Morphology and Rheological Properties of Polyacrylamide/Bentonite Organic Crosslinking Composite Gel
Abstract
:1. Introduction
2. Experimental Details
2.1. Raw Materials and Reagents
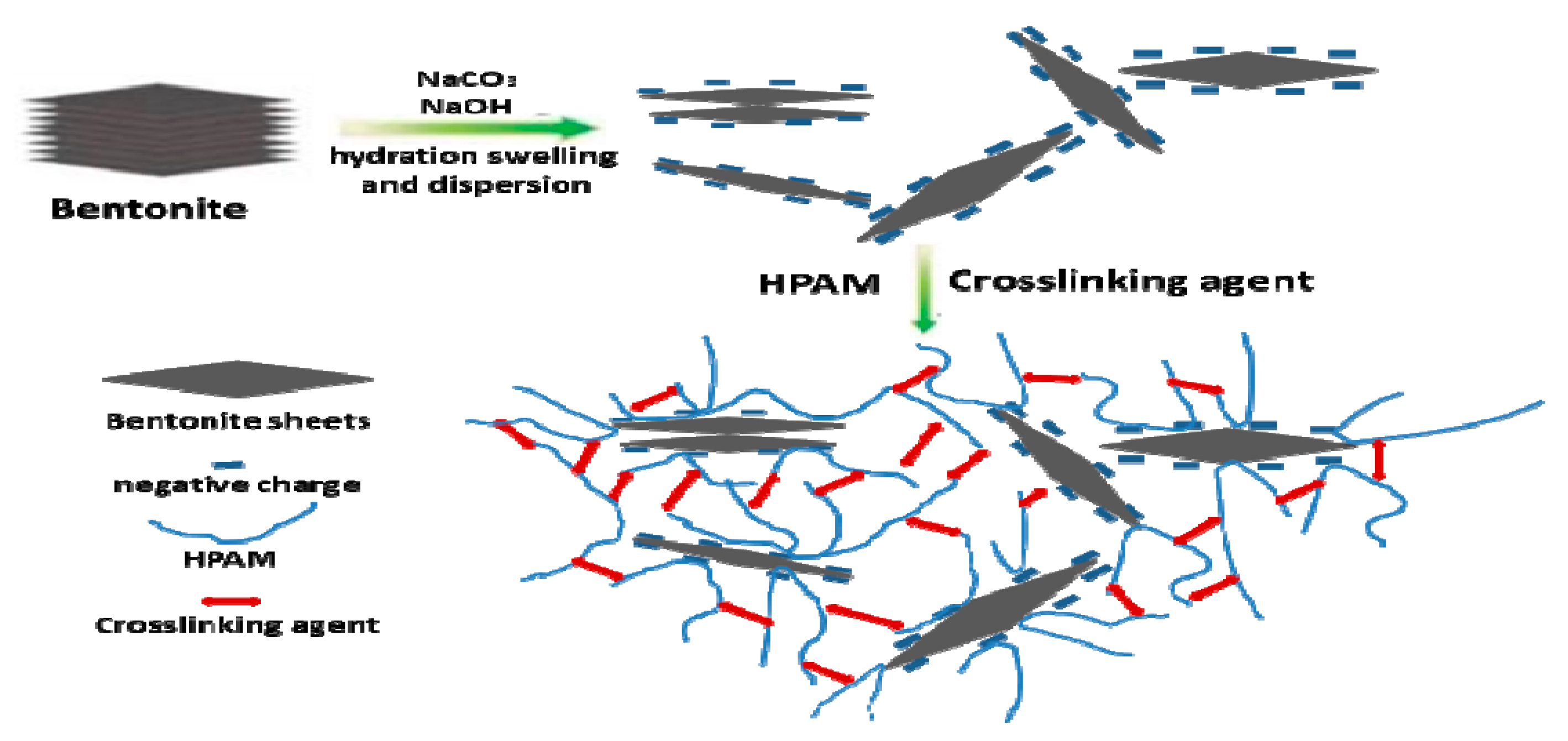
2.2. Preparation of Polyacrylamide/Bentonite Organic Crosslinked Composite Gel
2.3. Testing and Characterization
2.3.1. Particle-Size Analysis
2.3.2. Scanning Electron Microscopy Analysis
2.3.3. Rheology Tests
- Shear Thinning: The apparent viscosity of the composite gel was determined as a function of shear rate at 80 °C (shear rate from 0.01 to 50 s−1).
- Thixotropy: At 80 °C, first, the shear rate increased from 0 to 20 s−1 within 50 s. Then, shears at a shear rate of 20 s−1 for 20 s occurred. After that, the shear rate decreased from 20 to 0 s−1 within 50 s. Finally, we measured the area of the thixotropic ring, which consists of the curves of the shear rate and shear stress. The area represents the degree of thixotropic degree of the composite gel.
- Viscoelasticity: In the oscillation mode, the appropriate strain values for frequency scanning (strain γ = 1%, angular frequency ω = 0.1–10 rad/s), the elastic modulus G′, and loss modulus G″ were tested at 80 °C.
- Start-up stress test: At 80 °C, the fixed shear rate was 0.3 s−1, and the shear stress was measured instantaneously with time.
3. Experimental Results and Discussion
3.1. Effect of Crosslinker Concentration on Composite Gel Particle Size
3.2. Microstructure of Polyacrylamide/Bentonite Organic Crosslinked Composite Gel
3.3. Rheological Properties of Composite Gel
3.3.1. Test of the Flow Curve of Composite Gel
3.3.2. Test of Thixotropy of Composite Gel
3.3.3. Test of Viscoelasticity of Composite Gel
3.3.4. Test of Start-up Stress of Composite Gel
4. Conclusions
- The composite gel formed by polyacrylamide and bentonite and organic crosslinking agent and stabilizer under high-temperature conditions was formed by polymer adsorption in bentonite and intermolecular crosslinking. The aggregate structure with bentonite as the core was different from the polyacrylamide gel structure formed by polymer aggregates. A larger aggregate particle size was formed with a higher content of crosslinking agent.
- The composite gel exhibited shear thinning characteristics, the bentonite content increased, and more aggregates were formed. The distance between the aggregates became smaller, and the interaction increased and formed a strong spatial network structure. The composite gel had a higher viscosity under shear and shear resistance at a high shear rate. As concentration of the crosslinker increased, the aggregate formed by the composite gel became larger, and the hydrodynamic volume increased. Shear resistance ability was also enhanced.
- The composite gel exhibited positive thixotropic properties and good viscoelastic properties. The area of the thixotropic ring was significantly increased, and the thixotropy was obviously enhanced. When the content of the bentonite increased, the elastic modulus and loss modulus were increased. The elastic performance was increased more, and the network structure was more complete. When the crosslinking agent content increased, the aggregates gradually became larger, and the molecular chain entanglement between the aggregates was greatly reduced. The thixotropy was weakened, and the elastic modulus decreased. The loss modulus increased, and the viscosity characteristics were enhanced, owing to the hydrodynamic volume increase.
- When the shear rate began to be loaded onto the composite gel, the apparent shear stress increased significantly with time and reached the highest value. Then the internal structure of the composite gel began to break, and the shear stress decreased. The flow direction of the aggregate showed different degrees of orientation. At this time, the composite gel gradually showed shear flow, and the shear stress tended to be stable. Start-up stress was apparently improved with the content of bentonite increased. The maximum value of start-up stress first decreased and then increased with increasing crosslinking agent content. The time of the start-up stress became short.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| γ | strain |
| ω | angular frequency, rad/s |
| G′ | elastic modulus, Pa |
| G″ | loss modulus, Pa |
| Dv(10) | the particle cumulative distribution is 10%, μm |
| Dv(50) | the particle cumulative distribution is 50%, μm |
| Dv(90) | the particle cumulative distribution is 90%, μm |
| tanδ | Loss factor |
References
- Seright, R.S.; Liang, J. A Survey of field applications of gel treatments for water shutoff. SPE 1994, 26991, 221–231. [Google Scholar]
- Chou, S.I.; Bae, J.H.; Friedmann, F.; Dolan, J.D. Development of optimal water control strategies. SPE 1994, 28451, 41–45. [Google Scholar]
- Pappas, J.; Creel, P.; Crook, R. Problem identification and solution method for water flow problem. SPE 1996, 35249, 797–805. [Google Scholar]
- Fulleylove, R.J.; Morgan, J.C.; Stevens, D.G.; Thrasher, D.R. Water shut-off in oil production wells-lessons from 12 treatments. SPE 1996, 36211, 415–427. [Google Scholar]
- Zhao, F.L.; Zhang, G.C.; Sun, M.Q. Studies on channel plugging in a reservoir by using clay as a profile control agent in a double—Fluid method. Acta Pet. Sin. 1994, 15, 56–64. [Google Scholar]
- Bedaiwi, E.; Al-Anazi, B.D.; Al-Anazi, A.F.; Paiaman, A.M. Polymer ingection for water production control through permeability alteration in fractured reservoir. Nafta 2009, 60, 221–231. [Google Scholar]
- Conformance Improvement—Gel Treatment Design. Petro Wiki. Available online: http://petrowiki.Org/Conformance_improvement_gel_treatment_design (accessed on 11 November 2017).
- Kurenkov, V.; Hartan, H.-G.; Lobanov, F. Degradation of polyacrylamide and its derivatives in aqueous solutions. Russ. J. Appl. Chem. 2002, 75, 1039–1050. [Google Scholar] [CrossRef]
- Gosavi, U.R.; Deopurkar, R.L. Microbial degradation of super absorbent ASPAN gel by an indigenously isolated bacterial culture. Macromolecules 1999, 32, 4264–4271. [Google Scholar] [CrossRef]
- Li, A.; Wang, A.Q. Study on superabsorbent composite. Xll. Effect of ion—Exchanged attpulgite on water absorbency of poly (acrylic acid)/attapulgite superabsorbent composites. Appl. Polym. Sci. 2007, 105, 3476–3482. [Google Scholar] [CrossRef]
- Yalcin, B.; Cakmak, M. The role of plasticizer on the exfoliation and dispersion and fracture behavior of clay particles in PVC matrix: A comprehensive morphological study. Polymer 2004, 45, 6623–6638. [Google Scholar] [CrossRef]
- Zanetti, M.; Lomakin, S.; Camino, G. Polymer layered silicate nanocomposites. Macromol. Mater. Eng. 2000, 279, 1–9. [Google Scholar] [CrossRef]
- Ray, S.S.; Okamoto, K.; Okamoto, M. Structure-property relationship in biodegradable poly (butylene succinate)/layered silicate nanocomposities. Macromolecules 2003, 36, 2355–2367. [Google Scholar]
- Mohamadian, N.; Ghorbani, H. A hybrid nanocomposite of poly(styrene-methyl methacrylate-acrylic acid)/clay as a novel rheology-improvement additive for drilling fluids. J. Polym. Res. 2019, 26, 1696–1709. [Google Scholar] [CrossRef]
- Cheraghian, G.; Nezhad, S.S.K.; Kamari, M.; Hemmati, M.; Masihi, M.; Bazgir, S. Effect of nanoclay on improved rheology properties of polyacrylamide solutions used in enhanced oil recovery. J. Petrol. Explor. Prod. Technol. 2015, 5, 189–196. [Google Scholar] [CrossRef]
- Schramm, G. A Practical Approach to Rheology and Rheometry; Gebrueder HAAKE GmbH: Karlsruhe, Germany, 1998. [Google Scholar]

















| Sample | Bentonite (mg/L) | HPAM (mg/L) | Urotropine (mg/L) | Resorcinol (mg/L) | Oxalic Acid (mg/L) | Thiourea (mg/L) |
|---|---|---|---|---|---|---|
| BH | 40,000 | 1500 | 0 | 0 | 0 | 0 |
| BHCT-1 | 40,000 | 1500 | 100 | 80 | 100 | 30 |
| BHCT-2 | 40,000 | 1500 | 200 | 175 | 200 | 30 |
| BHCT-3 | 40,000 | 1500 | 400 | 350 | 400 | 30 |
| BHCT-4 | 40,000 | 1500 | 600 | 500 | 600 | 30 |
| BHCT-5 | 10,000 | 1500 | 200 | 175 | 200 | 30 |
| BHCT-6 | 20,000 | 1500 | 200 | 175 | 200 | 30 |
| BHCT-7 | 60,000 | 1500 | 200 | 175 | 200 | 30 |
| HCT | 0 | 1500 | 200 | 175 | 200 | 30 |
| Sample | Specific Surface Area (m³/kg) | Dv(10) (μm) | Dv(50) (μm) | Dv(90) (μm) |
|---|---|---|---|---|
| BH | 375.6 | 10.7 | 50.6 | 203 |
| BHCT-1 | 407.3 | 8.06 | 73.4 | 724 |
| BHCT-2 | 540.7 | 4.27 | 54.4 | 868 |
| BHCT-3 | 770.3 | 2.84 | 89.2 | 1850 |
| BHCT-4 | 807.8 | 2.51 | 950 | 2460 |
| Sample | BHCT-5 | BHCT-6 | BHCT-2 | BHCT-7 |
|---|---|---|---|---|
| Thixotropic ring area | 0.224 Pa/s | 2.785 Pa/s | 13.482 Pa/s | 38.597 Pa/s |
| Sample | BHCT-1 | BHCT-2 | BHCT-3 | BHCT-4 |
|---|---|---|---|---|
| Thixotropic ring area | 10.032 Pa/s | 13.482 Pa/s | 4.926 Pa/s | 4.741 Pa/s |
| Sample | BHCT-5 | BHCT-6 | BHCT-2 | BHCT-7 |
|---|---|---|---|---|
| Maximum shear stress | 0.090 Pa | 0.299 Pa | 0.760 Pa | 2.837 Pa |
| Sample | BHCT-1 | BHCT-2 | BHCT-3 | BHCT-4 |
|---|---|---|---|---|
| Maximum shear stress | 1.146 Pa | 0.760 Pa | 1.147 Pa | 1.450 Pa |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhou, W.; Qi, Z.; Luo, T.; Yan, W.; Xu, H.; Cheng, K.; Li, H. Morphology and Rheological Properties of Polyacrylamide/Bentonite Organic Crosslinking Composite Gel. Energies 2019, 12, 3648. https://doi.org/10.3390/en12193648
Li J, Zhou W, Qi Z, Luo T, Yan W, Xu H, Cheng K, Li H. Morphology and Rheological Properties of Polyacrylamide/Bentonite Organic Crosslinking Composite Gel. Energies. 2019; 12(19):3648. https://doi.org/10.3390/en12193648
Chicago/Turabian StyleLi, Jun, Wen Zhou, Zhilin Qi, Taotao Luo, Wende Yan, Honglin Xu, Keyang Cheng, and Hui Li. 2019. "Morphology and Rheological Properties of Polyacrylamide/Bentonite Organic Crosslinking Composite Gel" Energies 12, no. 19: 3648. https://doi.org/10.3390/en12193648
APA StyleLi, J., Zhou, W., Qi, Z., Luo, T., Yan, W., Xu, H., Cheng, K., & Li, H. (2019). Morphology and Rheological Properties of Polyacrylamide/Bentonite Organic Crosslinking Composite Gel. Energies, 12(19), 3648. https://doi.org/10.3390/en12193648





