Enhancing Bioenergy Yields from Sequential Bioethanol and Biomethane Production by Means of Solid–Liquid Separation of the Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bioethanol Production
2.1.1. Biomass and Pretreatment
2.1.2. Hydrolysis, Fermentation, and Distillation
2.2. Biomethane Potential
2.3. Chemical Analysis
2.4. Statistics
3. Results
3.1. Chemical Composition
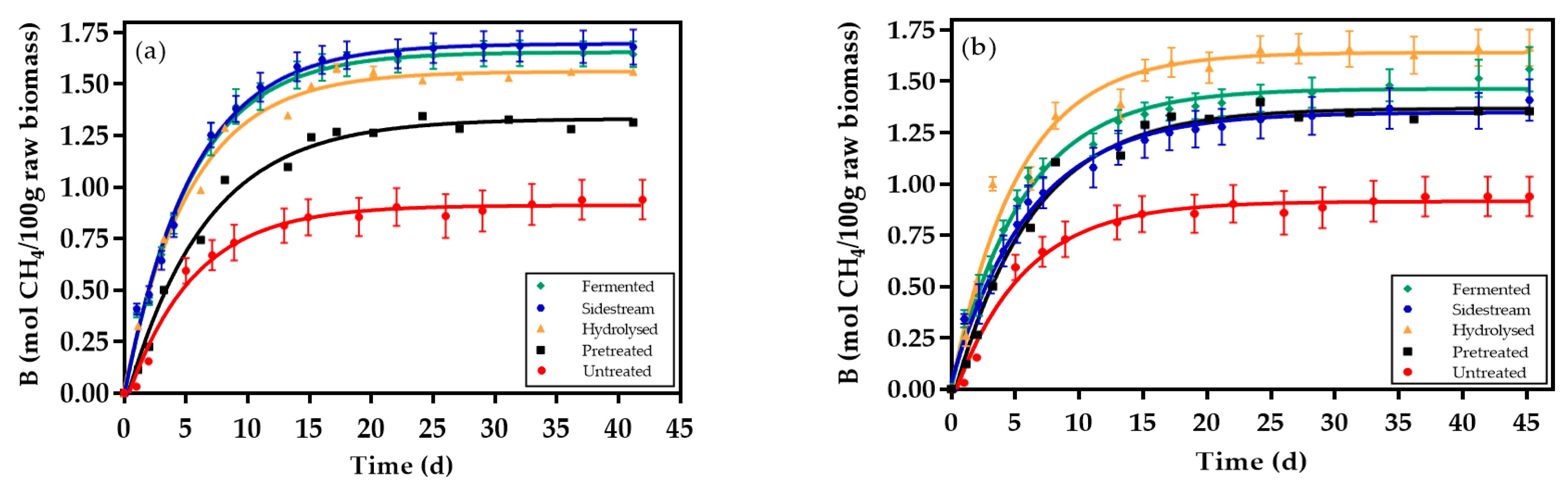
3.2. Methane Recovery from Solid and Liquid Fractions of the Bioethanol Production Process
3.3. Kinetics Rate and Digestion Time
3.4. The Ratio of Methane in the Produced Biogas
3.5. Mass Balances
4. Discussion
4.1. Chemical Composition
4.2. Methane Recovery From Solid and Liquid Fractions of Bioethanol Production Process
4.3. Kinetics Rate and Digestion Time
4.4. Mass Balances
5. Conclusions
Author Contributions
Funding

Acknowledgments
Conflicts of Interest
References
- Sims, R.E.H.; Mabee, W.; Saddler, J.N.; Taylor, M. An overview of second generation biofuel technologies. Bioresour. Technol. 2010, 101, 1570–1580. [Google Scholar] [CrossRef]
- Naik, S.N.; Goud, V.V.; Rout, P.K.; Dalai, A.K. Production of first and second generation biofuels: A comprehensive review. Renew. Sustain. Energy Rev. 2010, 14, 578–597. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Raud, M.; Orupõld, K.; Kikas, T. Potential of bioethanol production waste for methane recovery. Energy 2019, 173, 133–139. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Brancoli, P.; Agnihotri, S.; Bolton, K.; Taherzadeh, M.J. A review of integration strategies of lignocelluloses and other wastes in 1st generation bioethanol processes. Process Biochem. 2018, 75, 173–186. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Raud, M.; Orupõld, K.; Kikas, T. Second-generation bioethanol production: A review of strategies for waste valorisation. Agron. Res. 2017, 15, 830–847. [Google Scholar]
- Raud, M.; Olt, J.; Kikas, T. N2 explosive decompression pretreatment of biomass for lignocellulosic ethanol production. Biomass Bioenergy 2016, 90, 1–6. [Google Scholar] [CrossRef]
- Rocha-Meneses, L.; Ivanova, A.; Atouguia, G.; Ávila, I.; Raud, M.; Orupõld, K.; Kikas, T. The effect of flue gas explosive decompression pretreatment on methane recovery from bioethanol production waste. Ind. Crops Prod. 2019, 127, 66–72. [Google Scholar] [CrossRef]
- Raud, M.; Krennhuber, K.; Jäger, A.; Kikas, T. Nitrogen explosive decompression pre-treatmentpretreatment: An alternative to steam explosion. Energy 2019, 177, 175–182. [Google Scholar] [CrossRef]
- Song, Z.; Yang, G.; Liu, X.; Yan, Z.; Yuan, Y.; Liao, Y. Comparison of seven chemical pretreatments of corn straw for improving methane yield by anaerobic digestion. PLoS ONE 2014, 9, e93801. [Google Scholar] [CrossRef]
- Raud, M.; Rooni, V.; Kikas, T. The efficiency of nitrogen and flue gas as operating gases in explosive decompression pretreatment. Energies 2018, 11, 2074. [Google Scholar] [CrossRef]
- Fuess, L.T.; Garcia, M.L. Bioenergy from stillage anaerobic digestion to enhance the energy balance ratio of ethanol production. J. Environ. Manag. 2015, 162, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, M.; Abomohra, A.E.-F.; Ai, P.; Wang, D.; El-Mashad, H.M.; Zhang, Y. Biorefining of rice straw by sequential fermentation and anaerobic digestion for bioethanol and/or biomethane production: Comparison of structural properties and energy output. Bioresour. Technol. 2018, 268, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Calicioglu, O.; Brennan, R.A. Sequential ethanol fermentation and anaerobic digestion increases bioenergy yields from duckweed. Bioresour. Technol. 2018, 257, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Cestonaro do Amaral, A.; Kunz, A.; Radis Steinmetz, R.L.; Scussiato, L.A.; Tápparo, D.C.; Gaspareto, T.C. Influence of solid–liquid separation strategy on biogas yield from a stratified swine production system. J. Environ. Manag. 2016, 168, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Anjos, I.D.; Toneli, J.T.C.L.; Sagula, A.L.; Lucas Junior, J.d. Biogas production in dairy cattle systems, using batch digesters with and without solids separation in the substrates. Eng. Agric. 2017, 37, 426–432. [Google Scholar] [CrossRef]
- Owen, W.F.; Stuckey, D.C.; Healy, J.B.; Young, L.Y.; McCarty, P.L. Bioassay for monitoring biochemical methane potential and anaerobic toxicity. Water Res. 1979, 13, 485–492. [Google Scholar] [CrossRef]
- Luna-delRisco, M.; Normak, A.; Orupõld, K. Biochemical methane potential of different organic wastes and energy crops from Estonia. Agron. Res. 2011, 9, 331–342. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis of the Association of Official Analytical Chemists; AOAC International: Rockville, MD, USA, 1990; Volume 1. [Google Scholar]
- Agrawal, R.; Bhadana, B.; Mathur, A.S.; Kumar, R.; Gupta, R.P.; Satlewal, A. Improved enzymatic hydrolysis of pilot scale pretreated rice straw at high total solids loading. Energy Res. 2018, 6, 115. [Google Scholar] [CrossRef]
- Jingura, R.M.; Kamusoko, R. Methods for determination of biomethane potential of feedstocks: A review. Biofuel Res. J. 2017, 4, 573–586. [Google Scholar] [CrossRef]
- Meiramkulova, K.; Bayanov, A.; Ivanova, T.; Havrland, B.; Kára, J.; Hanzlíková, I. Effect of different compositions on anaerobic co-digestion of cattle manure and agro-industrial by-products. Agron. Res. 2018, 16, 176–187. [Google Scholar] [CrossRef]
- Xu, N.; Liu, S.; Xin, F.; Zhou, J.; Jia, H.; Xu, J.; Jiang, M.; Dong, W. Biomethane production from lignocellulose: biomass recalcitrance and its impacts on anaerobic digestion. Front. Bioeng. Biotechnol. 2019, 7, 191. [Google Scholar] [CrossRef] [PubMed]
- Drosg, B.; Fuchs, W.; Meixner, K.; Waltenberger, R.; Kirchmayr, R.; Braun, R.; Bochmann, G. Anaerobic digestion of stillage fractions–estimation of the potential for energy recovery in bioethanol plants. Water Sci. Technol. 2013, 67, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Town, J.; Annand, H.; Pratt, D.; Dumonceaux, T.; Fonstad, T. Microbial community composition is consistent across anaerobic digesters processing wheat-based fuel ethanol waste streams. Bioresour. Technol. 2014, 157, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Aslanzadeh, S. Pretreatment of Cellulosic Waste and High Rate Biogas Production. Ph.D. Thesis, University of Borås, Bross, Sweden, 2014. [Google Scholar]
- Fagbohungbe, M.O.; Dodd, I.C.; Herbert, B.M.J.; Li, H.; Ricketts, L.; Semple, K.T. High solid anaerobic digestion: Operational challenges and possibilities. Environ. Technol. Innov. 2015, 4, 268–284. [Google Scholar] [CrossRef]
- Lee, E.; Bittencourt, P.; Casimir, L.; Jimenez, E.; Wang, M.; Zhang, Q.; Ergas, S.J. Biogas production from high solids anaerobic co-digestion of food waste, yard waste and waste activated sludge. Waste Manag. 2019, 95, 432–439. [Google Scholar] [CrossRef] [PubMed]
- Miller, K. Solid–Liquid Separation Technologies in the Conversion of Bagasse to Liquid Fuel. Master’s Thesis, Louisiana State University, Baton Rouge, LA, USA, 2010. [Google Scholar]




| Fraction | Pretreatment | Variable | TS | VS |
|---|---|---|---|---|
| g/kg | g/kgTS | |||
| - | - | Untreated | 931 ± 0 | 963 ± 0 |
| Liquid Fraction | NED | Pretreated | 18.0 ± 0.07 | 997 ± 0 |
| Hydrolyzed | 34.2 ± 0.09a | 997 ± 0 | ||
| Fermented | 20.1 ± 0.8 | 997 ± 0 b | ||
| Sidestream | 23.4 ± 0.7a | 997 ± 0 b | ||
| Flue Gas | Pretreated | 17.1 ± 0.1 | 997 ± 0 | |
| Hydrolyzed | 37.8 ± 0.1 a | 997 ± 0 b | ||
| Fermented | 19.7 ± 0.4 | 997 ± 0 | ||
| Sidestream | 19.7 ± 0.7 | 997 ± 0 | ||
| Solid Fraction | NED | Pretreated | 118 ± 0 | 996 ± 0 b |
| Hydrolyzed | 139 ± 2 | 995 ± 0 b | ||
| Fermented | 123 ± 1 | 995 ± 0 | ||
| Sidestream | 128 ± 6 | 995 ± 0 | ||
| Flue Gas | Pretreated | 125 ± 4 | 995 ± 1 b | |
| Hydrolyzed | 144 ± 2 | 995 ± 0 | ||
| Fermented | 116 ± 3 a | 995 ± 0 b | ||
| Sidestream | 113 ± 3 a | 996 ± 0 b |
| Glucose (g/L) | Xylose (g/L) | Glycerol (g/L) | Acetic Acid (g/L) | Ethanol (g/L) | ||
|---|---|---|---|---|---|---|
| NED | Pretreated | 0.48 ± 0.02 | 0.6 ± 0.4 | <0.25 a | 1.53 ± 0.03 | - |
| Hydrolyzed | 13.7 ± 0.8 | 4.06 ± 0.18 | <0.25 a | 1.81 ± 0.01 | 1.15 ± 0.05 | |
| Fermented | 0.25 ± 0.09 | 3.6 ± 0.4 | 0.7 ± 0.1 | 2.2 ± 0.2 | 6.3 ± 0.0 | |
| Sidestream | 0.8 ± 0.3 | 3.8 ± 0.6 | 0.71 ± 0.06 | 2.6 ± 0.3 | 8.4 ± 0.7 | |
| Flue Gas | Pretreated | 0.64 ± 0.14 | <0.25 a | <0.25 a | 1.25 ± 0.07 | - |
| Hydrolyzed | 15.1 ± 1.7 | 4.1 ± 0.3 | <0.25 a | 1.62 ± 0.00 | 0.60 ± 0.00 | |
| Fermented | <0.25 a | 2.1 ± 0.2 | 0.53 ± 0.08 | 1.7 ± 0.3 | 4.4 ± 1.3 | |
| Sidestream | 0.39 ± 0.04 | 4.1 ± 0.6 | 0.79 ± 0.06 | 2.3 ± 0.3 | 7.3 ± 0.7 |
| Fraction | Pretreatment | Variable | Bmax mol CH4/100 g |
|---|---|---|---|
| Untreated | - | - | 0.91 ± 0.02 a,g,h |
| Liquid Fraction | NED | Pretreated | 0.19 ± 0.00 e |
| Hydrolyzed | 0.46 ± 0.00 e,f | ||
| Fermented | 0.53 ± 0.01 g | ||
| Sidestream | 0.58 ± 0.01 g,h | ||
| Flue Gas | Pretreated | 0.17 ± 0.00 e | |
| Hydrolyzed | 0.49 ± 0.01 f,g,l | ||
| Fermented | 0.49 ± 0.01 f,l,m | ||
| Sidestream | 0.52 ± 0.01 g,h,l,m | ||
| Solid Fraction | NED | Pretreated | 1.3 ± 0.03 a |
| Hydrolyzed | 1.5 ± 0.02 b | ||
| Fermented | 1.6 ± 0.02 b,c | ||
| Sidestream | 1.7 ± 0.02 b,c,d | ||
| Flue Gas | Pretreated | 1.4 ± 0.04 b,i | |
| Hydrolyzed | 1.6 ± 0.07 b,d,j | ||
| Fermented | 1.5 ± 0.02 a,b,c,i,j,k | ||
| Sidestream | 1.3 ± 0.03 a,i,k |
| Fraction | Pretreatment | Variable | 85% Bmax | 95% Bmax | ||
|---|---|---|---|---|---|---|
| mol CH4/100 g | Days | mol CH4/100 g | Days | |||
| Untreated | - | - | 0.88 | 14.0 | 0.98 | 21.7 |
| Liquid Fraction | NED | Pretreated | 0.16 | 2.6 | 0.18 | 4.1 |
| Hydrolyzed | 0.39 | 2.8 | 0.44 | 4.5 | ||
| Fermented | 0.45 | 2.4 | 0.51 | 3.8 | ||
| Sidestream | 0.49 | 2.4 | 0.55 | 3.6 | ||
| Flue Gas | Pretreated | 0.14 | 2.6 | 0.16 | 4.1 | |
| Hydrolyzed | 0.42 | 3.0 | 0.47 | 4.8 | ||
| Fermented | 0.42 | 2.4 | 0.47 | 3.6 | ||
| Sidestream | 0.45 | 2.3 | 0.50 | 3.5 | ||
| Solid Fraction | NED | Pretreated | 1.1 | 12.5 | 1.3 | 18.9 |
| Hydrolyzed | 1.3 | 10.2 | 1.5 | 16.1 | ||
| Fermented | 1.4 | 10.2 | 1.6 | 16.1 | ||
| Sidestream | 1.4 | 10.5 | 1.6 | 16.6 | ||
| Flue Gas | Pretreated | 1.2 | 12.0 | 1.3 | 18.6 | |
| Hydrolyzed | 1.4 | 9.9 | 1.6 | 15.6 | ||
| Fermented | 1.2 | 10.6 | 1.4 | 16.7 | ||
| Sidestream | 1.1 | 11.5 | 1.3 | 18.1 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha-Meneses, L.; Ferreira, J.A.; Bonturi, N.; Orupõld, K.; Kikas, T. Enhancing Bioenergy Yields from Sequential Bioethanol and Biomethane Production by Means of Solid–Liquid Separation of the Substrates. Energies 2019, 12, 3683. https://doi.org/10.3390/en12193683
Rocha-Meneses L, Ferreira JA, Bonturi N, Orupõld K, Kikas T. Enhancing Bioenergy Yields from Sequential Bioethanol and Biomethane Production by Means of Solid–Liquid Separation of the Substrates. Energies. 2019; 12(19):3683. https://doi.org/10.3390/en12193683
Chicago/Turabian StyleRocha-Meneses, Lisandra, Jorge A Ferreira, Nemailla Bonturi, Kaja Orupõld, and Timo Kikas. 2019. "Enhancing Bioenergy Yields from Sequential Bioethanol and Biomethane Production by Means of Solid–Liquid Separation of the Substrates" Energies 12, no. 19: 3683. https://doi.org/10.3390/en12193683
APA StyleRocha-Meneses, L., Ferreira, J. A., Bonturi, N., Orupõld, K., & Kikas, T. (2019). Enhancing Bioenergy Yields from Sequential Bioethanol and Biomethane Production by Means of Solid–Liquid Separation of the Substrates. Energies, 12(19), 3683. https://doi.org/10.3390/en12193683









