Impact of Water Content on Energy Potential and Combustion Characteristics of Methanol and Ethanol Fuels
Abstract
:1. Introduction
2. Materials and Methods
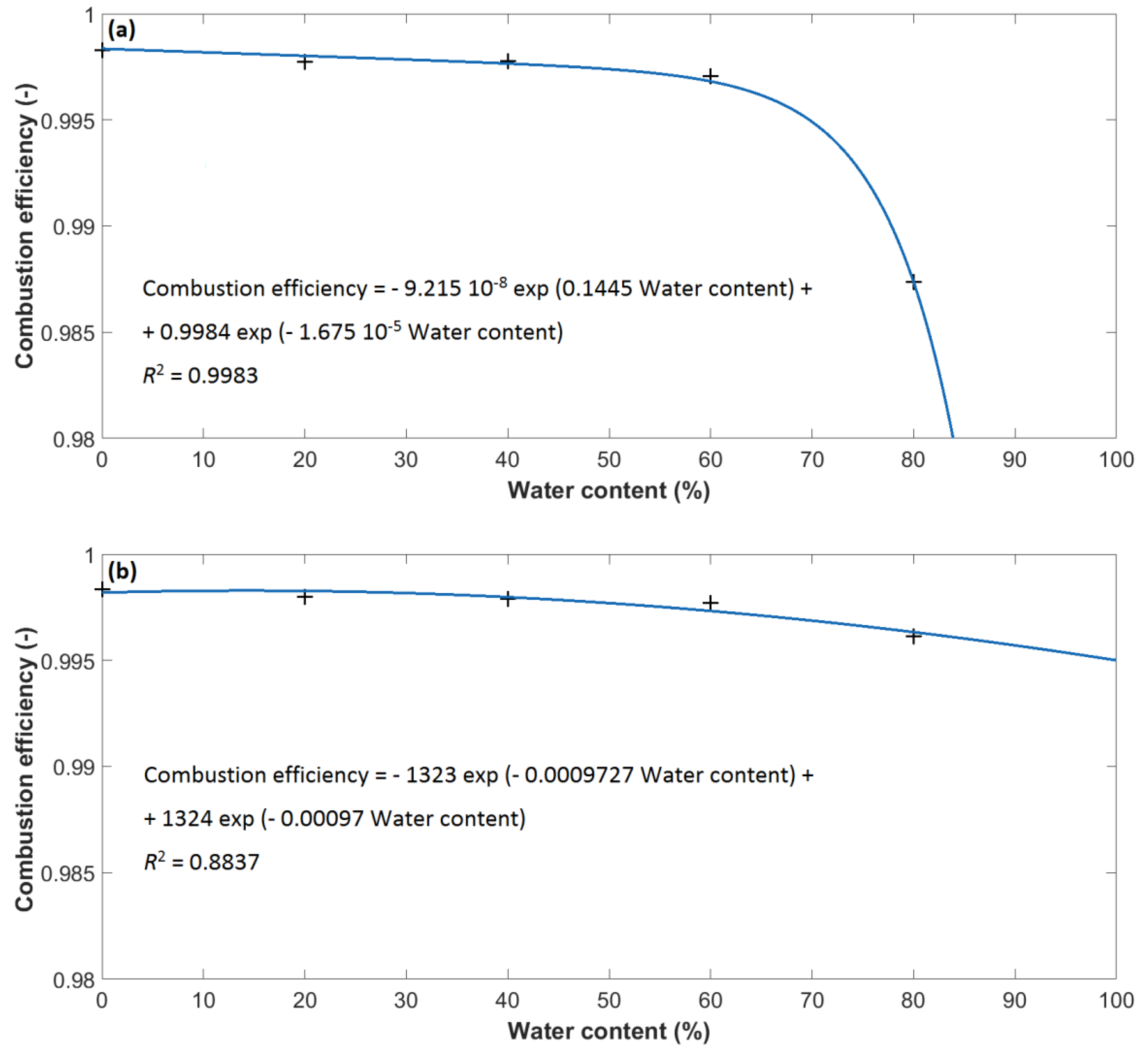
3. Results and Discussion
4. Conclusions
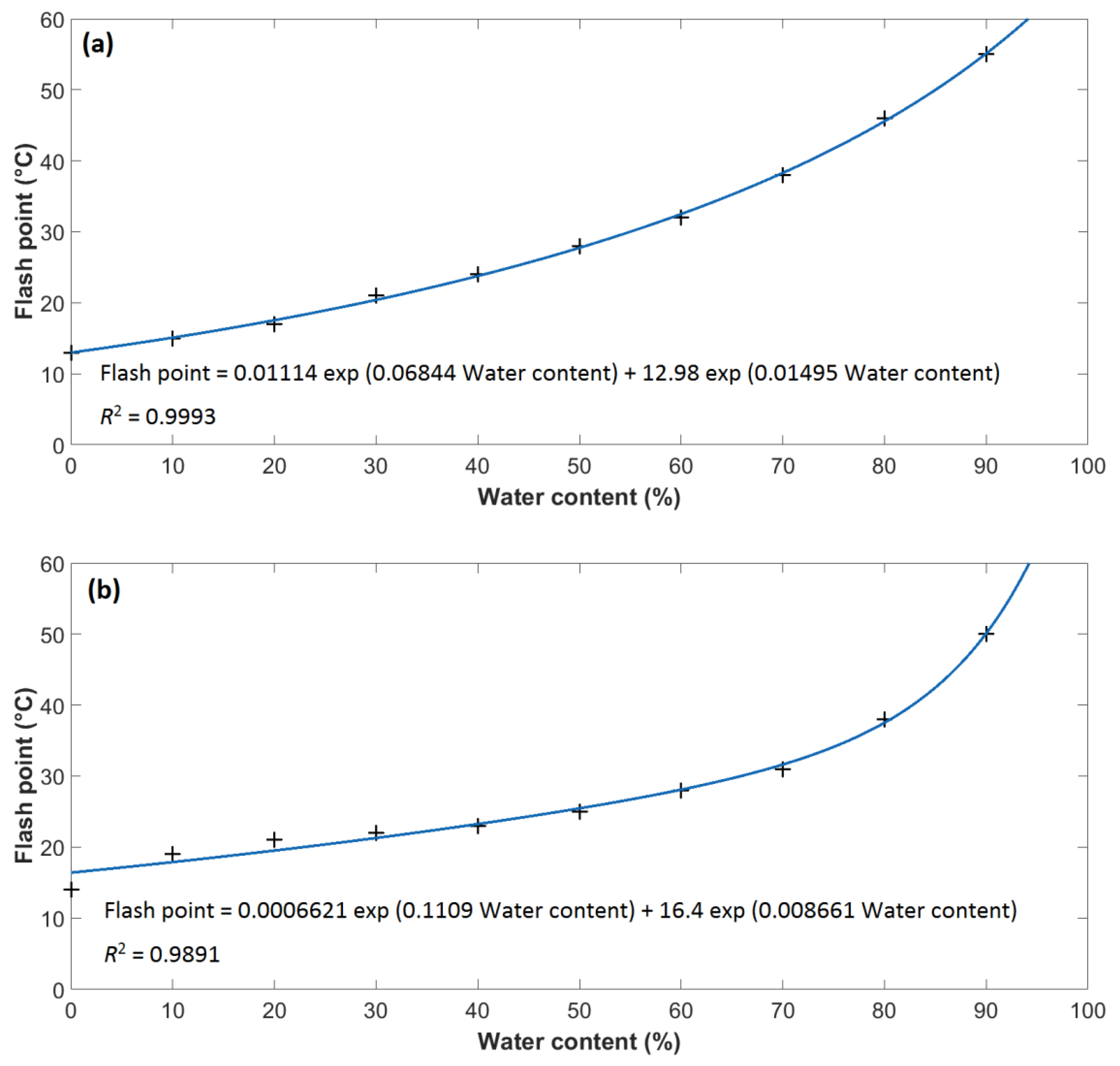
- With increasing water content, the flash point of both methanol and ethanol considerably increased. Both methanol and ethanol show an exponential dependence of flash point on water content. Despite the noticeable increase in the flash point (in an undiluted state, methanol and ethanol have flash points of 13 and 14 °C, while for a water content of 90%, the flash point rises to 55 and 50 °C, respectively), the examined alcohols behave as flammable liquids even at a water content of 90%.
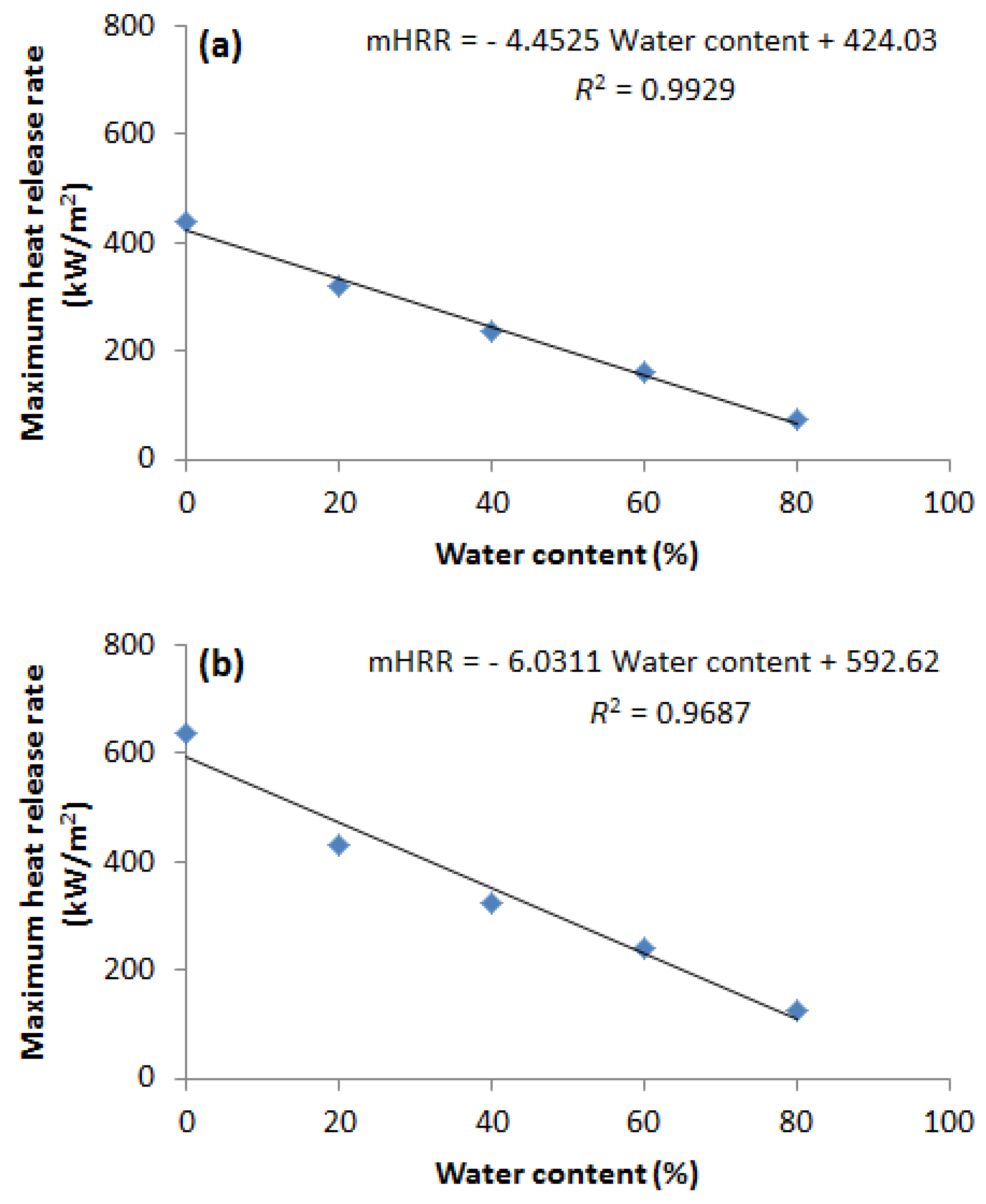
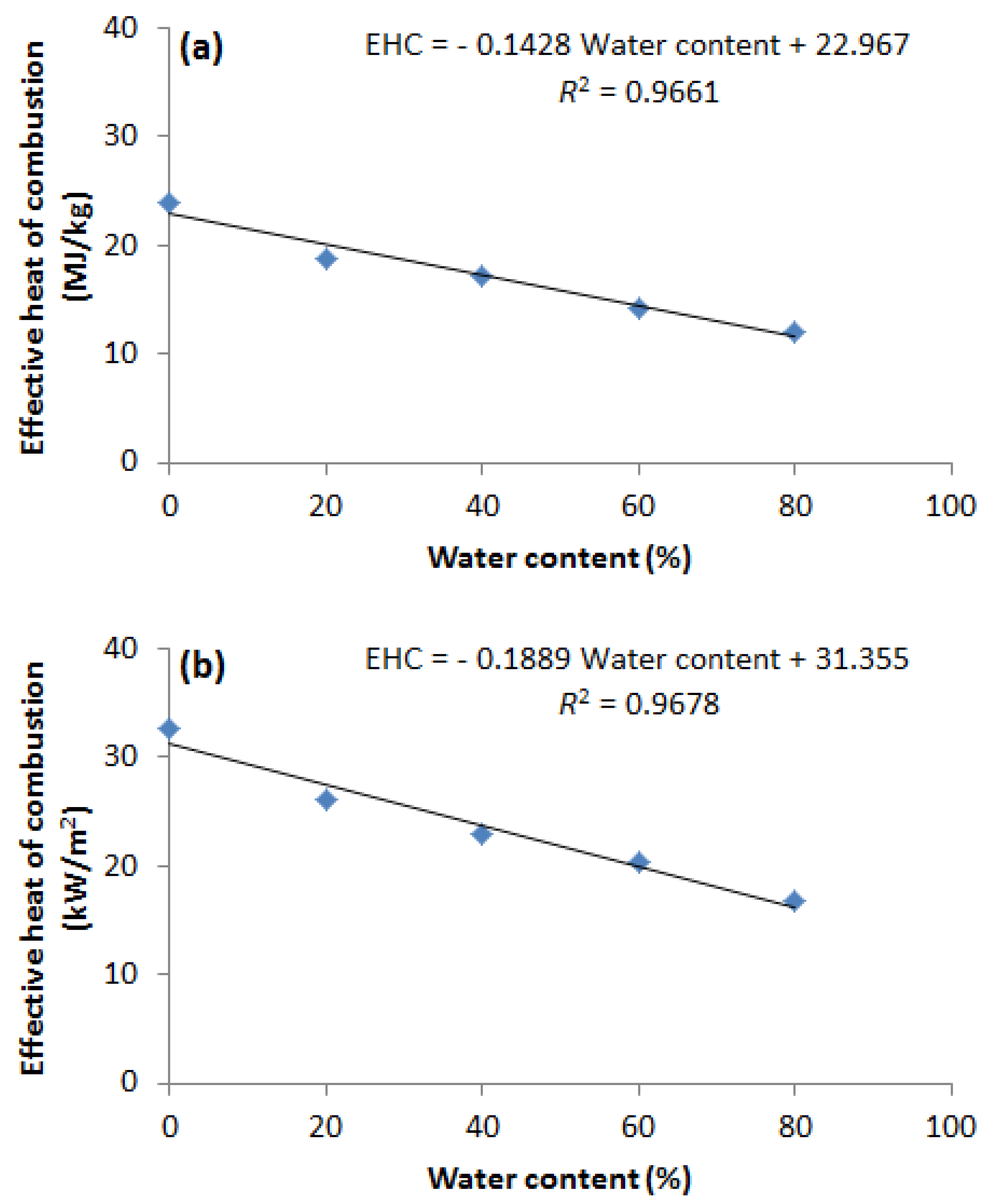
- The EHC, mHRR, and MARHE of methanol and ethanol decrease linearly with increasing water content.
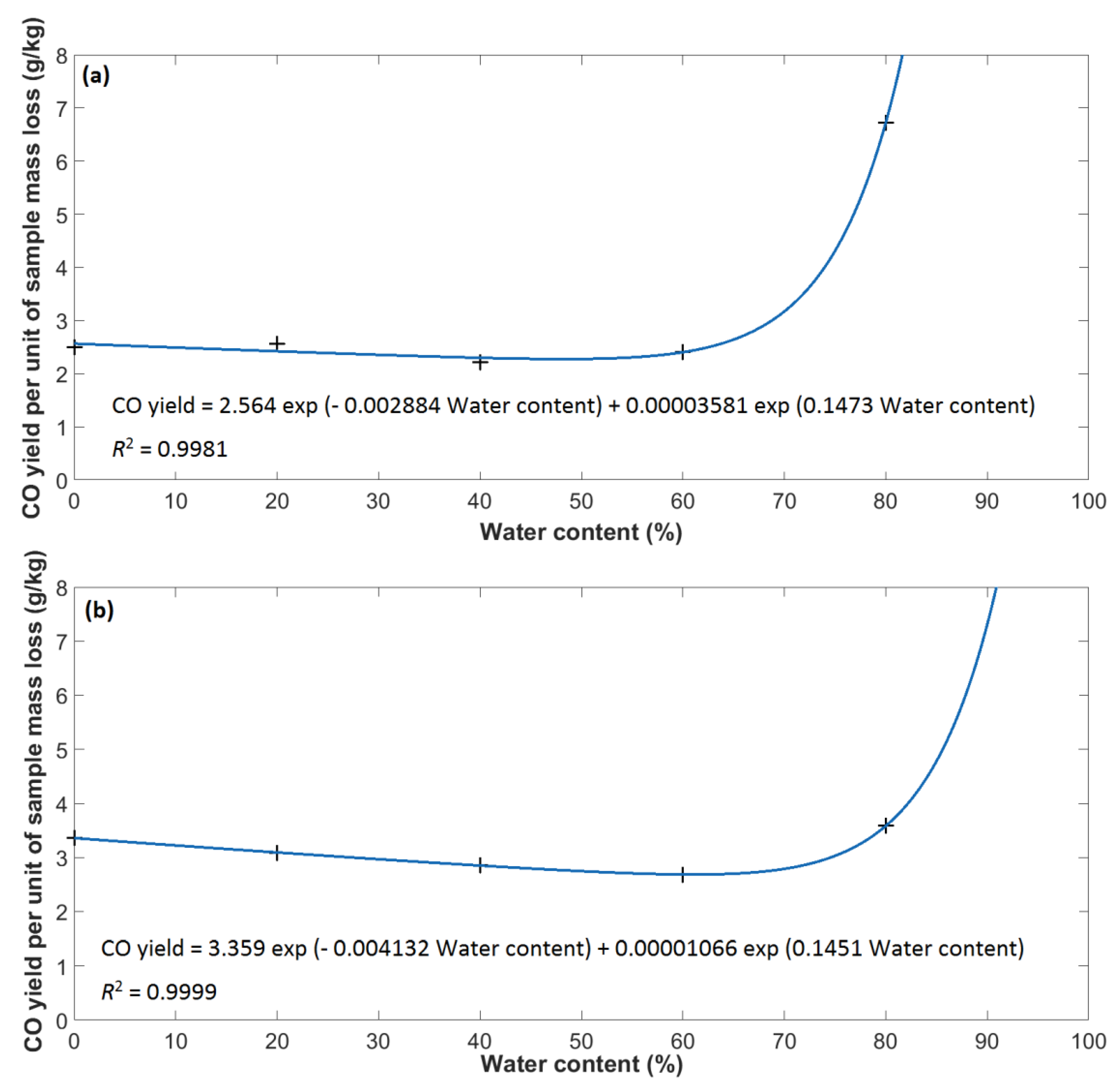
- The water content of methanol (in the range 0 to 60%) has no practical effect on the CO yield (per mass loss), while the CO yield (per mass loss) of ethanol with decreasing water content (in the range 0 to 60%) slightly decreases. The CO yield (per released heat) of both methanol and ethanol increases with increasing water content. The maximum CO yield (per both mass loss and released heat) of both methanol and ethanol was found to be at a water content of 80%. As the combustion of methanol and ethanol only produces a negligible amount soot, and from a toxicological point of view, CO is the most important combustion product, the impact of the combustion of methanol and ethanol on the environment is determined only the CO yield (mainly per released heat). Minimizing the impact of methanol and ethanol combustion on the environment can thus be achieved by minimizing the water content.
- The ratio of methanol to water (evaporated from the solution into the combustion zone) and the ratio of ethanol to water (evaporated from the solution into the combustion zone) both decrease linearly with increasing water content of the solution.
- A methanol solution with water exposed to a heat flux of 10 kw/m2 stops burning when the methanol content decreases to 15.6–17.3%. A solution of ethanol with water exposed to the same heat flux stops burning when the ethanol content decreases to approximately 11–14.2 %.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| EPF | Effective heat of combustion of pure fuel (kJ/kg) |
| EHC | Effective heat of combustion (MJ/kg) |
| ETBE | Ethyl tert-butyl ether (-) |
| FE | Mass of fuel evaporated (g) |
| HRR | Heat release rate (kW/m2) |
| IUPAC | International Union of Pure and Applied Chemistry (-) |
| MARHE | Maximum average rate of heat emission (kW/m2) |
| MEW | Mass of evaporated water (g) |
| mHRR | Maximum heat release rate (kW/m2) |
| MLS | Mass loss of the solution of fuel and water (g) |
| MTBE | Methyl tert-butyl ether (-) |
References
- Cooney, C.P.; Worm, J.J.; Naber, J.D. Combustion characterization in an internal combustion engine with ethanol—gasoline blended fuels varying compression ratios and ignition timing. Energy Fuel 2009, 23, 2319–2324. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Cheung, C.S.; Chan, T.L.; Yao, C.D. Experimental investigation on regulated and unregulated emissions of a diesel/methanol compound combustion engine with and without diesel oxidation catalyst. Sci. Total Environ. 2010, 408, 865–872. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Ji, C.; Liu, X. Combustion and emissions performance of a DME-enriched spark-ignited methanol engine at idle condition. Appl. Energy 2011, 88, 3704–3711. [Google Scholar] [CrossRef]
- Melo, T.C.C.D.; MacHado, G.B.; Belchior, C.R.P.; Colaco, M.J.; Barros, J.E.M.; De Oliveira, E.J.; De Oliveira, D.G. Hydrous ethanol-gasoline blends-combustion and emission investigations on a flex-fuel engine. Fuel 2012, 97, 796–804. [Google Scholar] [CrossRef]
- Liaw, H.J.; Tang, C.J.; Lia, S.J. A model for predicting the flash point of ternary flammable solutions of liquid. Combust Flame 2004, 138, 308–319. [Google Scholar] [CrossRef]
- Vidal, M.; Rogers, W.J.; Mannan, M.S. Prediction of minimum flash point behaviour for binary mixtures. Process Saf. Environ. 2006, 84, 1–9. [Google Scholar] [CrossRef]
- Guo, Y.; Wei, H.; Yang, F.; Li, D.; Fang, W.; Lin, R. Study on volatility and flash point of the pseudo-binary mixtures of sunflowerseed-based biodiesel + ethanol. J. Hazard. Mater. 2009, 167, 625–629. [Google Scholar] [CrossRef]
- Kurambhatti, C.V.; Kumar, D.; Rausch, K.D.; Tumbleson, M.E.; Singh, V. Ethanol production from corn fiber separated after liquefaction in the dry grind process. Energies 2018, 11, 2921. [Google Scholar] [CrossRef]
- Wang, X.; Martin, N.M.; Nilsson, J.; Carlson, S.; Gustafson, J.; Skoglundh, M.; Carlsson, P.A. Copper-modified zeolites and silica for conversion of methane to methanol. Catalysts 2018, 8, 545. [Google Scholar] [CrossRef]
- Simonelt, B.R.T.; Rogge, W.F.; Mazurek, M.A.; Standley, L.J.; Hildemannand, L.M.; Cass, G.R. Lignin pyrolysis products, lignans, and resin acids as specific tracers of plant classes in emissions from biomass combustion. Environ. Sci. Technol. 1993, 27, 2533–2541. [Google Scholar] [CrossRef]
- Luche, J.; Mathis, E.; Rogaume, T.; Richard, F.; Guillaume, E. High-density polyethylene thermal degradation and gaseous compound evolution in a cone calorimeter. Fire Saf. J. 2012, 54, 24–35. [Google Scholar] [CrossRef]
- Ometto, A.R.; Hauschild, M.Z.; Roma, W.N.L. Lifecycle assessment of fuel ethanol from sugarcane in Brazil. Int. J. Life Cycle Assess. 2009, 14, 236–247. [Google Scholar] [CrossRef]
- Gann, R.G.; Babrauskas, V.; Grayson, S.J.; Marsh, N.D. Hazards of combustion products: Toxicity, opacity, corrosivity, and heat release: The experts’ views on capability and issues. Fire Mater. 2011, 35, 115–127. [Google Scholar] [CrossRef]
- IEC 60695-7:2010. Fire Hazard Testing. Part 7-1: Toxicity of Fire Effluent—General Guidance, 3rd ed.; International Electrotechnical Commission: Geneva, Switzerland, 2010; p. 50. [Google Scholar]
- IEC 60695-7-3:2011. Fire Hazard Testing. Part 7-3: Toxicity of Fire Effluent—Use and Interpretation of Test Results, 1st ed.; International Electrotechnical Commission: Geneva, Switzerland, 2011; p. 68. [Google Scholar]
- C/VM2:2014. Verification Method: Framework for Fire Safety Design, 1st ed.; The Ministry of Business, Innovation and Employment: Wellington, New Zealand, 2014; p. 74. [Google Scholar]
- Karlsson, B.; Quintiere, J. Enclosure Fire Dynamics, 1st ed.; CRC Press: Boca Raton, FL, USA, 1999; p. 336. [Google Scholar]
- Maricq, M.M. Soot formation in ethanol/gasoline fuel blend diffusion flames. Combust Flame 2012, 159, 170–180. [Google Scholar] [CrossRef]
- ISO 2719:2016. Determination of Flash Point: Pensky-Martens Closed Cup Method, 4th ed.; International Organization for Standardization: Geneva, Switzerland, 2016; p. 22. [Google Scholar]
- ISO 5660-1:2015. Reaction to Fire Tests. Heat Release, Smoke Production and Mass Loss Rate. Part 1: Heat Release Rate (Cone Calorimeter Method) and Smoke Production Rate (Dynamic Measurement), 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2015; p. 55. [Google Scholar]
- Martinka, J.; Chrebet, T.; Balog, K. An assessment of petrol fire risk by oxygen consumption calorimetry. J. Therm. Anal. Calorim. 2014, 117, 325–332. [Google Scholar] [CrossRef]
- Ward, D.E.; Hao, W.M. Air Toxic Emissions from Burning of Biomass Globally: Preliminary Estimates. In Proceedings of the 85th Annual Meeting and Exhibition, Air and Waste Management Association, Kansas City, MO, USA, 16–25 June 1992; Air and Waste Management Association: Kansas City, MO, USA, 1992; pp. 92–98. [Google Scholar]
- Ferek, R.J.; Reid, J.S.; Hobbs, P.V.; Blake, D.R.; Liousse, C. Emission factors of hydrocarbons, halocarbons, trace gases and particles from biomass burning in Brazil. J. Geophys. Res. Atmos. 1998, 103, 32107–32118. [Google Scholar] [CrossRef] [Green Version]
- Janssens, M. Rate of heat release of wood products. Fire Saf. J. 1991, 17, 217–237. [Google Scholar] [CrossRef]
- Maciulaitis, R.; Praniauskas, V. Fire tests on wood products subjected to different heat fluxes. J. Civ. Eng. Manag. 2010, 16, 484–490. [Google Scholar] [CrossRef]
- Akaki, T.; Maehara, H.; Tooyama, M. Development of wood and wood ash-based hydroxyapatite composites and their fire-retarding properties. J. Wood Sci. 2012, 58, 532–537. [Google Scholar] [CrossRef]
- Carosio, F.; Cuttica, F.; Medina, L.; Berglund, L.A. Clay nanopaper as multifunctional brick and mortar fire protection coating-Wood case study. Mater. Des. 2016, 93, 357–363. [Google Scholar] [CrossRef]
- Zhang, J. Study of Polyamide 6-Based Nanocomposites. Ph.D. Thesis, Polytechnic University, New York, NY, USA, 27 November 2008. [Google Scholar]
- Beyer, G. Flame retardant properties of EVA-nanocomposites and improvements by combination of nanofillers with aluminium trihydrate. Fire Mater. 2001, 25, 193–197. [Google Scholar] [CrossRef]
- Wang, S.; Hu, Y.; Li, Z.; Wang, Z.; Zhuang, Y.; Chen, Z.; Fan, W. Flammability and phase-transition studies of nylon 6/montmorillonite nanocomposites. Colloid Polym. Sci. 2003, 281, 951–956. [Google Scholar] [CrossRef]
- Chigwada, G.; Jash, P.; Jiang, D.D.; Wilkie, C.A. Fire retardancy of vinyl ester nanocomposites: Synergy with phosphorus-based fire retardants. Polym. Degrad. Stab. 2005, 89, 85–100. [Google Scholar] [CrossRef] [Green Version]
- Varley, R.J.; Groth, A.M.; Leong, K.H. The role of nanodispersion on the fire performance of organoclay-polyamide nanocomposites. Compos. Sci. Technol. 2008, 68, 2882–2891. [Google Scholar] [CrossRef]
- Srinivasan, K.K.; Mago, P.J.; Krishnan, S.R. Analysis of exhaust waste heat recovery from a dual fuel low temperature combustion engine using an organic Rankine cycle. Energy 2010, 35, 2387–2399. [Google Scholar] [CrossRef]
- Maurya, R.K.; Agarwal, A.K. Experimental study of combustion and emission characteristics of ethanol fuelled port injected homogeneous charge compression ignition (HCCI) combustion engine. Appl. Energy 2011, 88, 1169–1180. [Google Scholar] [CrossRef]
- Liang, C.; Ji, C.; Gao, B.; Liu, X.; Zhu, Y. Investigation on the performance of a spark-ignited ethanol engine with DME enrichment. Energy Convers. Manag. 2012, 58, 19–25. [Google Scholar] [CrossRef]
- Zachar, M.; Mitterova, I.; Xu, Q.; Majlingova, A.; Cong, J.; Galla, S. Determination of fire and burning properties of spruce wood. Drvna Ind. 2012, 63, 217–223. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.H.; Kim, S. Estimating the fire behavior of wood flooring using a cone calorimeter. J. Therm. Anal. Calorim. 2012, 110, 677–683. [Google Scholar] [CrossRef]
- Pfeil, M.A.; Groven, L.J.; Lucht, R.P.; Son, S.F. Effects of ammonia borane on the combustion of an ethanol droplet at atmospheric pressure. Combust Flame 2013, 160, 2194–2203. [Google Scholar] [CrossRef]
- Cekovska, H.; Gaff, M.; Osvaldova, L.M.; Kacik, F.; Kaplan, L.; Kubs, J. Tectona grandis Linn. and its fire characteristics affected by the thermal modification of wood. BioRes 2017, 12, 2805–2817. [Google Scholar] [CrossRef]
- Martinka, J.; Martinka, F.; Rantuch, P.; Hrusovsky, I.; Blinova, L.; Balog, K. Calorific value and fire risk of selected fast-growing wood species. J. Therm. Anal. Calorim. 2018, 131, 899–906. [Google Scholar] [CrossRef]
- Haynes, W.M. Handbook of Chemistry and Physics, 96th ed.; CRC Press: Boca Raton, FL, USA, 2015; p. 2677. [Google Scholar]
- ISO 1716:2018. Reaction to Fire Tests for Products: Determination of the Gross Heat of Combustion (Calorific Value), 4th ed.; International Organization for Standardization: Geneva, Switzerland, 2018; p. 30. [Google Scholar]
- Thornton, W.M. The relation of oxygen to the heat of combustion of organic compounds. Philos. Mag. 1917, 33, 196–203. [Google Scholar] [CrossRef]
- Gunther, B.; Gebauer, K.; Barkowski, R.; Rosenthal, M.; Bues, C.T. Calorific value of selected wood species and wood products. Eur. J. Wood Prod. 2012, 70, 755–757. [Google Scholar] [CrossRef]
- Todaro, L.; Rita, A.; Cetera, P.; D’Auria, M. Thermal treatment modifies the calorific value and ash content in some wood species. Fuel 2015, 140, 1–3. [Google Scholar] [CrossRef]
- DiNenno, P.J. SFPE Handbook of Fire Protection Engineering, 3rd ed.; National Fire Protection Association: Quincy, MD, USA, 2002; p. 1606. [Google Scholar]
- Ozgen, S.; Caserini, S.; Galante, S.; Giugliano, M.; Angelino, E.; Marongiu, A.; Hugony, F.; Migliavacca, G.; Morreale, C. Emission factors from small scale appliances burning wood and pellets. Atmos. Environ. 2014, 94, 144–153. [Google Scholar] [CrossRef]
- Gardiner, W.C. Gas-Phase Combustion Chemistry, 2nd ed.; Springer: New York, NY, USA, 2000; p. 543. [Google Scholar]




| Water Content in Solution (%) | 60 | 80 |
|---|---|---|
| Mass loss of methanol solution (g) | 35.5 | 8.1 |
| Mass loss of ethanol solution (g) | 35.4 | 14.8 |
| Mass of burned methanol (g) | 20.9 | 4.1 |
| Mass of burned ethanol (g) | 21.9 | 7.5 |
| Mass of water evaporated from methanol solution (g) | 14.6 | 4 |
| Mass of water evaporated from ethanol solution (g) | 13.5 | 7.3 |
| Water Content in Solution (%) | 60 | 80 |
|---|---|---|
| Critical methanol content in solution (%) | 17.3 | 15.6 |
| Critical ethanol content in solution (%) | 14.2 | 11 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinka, J.; Rantuch, P.; Wachter, I. Impact of Water Content on Energy Potential and Combustion Characteristics of Methanol and Ethanol Fuels. Energies 2019, 12, 3491. https://doi.org/10.3390/en12183491
Martinka J, Rantuch P, Wachter I. Impact of Water Content on Energy Potential and Combustion Characteristics of Methanol and Ethanol Fuels. Energies. 2019; 12(18):3491. https://doi.org/10.3390/en12183491
Chicago/Turabian StyleMartinka, Jozef, Peter Rantuch, and Igor Wachter. 2019. "Impact of Water Content on Energy Potential and Combustion Characteristics of Methanol and Ethanol Fuels" Energies 12, no. 18: 3491. https://doi.org/10.3390/en12183491
APA StyleMartinka, J., Rantuch, P., & Wachter, I. (2019). Impact of Water Content on Energy Potential and Combustion Characteristics of Methanol and Ethanol Fuels. Energies, 12(18), 3491. https://doi.org/10.3390/en12183491







