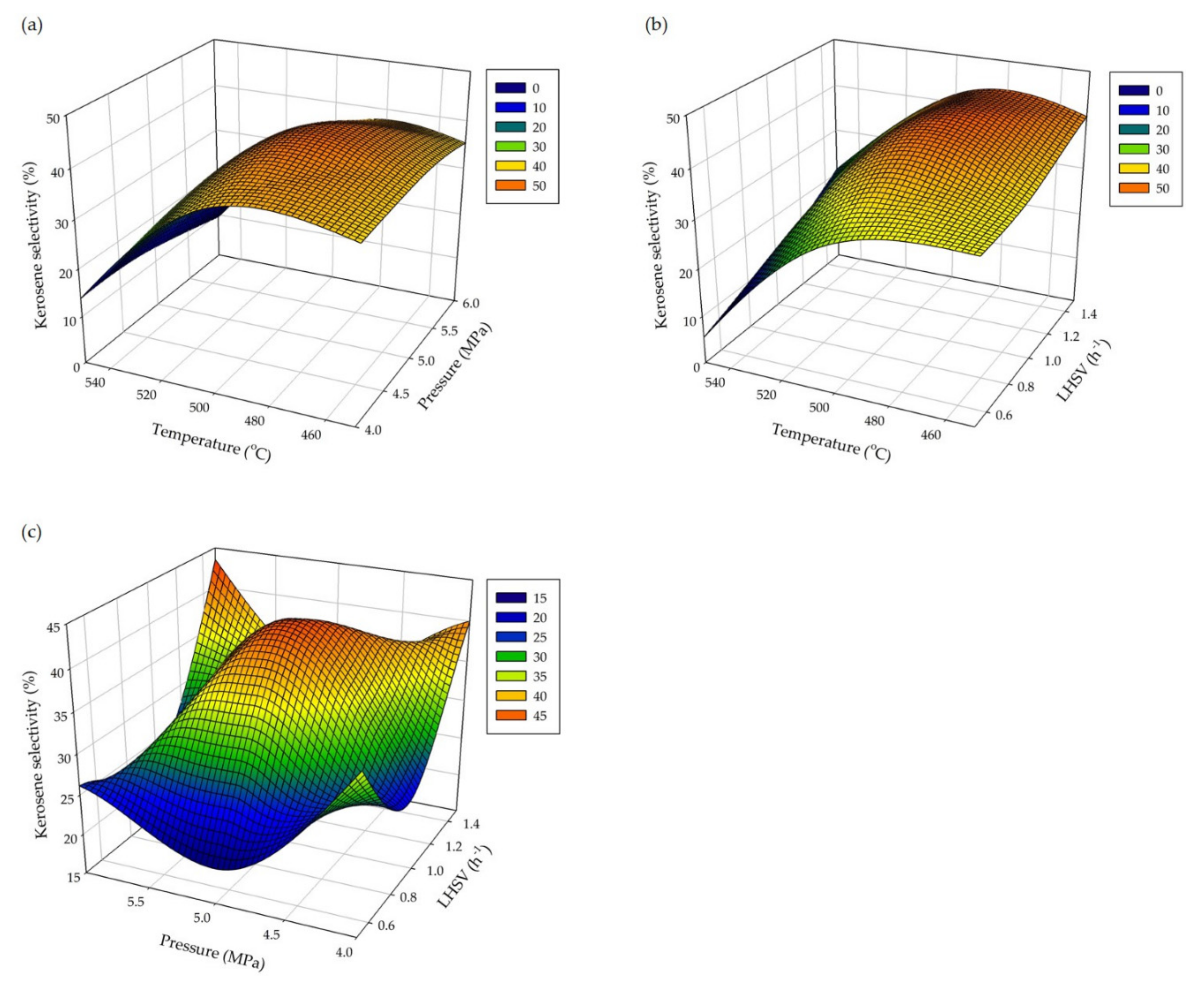
3.2. Reaction and Optimization
3.3. Bio-Hydrogenated Kerosene Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, L.; Li, H.; Fu, J.; Miao, C.; Lv, P.; Yuan, Z. Catalytic hydroprocessing of fatty acid methyl esters to renewable alkane fuels over Ni/HZSM-5 catalyst. Catal. Today 2016, 259, 266–276. [Google Scholar] [CrossRef]
- Srifa, A.; Faungnawakij, K.; Itthibenchapong, V.; Viriya-empikul, N.; Charinpanitkul, T.; Assabumrungrat, S. Production of bio-hydrogenated diesel by catalytic hydrotreating of palm oil over NiMoS2/γ-Al2O3 catalyst. Bioresour. Technol. 2014, 158, 81–90. [Google Scholar] [CrossRef]
- Twaiq, F.A.; Mohamed, A.R.; Bhatia, S. Liquid hydrocarbon fuels from palm oil by catalytic cracking over aluminosilicate mesoporous catalysts with various Si/Al ratios. Microporous Mesoporous Mater. 2003, 64, 95–107. [Google Scholar] [CrossRef]
- Vásquez, M.C.; Silva, E.E.; Castillo, E.F. Hydrotreatment of vegetable oils: A review of the technologies and its developments for jet biofuel production. Biomass Bioenergy 2017, 105, 197–206. [Google Scholar] [CrossRef]
- Gutiérrez-Antonio, C.; Gómez-Castro, F.I.; De Lira-Flores, J.A.; Hernández, S. A review on the production processes of renewable jet fuel. Renew. Sustain. Energy Rev. 2017, 79, 709–729. [Google Scholar] [CrossRef]
- Ancheyta, J.; Sánchez, S.; Rodríguez, M.A. Kinetic modeling of hydrocracking of heavy oil fractions: A review. Catal. Today 2005, 109, 76–92. [Google Scholar] [CrossRef]
- Looi, P.Y.; Mohamed, A.R.; Tye, C.T. Hydrocracking of residual oil using molybdenum supported over mesoporous alumina as a catalyst. Chem. Eng. J. 2012, 181–182, 717–724. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.M.; Briones, L.; Arroyo, M. Hydroprocessing of the LDPE thermal cracking oil into transportation fuels over Pd supported on hierarchical ZSM-5 catalyst. Fuel 2017, 206, 190–198. [Google Scholar] [CrossRef]
- Verma, D.; Rana, B.S.; Kumar, R.; Sibi, M.G.; Sinha, A.K. Diesel and aviation kerosene with desired aromatics from hydroprocessing of jatropha oil over hydrogenation catalysts supported on hierarchical mesoporous SAPO-11. Appl. Catal. A Gen. 2015, 490, 108–116. [Google Scholar] [CrossRef]
- Mat Yasin, M.H.; Mamat, R.; Najafi, G.; Ali, O.M.; Yusop, A.F.; Ali, M.H. Potentials of palm oil as new feedstock oil for a global alternative fuel: A review. Renew. Sustain. Energy Rev. 2017, 79, 1034–1049. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury, K.; Banu, L.; Khan, S.; Latif, A. Studies on the Fatty Acid Composition of Edible Oil. Bangladesh J. Sci. Ind. Res. 1970, 42, 311–316. [Google Scholar] [CrossRef]
- Aslan, N.; Cebeci, Y. Application of Box-Behnken design and response surface methodology for modeling of some Turkish coals. Fuel 2007, 86, 90–97. [Google Scholar] [CrossRef]
- Dujjanutat, P.; Neramittagapong, A.; Kaewkannetra, P. H2-assisted chemical reaction for green-kerosene production. Defect Diffus. Forum 2015, 364, 104–111. [Google Scholar] [CrossRef]
- Ferris, A.M.; Rothamer, D.A. Methodology for the experimental measurement of vapor-liquid equilibrium distillation curves using a modified ASTM D86 setup. Fuel 2016, 182, 467–479. [Google Scholar] [CrossRef]
- Groen, J.C.; Peffer, L.A.A.; Pérez-Ramírez, J. Pore size determination in modified micro- and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis. Microporous Mesoporous Mater. 2003, 60, 1–17. [Google Scholar] [CrossRef]
- Tiwari, R.; Rana, B.S.; Kumar, R.; Verma, D.; Kumar, R.; Joshi, R.K.; Garg, M.O.; Sinha, A.K. Hydrotreating and hydrocracking catalysts for processing of waste soya-oil and refinery-oil mixtures. Catal. Commun. 2011, 12, 559–562. [Google Scholar] [CrossRef]
- Callejas, M.A.; Martínez, M.T.; Blasco, T.; Sastre, E. Coke characterisation in aged residue hydrotreating catalysts by solid-state 13C-NMR spectroscopy and temperature-programmed oxidation. Appl. Catal. A Gen. 2001, 218, 181–188. [Google Scholar] [CrossRef]
- Vozka, P.; Orazgaliyeva, D.; Šimáček, P.; Blažek, J.; Kilaz, G. Activity comparison of Ni-Mo/Al2O3 and Ni-Mo/TiO2 catalysts in hydroprocessing of middle petroleum distillates and their blend with rapeseed oil. Fuel Process. Technol. 2017, 167, 684–694. [Google Scholar] [CrossRef]
- Breysse, M.; Portefaix, J.L.; Vrinat, M. Support effects on hydrotreating catalysts. Catal. Today 1991, 10, 489–505. [Google Scholar] [CrossRef]
- Morales-Ortuño, J.C.; Klimova, T.E. Development of new hydrodesulfurization NiMo catalysts supported on Al2O3-TiSBA-15 hybrid materials. Fuel 2017, 198, 99–109. [Google Scholar] [CrossRef]
- Morales-Ortuño, J.C.; Ortega-Domínguez, R.A.; Hernández-Hipólito, P.; Bokhimi, X.; Klimova, T.E. HDS performance of NiMo catalysts supported on nanostructured materials containing titania. Catal. Today 2016, 271, 127–139. [Google Scholar] [CrossRef]
- Castillo-Villalón, P.; Ramírez, J.; Cuevas, R.; Vázquez, P.; Castañeda, R. Influence of the support on the catalytic performance of Mo, CoMo, and NiMo catalysts supported on Al2O3 and TiO2 during the HDS of thiophene, dibenzothiophene, or 4,6-dimethyldibenzothiophene. Catal. Today 2016, 259, 140–149. [Google Scholar] [CrossRef]
- Liu, C.; Zhou, Z.; Huang, Y.; Cheng, Z.; Yuan, W. Support effects on thiophene hydrodesulfurization over Co-Mo-Ni/Al 2O3 and Co-Mo-Ni/TiO2-Al2O3 catalysts. Chin. J. Chem. Eng. 2014, 22, 383–391. [Google Scholar] [CrossRef]
- Chu, P.L.; Vanderghem, C.; MacLean, H.L.; Saville, B.A. Process modeling of hydrodeoxygenation to produce renewable jet fuel and other hydrocarbon fuels. Fuel 2017, 196, 298–305. [Google Scholar] [CrossRef]
- Bezergianni, S.; Kalogianni, A. Hydrocracking of used cooking oil for biofuels production. Bioresour. Technol. 2009, 100, 3927–3932. [Google Scholar] [CrossRef] [PubMed]
- Kiatkittipong, W.; Phimsen, S.; Kiatkittipong, K.; Wongsakulphasatch, S.; Laosiripojana, N.; Assabumrungrat, S. Diesel-like hydrocarbon production from hydroprocessing of relevant refining palm oil. Fuel Process. Technol. 2013, 116, 16–26. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, J.; Zhao, J. Thermochemical conversion of triglycerides for production of drop-in liquid fuels. Renew. Sustain. Energy Rev. 2016, 58, 331–340. [Google Scholar] [CrossRef]
- Galadima, A.; Muraza, O. Catalytic upgrading of vegetable oils into jet fuels range hydrocarbons using heterogeneous catalysts: A review. J. Ind. Eng. Chem. 2015, 29, 12–23. [Google Scholar] [CrossRef]
- Bezergianni, S.; Kalogianni, A.; Vasalos, I.A. Hydrocracking of vacuum gas oil-vegetable oil mixtures for biofuels production. Bioresour. Technol. 2009, 100, 3036–3042. [Google Scholar] [CrossRef] [PubMed]
- Anand, M.; Farooqui, S.A.; Kumar, R.; Joshi, R.; Kumar, R.; Sibi, M.G.; Singh, H.; Sinha, A.K. Optimizing renewable oil hydrocracking conditions for aviation bio-kerosene production. Fuel Process. Technol. 2016, 151, 50–58. [Google Scholar] [CrossRef]
- Li, T.; Cheng, J.; Huang, R.; Yang, W.; Zhou, J.; Cen, K. Hydrocracking of palm oil to jet biofuel over different zeolites. Int. J. Hydrog. Energy 2016, 41, 21883–21887. [Google Scholar] [CrossRef]
- Emori, E.Y.; Hirashima, F.H.; Zandonai, C.H.; Ortiz-Bravo, C.A.; Fernandes-Machado, N.R.C.; Olsen-Scaliante, M.H.N. Catalytic cracking of soybean oil using ZSM5 zeolite. Catal. Today 2017, 279, 168–176. [Google Scholar] [CrossRef]
- Wang, F.; Xu, J.; Jiang, J.; Liu, P.; Li, F.; Ye, J.; Zhou, M. Hydrotreatment of vegetable oil for green diesel over activated carbon supported molybdenum carbide catalyst. Fuel 2018, 216, 738–746. [Google Scholar] [CrossRef]
- Hengst, K.; Arend, M.; Pfützenreuter, R.; Hoelderich, W.F. Deoxygenation and cracking of free fatty acids over acidic catalysts by single step conversion for the production of diesel fuel and fuel blends. Appl. Catal. B Environ. 2015, 174–175, 383–394. [Google Scholar] [CrossRef]
- Sankaranarayanan, T.M.; Banu, M.; Pandurangan, A.; Sivasanker, S. Hydroprocessing of sunflower oil-gas oil blends over sulfided Ni-Mo-Al-zeolite beta composites. Bioresour. Technol. 2011, 102, 10717–10723. [Google Scholar] [CrossRef]
- Huber, G.W.; O’Connor, P.; Corma, A. Processing biomass in conventional oil refineries: Production of high quality diesel by hydrotreating vegetable oils in heavy vacuum oil mixtures. Appl. Catal. A Gen. 2007, 329, 120–129. [Google Scholar] [CrossRef]
- Chuck, C.J.; Donnelly, J. The compatibility of potential bioderived fuels with Jet A-1 aviation kerosene. Appl. Energy 2014, 118, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Rye, L.; Blakey, S.; Wilson, C.W. Sustainability of supply or the planet: A review of potential drop-in alternative aviation fuels. Energy Environ. Sci. 2010, 3, 17–27. [Google Scholar] [CrossRef]





| Variable | Low (–) | Medium (0) | High (+) |
|---|---|---|---|
| Temperature (X1; °C) | 450 | 500 | 550 |
| Pressure (X2; MPa) | 4 | 5 | 6 |
| LHSV (X3; h–1) | 0.5 | 1.0 | 1.5 |
| Standard Order | Temperature (°C) | Pressure (MPa) | LHSV (h–1) | Kerosene Selectivity (%) |
|---|---|---|---|---|
| 1 | 450 | 4 | 1.0 | 51.28 |
| 2 | 550 | 4 | 1.0 | 26.04 |
| 3 | 450 | 6 | 1.0 | 50.00 |
| 4 | 550 | 6 | 1.0 | 25.81 |
| 5 | 450 | 5 | 0.5 | 49.46 |
| 6 | 550 | 5 | 0.5 | 17.53 |
| 7 | 450 | 5 | 1.5 | 47.83 |
| 8 | 550 | 5 | 1.5 | 35.87 |
| 9 | 500 | 4 | 0.5 | 47.83 |
| 10 | 500 | 6 | 0.5 | 29.17 |
| 11 | 500 | 4 | 1.5 | 57.89 |
| 12 | 500 | 6 | 1.5 | 54.35 |
| 13 | 500 | 5 | 1.0 | 51.06 |
| 14 | 500 | 5 | 1.0 | 52.69 |
| 15 | 500 | 5 | 1.0 | 54.84 |
| Source | DF | Fvalue | Pvalue | Result |
|---|---|---|---|---|
| Regression | 9 | 11.47 | 0.008 | significant |
| Linear | 3 | 23.20 | 0.002 | significant |
| Temperature | 1 | 50.64 | 0.001 | significant |
| Pressure | 1 | 3.27 | 0.130 | insignificant |
| LHSV | 1 | 15.70 | 0.011 | significant |
| Square | 3 | 8.77 | 0.020 | significant |
| Temperature × Temperature | 1 | 25.19 | 0.004 | significant |
| Pressure × Pressure | 1 | 1.05 | 0.353 | insignificant |
| LHSV × LHSV | 1 | 1.63 | 0.257 | insignificant |
| Interaction | 3 | 2.44 | 0.180 | insignificant |
| Temperature × Pressure | 1 | 0.01 | 0.915 | insignificant |
| Temperature × LHSV | 1 | 4.64 | 0.084 | insignificant |
| Pressure × LHSV | 1 | 2.66 | 0.164 | insignificant |
| Residual Error | 5 | 9.33 | 0.098 | insignificant |
| Lack-of-Fit | 3 | |||
| Pure error | 2 | |||
| Total | 14 | |||
| Source | DF1 | DF2 | Fcritical | |
| F(0.05, DF1, DF2) | 3 | 5 | 5.41 | |
| F(0.05, DF1, DF2) | 3 | 2 | 19.16 |
| Conditions | Feedstock | Catalysts | Kerosene | Ref. | |
|---|---|---|---|---|---|
| Yield (%) | Selectivity (%) | ||||
| 483 °C, 5.0 MPa, and 1.4 h−1 LHSV | RPO | Pd/Al2O3 | 47.46 | 57.30 | This study |
| 280 °C, 0.8 MPa, and LHSV 4 h−1 | FAME | 10 wt.% Ni/HZSM-5 | NR | 32.5 | [1] |
| 390 °C, 3 MPa, and 8-h RT | Palm oil | Ni/SAPO-34 | 42 | 69 | [31] |
| 450° C for 45 min with 0.07 g/min feed and gas flow rate 42 mL/min | Soybean oil | Zeolite ZSM-5 | 14.54 | NR | [32] |
| 450 °C, 6 MPa, and 1 h−1 LHSV | Jatropha oil | NiW | 37.5 | NR | [9] |
| 380 °C, 2 MPa, and 3 h RT | FAME | Mo/Al2O3 | NR | 33.2 | [33] |
| MoO/Al2O3 | NR | 25.79 | |||
| Ni2P/Al2O3 | NR | 3.49 | |||
| Mo2C/AC | NR | 21.02 | |||
| Mo/AC | NR | 18.55 | |||
| MoS2/Al2O3 | NR | 11.89 | |||
| 380 °C, WHSV 1.85 h−1, ambient pressure | Oleic acid | 1 wt.% Pd/Siral70 | NR | ≈10 (C10–C14) | [34] |
| Property | Specification | BHK | |
|---|---|---|---|
| Min | Max | ||
| Heat of combustion (MJ/kg) | 42.8 | - | 45.8 |
| Flash point (°C) | 38 | - | 41 |
| Freezing point (°C) | - | −47 | −8 |
| Carbon and hydrogen (mass percent) | 99.5 | - | 99.1 |
| Nitrogen (mass percent) | - | - | <0.10 |
| Oxygen (mass percent) | - | - | 0.8 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dujjanutat, P.; Neramittagapong, A.; Kaewkannetra, P. Optimization of Bio-Hydrogenated Kerosene from Refined Palm Oil by Catalytic Hydrocracking. Energies 2019, 12, 3196. https://doi.org/10.3390/en12163196
Dujjanutat P, Neramittagapong A, Kaewkannetra P. Optimization of Bio-Hydrogenated Kerosene from Refined Palm Oil by Catalytic Hydrocracking. Energies. 2019; 12(16):3196. https://doi.org/10.3390/en12163196
Chicago/Turabian StyleDujjanutat, Praepilas, Arthit Neramittagapong, and Pakawadee Kaewkannetra. 2019. "Optimization of Bio-Hydrogenated Kerosene from Refined Palm Oil by Catalytic Hydrocracking" Energies 12, no. 16: 3196. https://doi.org/10.3390/en12163196
APA StyleDujjanutat, P., Neramittagapong, A., & Kaewkannetra, P. (2019). Optimization of Bio-Hydrogenated Kerosene from Refined Palm Oil by Catalytic Hydrocracking. Energies, 12(16), 3196. https://doi.org/10.3390/en12163196




