Effect of Biodiesel Mixture Derived from Waste Frying-Corn, Frying-Canola-Corn and Canola-Corn Cooking Oils with Various Ages on Physicochemical Properties
Abstract
:1. Introduction
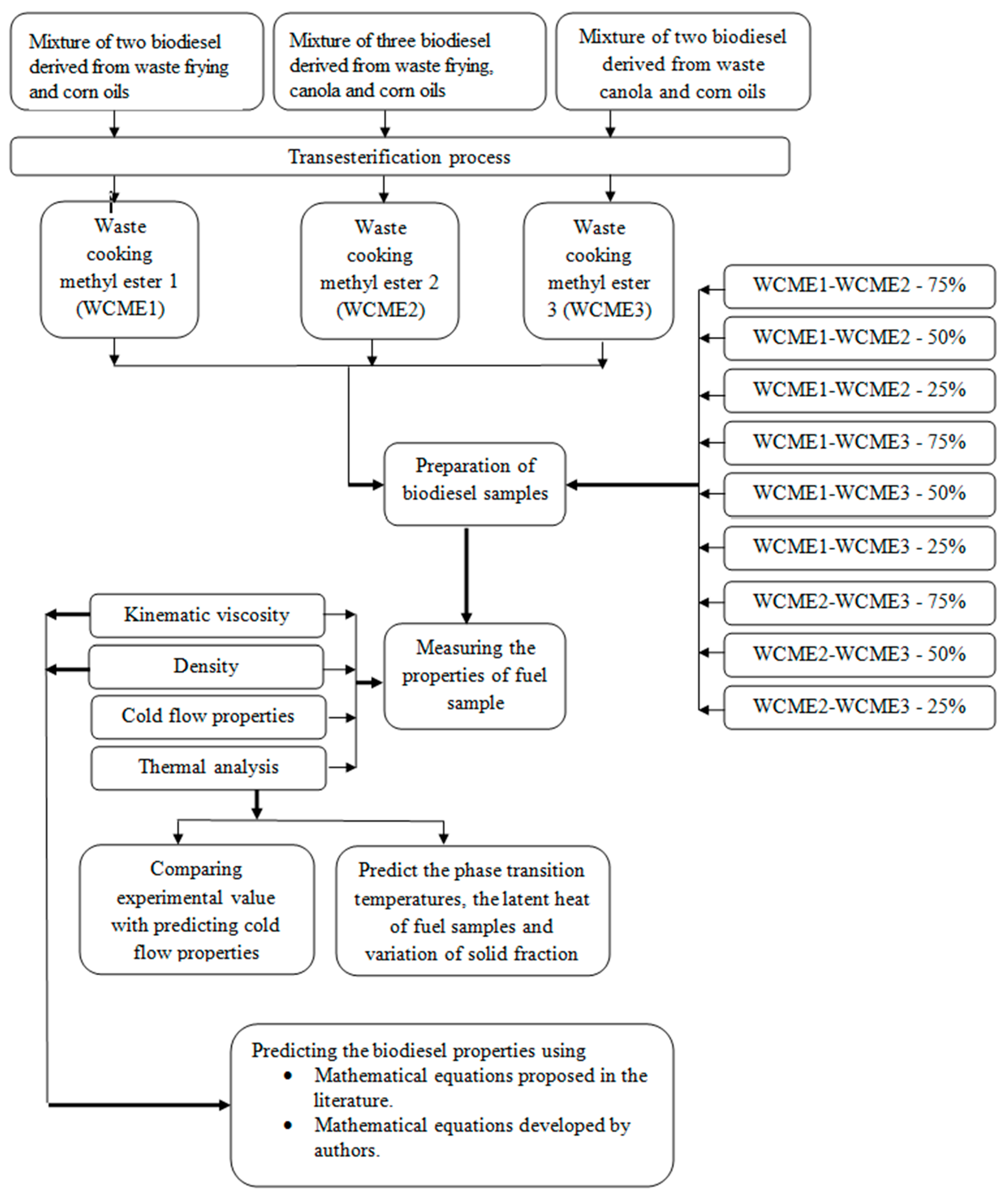
2. Materials and Methods
2.1. Biodiesel Sample Preparation
- WCME1 was prepared by mixing 50 vol% WFME and 50 vol% WCAME.
- WCME2 was obtained by blending 35 vol% WFME, 35 vol% WCAME and 35 vol% WCOME.
- WCME3 was the blended using50 vol% CAME and 50 vol% WCOME.
2.2. Storage Test Procedures
2.3. Kinematic Viscosity and Density Measurement
2.4. Cold Flow Measurement
2.5. Computer-Aided Cooling Curve Thermal Analysis (CA-CCA)
2.6. Oxidative Stability and Acid Value
2.7. Empirical Models
- Equations (5), (6) and (17)–(21): viscosity () correlations as a function of temperature (T) only.
- Equations (12) and (14)–(16): viscosity () correlations as a function of volume fraction (VF) only.
- Equations (11), (22) and (27)–(31): viscosity () correlations as a function of temperature and volume fraction.
- Equations (7) and (9): viscosity () correlations as a function of temperature and viscosity of pure fuels.
- Equations (13) and (26): viscosity () correlations as a function of volume fraction and viscosity of pure fuels.
- Equations (32) and (35)–(37): density () correlations as a function of temperature (T) only.
- Equations (34) and (38)–(40): density () correlations as a function of temperature and volume fraction.
- Equation (33): density () correlations as a function of volume fraction and viscosity of pure fuels.
- Equations (41) and (42): viscosity as a function of density.
- Equations (43) and (44): density as a function of viscosity.
3. Results and Discussion
3.1. Effect of Storage Period on WCME1 and WCME2 Properties
3.1.1. Kinematic Viscosity and Density of WCME1 and WCME2
3.1.2. Cold Flow Properties of WCME1 and WCME2
3.1.3. Oxidative Stability and an Acid Value of WCME1 and WCME2
3.2. Analyzing the Properties of Biodiesel Mixture
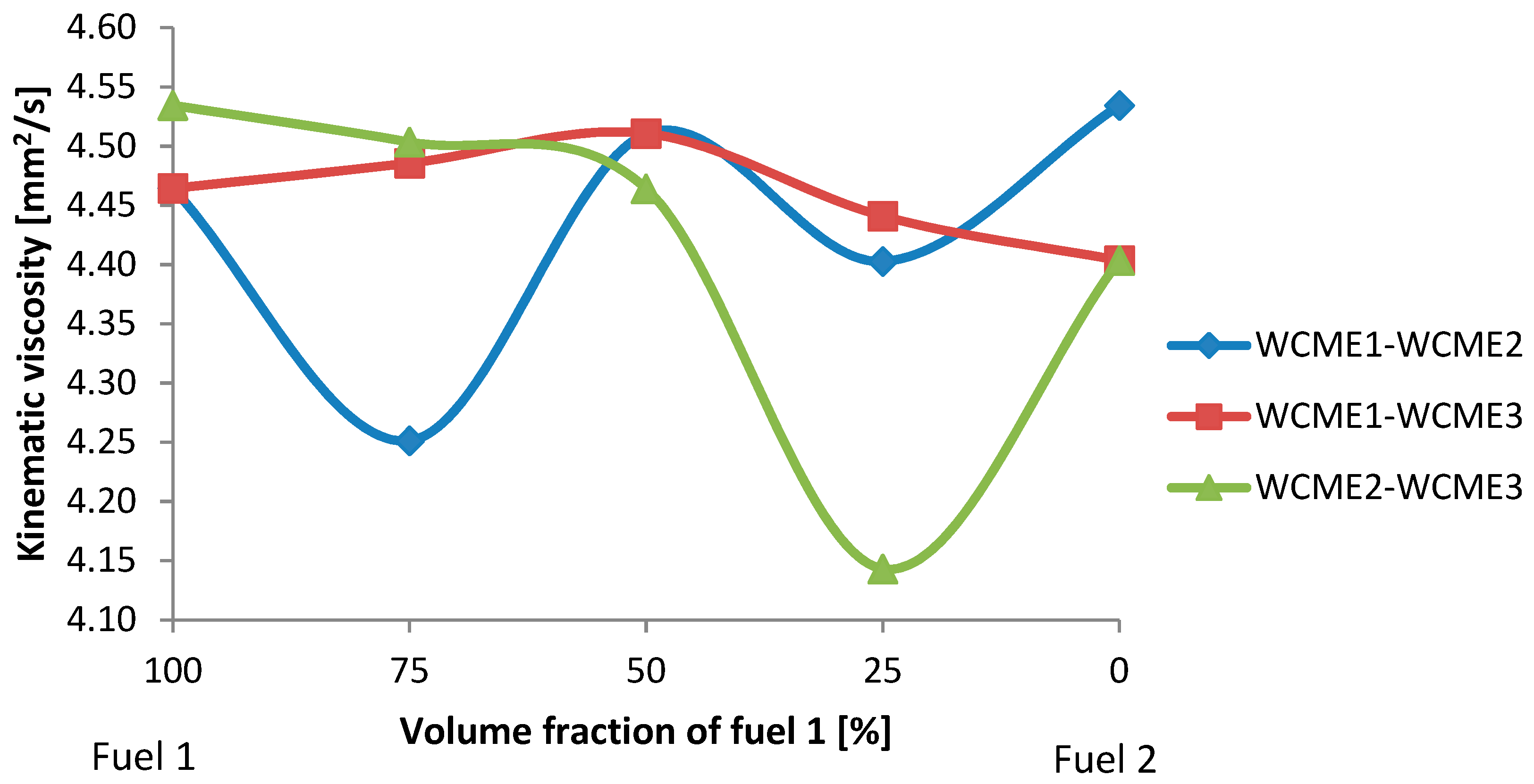
3.2.1. Kinematic Viscosities
- For WCME1-WCME2; the kinematic viscosities of pure WCME1 and WCME2 were higher than the viscosity of the blends. Additionally, WCME1-WCME2-50% had the highest value of viscosity compared to WCME1-WCME2-75% and WCME1-WCME2-25%.
- For WCME1-WCME3; based on the results, it was found that the increasing percentage amount of WCME2 WCME1-WCME3 blend (from 0% to 50%) increases the kinematic viscosity of biodiesel blends almost linearly, i.e., WCME1-WCME3-50% had the highest maximum kinematic viscosity compared to the other samples.
- For WCME2-WCME3; it was observed that increasing the amount of WCME3 leads to a decrease in the kinematic viscosity of WCME2-WCME3, i.e., WCME2-WCME3-25% had the minimum kinematic viscosity compared to the other samples.
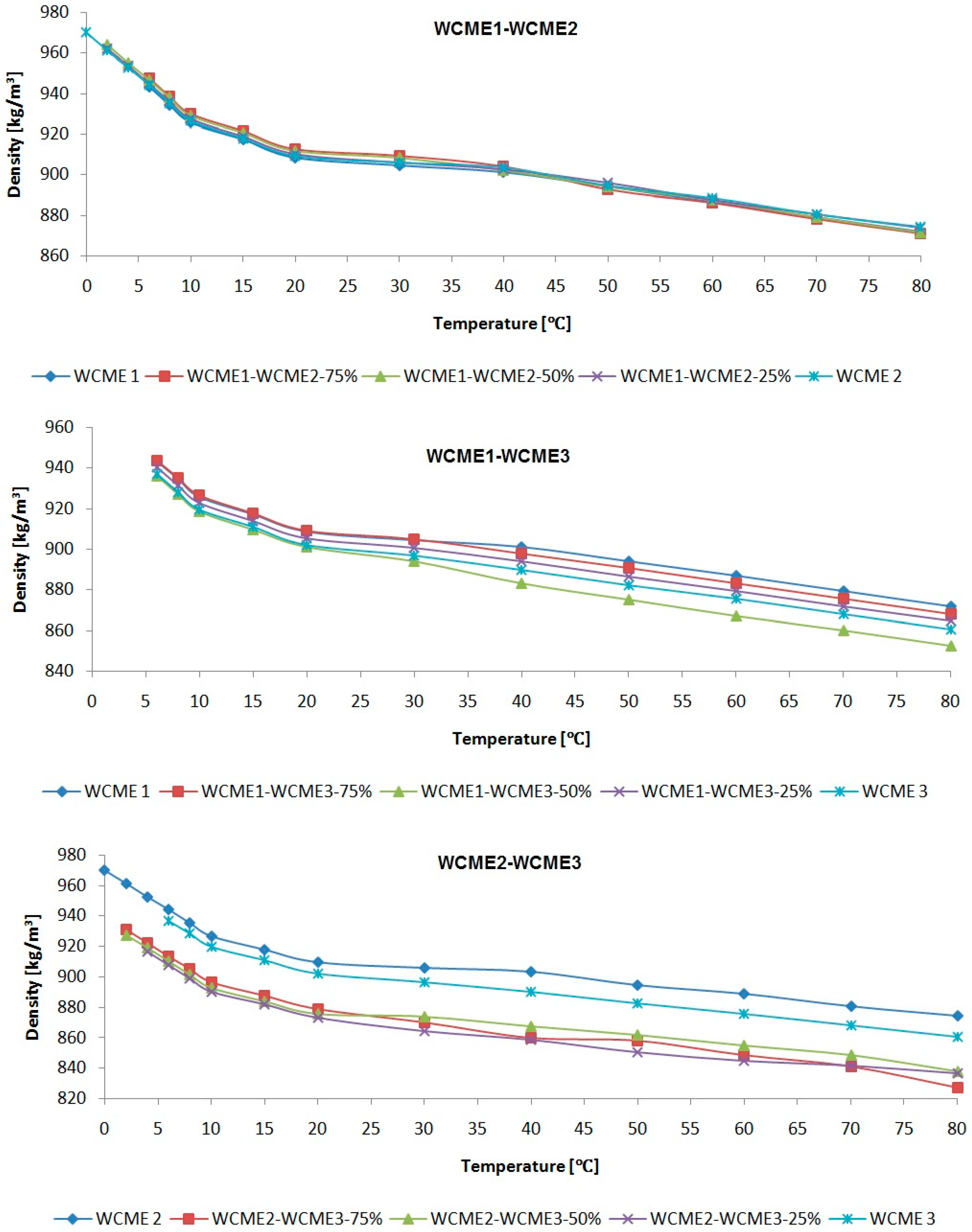
3.2.2. Density of Biodiesel
3.2.3. Kinematic Viscosity and Density Phenomenon
3.2.4. Cold Flow Properties of Biodiesel Samples
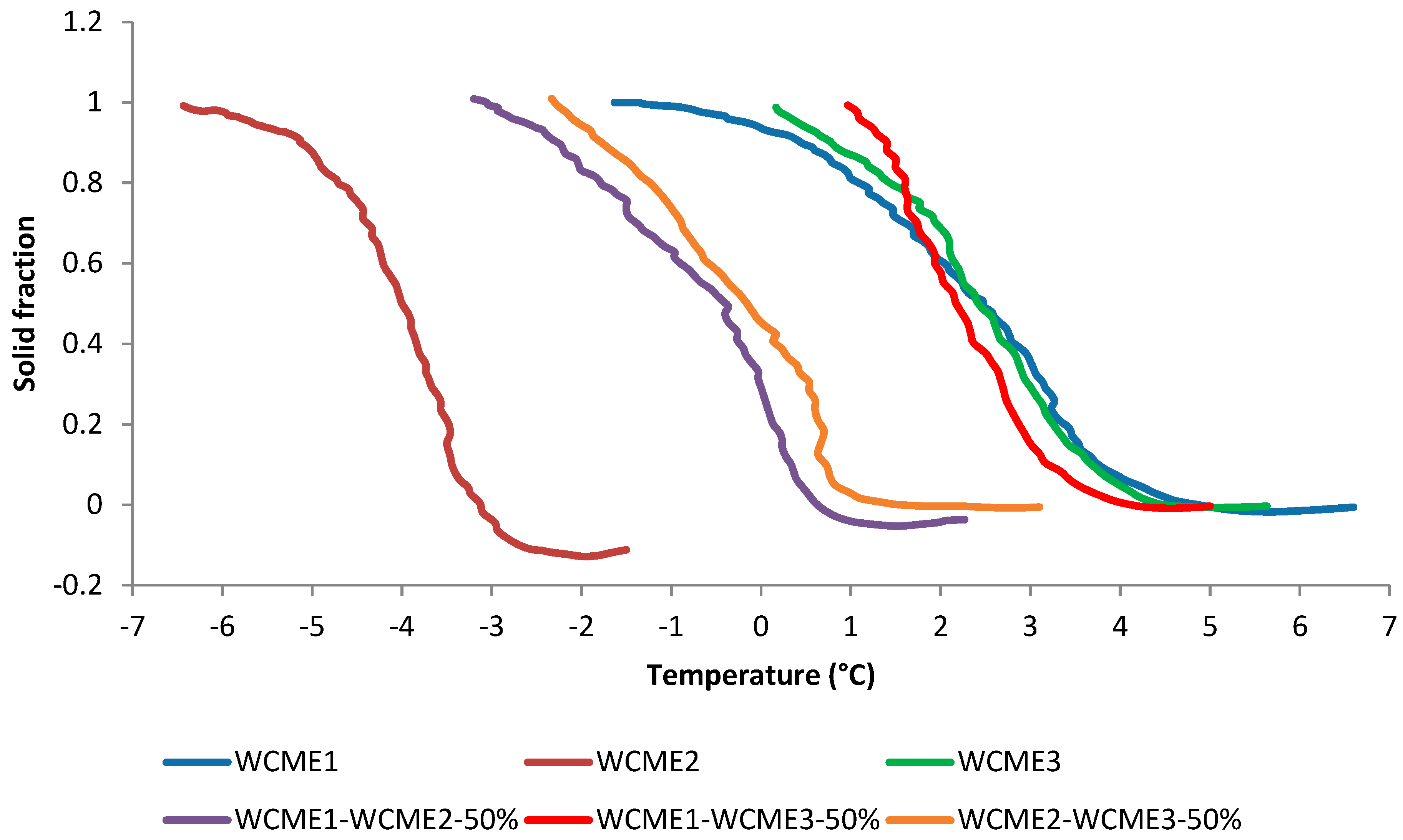
3.2.5. Computer-Aided Cooling Curve Analysis of Biodiesel Samples
3.3. Biodiesel Correlations
3.3.1. Kinematic Viscosity Correlations
3.3.2. Density Correlations
3.3.3. Kinematic Viscosity—Density Correlations
3.3.4. Empirical Modeling of Kinematic Viscosity and Density Developed by Authors
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Babaki, M.; Yousefi, M.; Habibi, Z.; Mohammadi, M. Process optimization for biodiesel production from waste cooking oil using multi−enzyme systems through response surface methodology. Renew. Energy 2017, 105, 465–472. [Google Scholar] [CrossRef]
- Oumer, A.; Hasan, M.; Baheta, A.T.; Mamat, R.; Abdullah, A. Bio−based liquid fuels as a source of renewable energy: A review. Renew. Sustain. Energy Rev. 2018, 88, 82–98. [Google Scholar] [CrossRef]
- Melo−Espinosa, E.A.; Piloto−Rodríguez, R.; Goyos−Pérez, L.; Sierens, R.; Verhelst, S. Emulsification of animal fats and vegetable oils for their use as a diesel engine fuel: An overview. Renew. Sustain. Energy Rev. 2015, 47, 623–633. [Google Scholar] [CrossRef]
- Bayındır, H.; Işık, M.Z.; Argunhan, Z.; Yücel, H.L.; Aydın, H. Combustion, performance, and emissions of a diesel power generator fueled with biodiesel−kerosene and biodiesel−kerosene−diesel blends. Energy 2017, 123, 241–251. [Google Scholar] [CrossRef]
- Knothe, G.; Razon, L.F. Biodiesel fuels. Prog. Energy Combust. Sci. 2017, 58, 36–59. [Google Scholar] [CrossRef]
- Aldhaidhawi, M.; Chiriac, R.; Bădescu, V.; Descombes, G.; Podevin, P. Investigation on the mixture formation, combustion characteristics and performance of a Diesel engine fueled with Diesel, Biodiesel B20 and hydrogen addition. Int. J. Hydrog. Energy 2017, 42, 16793–16807. [Google Scholar] [CrossRef] [Green Version]
- Dehaghani, A.H.; Rahimi, R. An experimental study of diesel fuel cloud and pour point reduction using different additives. Petroleum 2018. [CrossRef]
- Ghaly, A.E.; Dave, D. Production of Biodiesel by Enzymatic Transesterification: Review. Am. J. Biochem. Biotechnol. 2010, 6, 54–76. [Google Scholar] [CrossRef]
- Raman, L.A.; Deepanraj, B.; Rajakumar, S.; Sivasubramanian, V. Experimental investigation on performance, combustion and emission analysis of a direct injection diesel engine fuelled with rapeseed oil biodiesel. Fuel 2019, 246, 69–74. [Google Scholar] [CrossRef]
- Asokan, M.; Senthur Prabu, S.; Bade, P.K.; Nekkanti, V.M.; Gutta, S.S. Performance, combustion and emission characteristics of juliflora biodiesel fuelled DI diesel engine. Energy 2019, 173, 883–892. [Google Scholar] [CrossRef]
- Malvade, A.V.; Satpute, S.T. Production of Palm Fatty Acid Distillate Biodiesel and Effects of its Blends on Performance of Single Cylinder Diesel Engine. Procedia Eng. 2013, 64, 1485–1494. [Google Scholar] [CrossRef] [Green Version]
- Goga, G.; Chauhan, B.S.; Mahla, S.K.; Cho, H.M. Performance and emission characteristics of diesel engine fueled with rice bran biodiesel and n−butanol. Energy Rep. 2019, 5, 78–83. [Google Scholar] [CrossRef]
- Rajak, U.; Nashine, P.; Verma, T.N. Assessment of diesel engine performance using spirulina microalgae biodiesel. Energy 2019, 166, 1025–1036. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M. Impact of cold flow properties of biodiesel on engine performance. Renew. Sustain. Energy Rev. 2014, 31, 650–656. [Google Scholar] [CrossRef]
- Monirul, I.M.; Masjuki, H.H.; Kalam, M.A.; Zulkifli, N.W.; Rashedul, H.K.; Rashed, M.M.; Imdadul, H.K.; Mosarof, M.H. A comprehensive review on biodiesel cold flow properties and oxidation stability along with their improvement processes. Rsc Adv. 2015, 5, 86631–86655. [Google Scholar] [CrossRef]
- Magalhães, A.M.; Pereira, E.; Meirelles, A.J.; Sampaio, K.A.; Maximo, G.J. Proposing blends for improving the cold flow properties of ethylic biodiesel. Fuel 2019, 253, 50–59. [Google Scholar] [CrossRef]
- Bhale, P.V.; Deshpande, N.V.; Thombre, S.B. Experimental Investigations on Lubricity and Cold Flow Properties of Biodiesel. In Proceedings of the ASME 2008 Internal Combustion Engine Division Spring Technical Conference, Chicago, IL, USA, 27–30 April 2008. [Google Scholar] [CrossRef]
- Nainwal, S.; Sharma, N.; Sharma, A.S.; Jain, S.; Jain, S. Cold flow properties improvement of Jatropha curcas biodiesel and waste cooking oil biodiesel using winterization and blending. Energy 2015, 89, 702–707. [Google Scholar] [CrossRef]
- DeMarini, D.M.; Mutlu, E.; Warren, S.H.; King, C.; Gilmour, M.I.; Linak, W.P. Mutagenicity emission factors of canola oil and waste vegetable oil biodiesel: Comparison to soy biodiesel. Mutat. Res./Genet. Toxicol. Environ. Mutagen. 2019. [Google Scholar] [CrossRef]
- Fonseca, J.M.; Teleken, J.G.; De Cinque Almeida, V.; Da Silva, C. Biodiesel from waste frying oils: Methods of production and purification. Energy Convers. Manag. 2019, 184, 205–218. [Google Scholar] [CrossRef]
- Silva Filho, S.C.; Miranda, A.C.; Silva, T.A.; Calarge, F.A.; Souza, R.R.; Santana, J.C.; Tambourgi, E.B. Environmental and techno−economic considerations on biodiesel production from waste frying oil in São Paulo city. J. Clean. Prod. 2018, 183, 1034–1042. [Google Scholar] [CrossRef]
- Fawaz, E.G.; Salam, D.A. Preliminary economic assessment of the use of waste frying oils for biodiesel production in Beirut, Lebanon. Sci. Total Environ. 2018, 637–638, 1230–1240. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Bux, F.; Sharma, Y.C. A low cost one pot synthesis of biodiesel from waste frying oil (WFO) using a novel material, β−potassium dizirconate (β−K 2 Zr 2 O 5 ). Appl. Energy 2016, 172, 23–33. [Google Scholar] [CrossRef]
- Talebian−Kiakalaieh, A.; Amin, N.A.; Mazaheri, H. A review on novel processes of biodiesel production from waste cooking oil. Appl. Energy 2013, 104, 683–710. [Google Scholar] [CrossRef]
- Yaakob, Z.; Mohammad, M.; Alherbawi, M.; Alam, Z.; Sopian, K. Overview of the production of biodiesel from Waste cooking oil. Renewable and Sustainable Energy Rev. 2013, 18, 184–193. [Google Scholar] [CrossRef]
- Farooq, M.; Ramli, A.; Naeem, A. Biodiesel production from low FFA waste cooking oil using heterogeneous catalyst derived from chicken bones. Renew. Energy 2015, 76, 362–368. [Google Scholar] [CrossRef]
- Sahar Sadaf, S.; Iqbal, J.; Ullah, I.; Bhatti, H.N.; Nouren, S.; Nisar, J.; Iqbal, M. Biodiesel production from waste cooking oil: An efficient technique to convert waste into biodiesel. Sustain. Cities Soc. 2018, 41, 220–226. [Google Scholar] [CrossRef]
- Atapour, M.; Kariminia, H.; Moslehabadi, P.M. Optimization of biodiesel production by alkali−catalyzed transesterification of used frying oil. Process Saf. Environ. Prot. 2014, 92, 179–185. [Google Scholar] [CrossRef]
- Vieitez, I.; Callejas, N.; Irigaray, B.; Pinchak, Y.; Merlinski, N.; Jachmanián, I.; Grompone, M.A. Acid Value, Polar Compounds and Polymers as Determinants of the Efficient Conversion of Waste Frying Oils to Biodiesel. J. Am. Oil Chem. Soc. 2013, 91, 655–664. [Google Scholar] [CrossRef]
- Kassem, Y.; Çamur, H. Effects of storage under different conditions on the fuel properties of biodiesel mixture derived from waste frying and canola oils. Biomass Convers. Biorefinery 2018, 8, 825–845. [Google Scholar] [CrossRef]
- Kassem, Y.; Çamur, H. A Laboratory Study of the Effects of Wide Range Temperature on the Properties of Biodiesel Produced from Various Waste Vegetable Oils. Waste Biomass Valorization 2017, 8, 1995–2007. [Google Scholar] [CrossRef]
- ASTM. ASTM D445—09. Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity); ASTM International: West Conshohocken, PA, USA, 2009. [Google Scholar]
- ASTM. ASTM D854—14. Standard Test Methods for Specific Gravity of Soil Solids by Water Pycnometer; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM. ASTM D2500—17a. Standard Test Method for Cloud Point of Petroleum Products, ASTM, West Conshohocken; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM. ASTM D97—17b. Standard Test Method for Pour Point of Petroleum Products. West Conshohocken; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM. ASTM D6371. Standard Test Method for Cold Filter Plugging Point of Diesel and Heating Fuels; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Evcil, A.; Al−Shanableh, F.; Savas, M.A. Variation of solid fraction with cold flow properties of biodiesel produced from waste frying oil. Fuel 2018, 215, 522–527. [Google Scholar] [CrossRef]
- Emadi, D.; Whiting, L.V.; Djurdjevic, M.; Kierkus, W.T.; Sokolowski, J. Comparison of Newtonian and Fourier thermal analysis techniques for calculation of latent heat and solid fraction of aluminum alloys. Metall. J. Metall. 2004, 10, 91–106. [Google Scholar] [CrossRef] [Green Version]
- Sudheer, R.; Prabhu, K. A Computer Aided Cooling Curve Analysis method to study phase change materials for thermal energy storage applications. Mater. Des. 2016, 95, 198–203. [Google Scholar] [CrossRef]
- Tate, R.; Watts, K.; Allen, C.; Wilkie, K. The viscosities of three biodiesel fuels at temperatures up to 300 °C. Fuel 2006, 85, 1010–1015. [Google Scholar] [CrossRef]
- Tat, M.E.; Gerpen, J.H. The kinematic viscosity of biodiesel and its blends with diesel fuel. J. Am. Oil Chem. Soc. 1999, 76, 1511–1513. [Google Scholar] [CrossRef]
- Yuan, W.; Hansen, A.C.; Zhang, Q.; Tan, Z. Temperature−dependent kinematic viscosity of selected biodiesel fuels and blends with diesel fuel. J. Am. Oil Chem. Soc. 2005, 82, 195–199. [Google Scholar] [CrossRef]
- Moradi, G.R.; Karami, B.; Mohadesi, M. Densities and Kinematic Viscosities in Biodiesel–Diesel Blends at Various Temperatures. J. Chem. Eng. Data 2012, 58, 99–105. [Google Scholar] [CrossRef]
- Mejía, J.; Salgado, N.; Orrego, C. Effect of blends of Diesel and Palm−Castor biodiesels on viscosity, cloud point and flash point. Ind. Crop. Prod. 2013, 43, 791–797. [Google Scholar] [CrossRef]
- Alptekin, E.; Canakci, M. Determination of the density and the viscosities of biodiesel–diesel fuel blends. Renew. Energy 2008, 33, 2623–2630. [Google Scholar] [CrossRef]
- Grunberg, L.; Nissan, A. Mixture Law for Viscosity. Nature 1949, 164, 799–800. [Google Scholar] [CrossRef]
- Gülüm, M.; Bilgin, A. Measurements and empirical correlations in predicting biodiesel-diesel blends’ viscosity and density. Fuel 2017, 199, 567–577. [Google Scholar] [CrossRef]
- Kassem, Y.; Aktuğ, B.; Ghisher, M.; Çamur, H. Measurements, Correlations and Comparison of Biodiesel Blend Properties with three Commercial Diesel Fuels, Kerosene and Benzene. Int. J. Appl. Eng. Res. 2018, 13, 7019–7032. [Google Scholar]
- Kendall, J.; Monroe, K.P. The Viscosity of Liquids. Ii. The Viscosity−Composition Curve for Ideal Liquid Mixtures.1. J. Am. Chem. Soc. 1917, 39, 1787–1802. [Google Scholar] [CrossRef]
- Kanaveli, I.; Atzemi, M.; Lois, E. Predicting the viscosity of diesel/biodiesel blends. Fuel 2017, 199, 248–263. [Google Scholar] [CrossRef]
- Tesfa, B.; Mishra, R.; Gu, F.; Powles, N. Prediction models for density and viscosity of biodiesel and their effects on fuel supply system in CI engines. Renew. Energy 2010, 35, 2752–2760. [Google Scholar] [CrossRef] [Green Version]
- Ramírez-Verduzco, L.F.; García−Flores, B.E.; Rodríguez−Rodríguez, J.E.; Jaramillo−Jacob, A.D. Prediction of the density and viscosity in biodiesel blends at various temperatures. Fuel 2011, 90, 1751–1761. [Google Scholar] [CrossRef]
- Tat, M.E.; Gerpen, J.H. The specific gravity of biodiesel and its blends with diesel fuel. J. Am. Oil Chem. Soc. 2000, 77, 115–119. [Google Scholar] [CrossRef]
- Pratas, M.J.; Freitas, S.V.; Oliveira, M.B.; Monteiro, S.C.; Lima, A.S.; Coutinho, J.A. Biodiesel Density: Experimental Measurements and Prediction Models. Energy Fuels 2011, 25, 2333–2340. [Google Scholar] [CrossRef]
- Gülüm, M.; Bilgin, A. Density, flash point and heating value variations of corn oil biodiesel–diesel fuel blends. Fuel Process. Technol. 2015, 134, 456–464. [Google Scholar] [CrossRef]
- Fahd, M.E.; Lee, P.; Chou, S.K.; Wenming, Y.; Yap, C. Experimental study and empirical correlation development of fuel properties of waste cooking palm biodiesel and its diesel blends at elevated temperatures. Renew. Energy 2014, 68, 282–288. [Google Scholar] [CrossRef]
- Ramírez Verduzco, L.F. Density and viscosity of biodiesel as a function of temperature: Empirical models. Renew. Sustain. Energy Rev. 2013, 19, 652–665. [Google Scholar] [CrossRef]
- Rodenbush, C.M.; Hsieh, F.H.; Viswanath, D.S. Density and viscosity of vegetable oils. J. Am. Oil Chem. Soc. 1999, 76, 1415–1419. [Google Scholar] [CrossRef]
- BSI. Automotive Fuels—Fatty Acid Methyl Esters (FAME) for Diesel Engines—Requirements and Test Methods; EN−14214; BSI: London, UK, 2007. [Google Scholar]
- BSI. Fat and Oil Derivatives. Fatty Acid Methyl esters (FAME). Determination of Oxidation Stability (Accelerated Oxidation Test); EN 14112; BSI: London, UK, 2003. [Google Scholar]
- Moser, B.R. Influence of extended storage on fuel properties of methyl esters prepared from canola, palm, soybean and sunflower oils. Renew. Energy 2011, 36, 1221–1226. [Google Scholar] [CrossRef]
- Knothe, G. Improving biodiesel fuel properties by modifying fatty ester composition. Energy Environ. Sci. 2009, 2, 759. [Google Scholar] [CrossRef]
- ASTM. ASTM D664−17a. Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Bouaid, A.; Martinez, M.; Aracil, J. Long storage stability of biodiesel from vegetable and used frying oils. Fuel 2007, 86, 2596–2602. [Google Scholar] [CrossRef]
- Jose, T.K.; Anand, K. Effects of biodiesel composition on its long term storage stability. Fuel 2016, 177, 190–196. [Google Scholar] [CrossRef]
- Rawajfeh, K.; Al-Hamamre, Z. Study on the viscosity of jojoba oil blends with biodiesel or petroleum diesel. Energy Sources Part A Recovery Util. Environ. Eff. 2016, 38, 3290–3299. [Google Scholar] [CrossRef]
- Machado, M.; Zuvanov, V.; Rojas, E.; Zuniga, A.; Costa, B. Thermo physical Properties of Biodiesel Obtained from Vegetable Oils: Corn, Soy, Canola and Sunflower. Encicl. Biosf. 2012, 8, 917. [Google Scholar]
- Moradi, G.; Mohadesi, M.; Karami, B.; Moradi, R. Using Artificial Neural Network for Estimation of Density and Viscosities of Biodiesel–Diesel Blends. J. Chem. Pet. Eng. 2015, 49, 153–165. [Google Scholar]
- Amin, A.; Gadallah, A.; Morsi, A.E.; El−Ibiari, N.; El−Diwani, G. Experimental and empirical study of diesel and castor biodiesel blending effect, on kinematic viscosity, density and calorific value. Egypt. J. Pet. 2016, 25, 509–514. [Google Scholar] [CrossRef] [Green Version]






| wt% | Pure Biodiesel | Mixture of Biodiesel | ||||
|---|---|---|---|---|---|---|
| WFME (0 months) | WCAME (0 months) | WCOME (0 months) | WCME1 (8 months) | WCME2 (30 months) | WCME3 (0 months) | |
| C8:0 | 0.05 | 0.00 | 0.29 | 0.00 | 0.00 | 0.15 |
| C10:0 | 0.33 | 0.00 | 0.32 | 0.00 | 0.00 | 0.16 |
| C12:0 | 1.18 | 0.08 | 4.03 | 0.10 | 0.18 | 2.06 |
| C14:0 | 0.10 | 0.00 | 2.10 | 0.70 | 0.67 | 1.05 |
| C16:0 | 36.29 | 13.50 | 13.73 | 24.80 | 28.53 | 13.62 |
| C16:1 | 0.00 | 0.00 | 0.90 | 1.00 | 0.32 | 0.45 |
| C16:2 | 0.00 | 0.00 | 0.00 | 0.20 | 0.00 | 0.00 |
| C17:0 | 0.00 | 0.00 | 0.00 | 0.10 | 0.00 | 0.00 |
| C18:0 | 4.04 | 2.19 | 5.10 | 5.10 | 3.91 | 3.65 |
| C18:1 | 40.30 | 57.33 | 46.17 | 41.60 | 44.36 | 51.75 |
| C18:2 | 17.53 | 20.47 | 20.10 | 22.30 | 18.58 | 20.28 |
| C18:3 | 0.18 | 5.29 | 5.10 | 2.90 | 2.70 | 5.20 |
| C20:0 | 0.00 | 0.35 | 0.53 | 0.40 | 0.35 | 0.44 |
| C20:1 | 0.00 | 0.78 | 0.76 | 0.40 | 0.40 | 0.77 |
| C22:0 | 0.00 | 0.00 | 0.00 | 0.10 | 0.00 | 0.00 |
| MUFAMEs | 40.30 | 58.11 | 47.83 | 43.00 | 45.08 | 52.97 |
| PUFAMEs | 17.71 | 25.76 | 25.20 | 25.20 | 21.28 | 25.48 |
| SFAMEs | 41.61 | 16.12 | 25.49 | 31.20 | 33.64 | 20.82 |
| MUFAMEs | Monounsaturated FAMEs; | |||||
| PUFAMEs | Polyunsaturated FAMEs; | |||||
| SFAMEs | Saturated FAMEs; | |||||
| Property | Unit | Test Method | Limits | WFME | WCAME | WCOME |
| Kinematic viscosity at 40 °C | mm2/s | ASTM D445 | 1.9–6.0 | 4.67 | 4.68 | 4.35 |
| Density at 15 °C | kg/m3 | ASTM D854 | 867 min. | 876.4 | 895.8 | 912.7 |
| Cloud Point | °C | ASTM D2500 | Report | 16.5 | −1.0 | −7.5 |
| Cold Filter Plugging Point | °C | ASTM D6371 | Report | 7.4 | −7.5 | −8.0 |
| Pour Point | °C | ASTM D97 | Report | 10.5 | −11.0 | −12.0 |
| Acid value | mg KOH/g | ASTM D664 | 0.5 max. | 0.37 | 0.42 | 0.41 |
| Oxidation Stability (at 110 °C) | h | EN 14112 | 3.0 min. | 7.56 | 7.25 | 9.45 |
| Property | Unit | Test Method | Limits | WCME1 | WCME2 | WCME3 |
| Kinematic viscosity at 40 °C | mm2/s | ASTM D445 | 1.9-6.0 | 4.46 | 4.46 | 4.53 |
| Density at 15 °C | kg/m3 | ASTM D854 | 867 min. | 917.10 | 918.03 | 910.88 |
| Cloud Point | °C | ASTM D2500 | Report | 5.2 | −2.0 | 4.5 |
| Cold Filter Plugging Point | °C | ASTM D6371 | Report | 4.6 | −2.3 | 4.2 |
| Pour Point | °C | ASTM D97 | Report | −2.0 | −5.5 | 1.4 |
| Acid value | mg KOH/g | ASTM D664 | 0.5 max. | 0.51 | 0.90 | 0.30 |
| Oxidation Stability (at 110 °C) | h | EN 14112 | 3.0 min. | 5.0 | 2.2 | 14.0 |
| Equation Number | Investigators | Equation |
|---|---|---|
| 5 | Tate et al. [40], Andrade’s equation [41] | |
| 6 | Yuan et al. [42] | |
| 7 | Moradi et al. [43] | |
| 8 | ||
| 9 | ||
| 10 | ||
| 11 | Mejia et al. [44] | |
| 12 | Alptekin and Canakci [45] | |
| 13 | Grunberg and Nissan [46] | |
| 14 | Gülüm and Bilgin [47] | |
| 15 | ||
| 16 | ||
| 17 | ||
| 18 | ||
| 19 | ||
| 20 | ||
| 21 | ||
| 22 | Kassem et al. [48] | |
| 23 | ||
| 24 | Kendall and Monroe [49] | |
| 25 | Kanaveli et al. [50] | |
| 26 | ||
| 27 | Tesfa et al. [51] | |
| 28 | Ramírez-Verduzco et al. [52] | |
| 29 | ||
| 30 | ||
| 31 | Kassem and Çamur [30] | |
| 32 | Tat and Van Gerpen [53] | |
| 33 | Kay mixing rule [54] | |
| 34 | Ramírez-Verduzco et al. [52] | |
| 35 | Gülüm and Bilgin [55] | |
| 36 | ||
| 37 | ||
| 38 | ||
| 39 | ||
| 40 | Fahd et al. [56] | |
| 41 | Kassem and Çamur [30] | |
| 42 | Ramírez Verduzco [57] | |
| 43 | Gülüm and Bilgin [47] | |
| 44 | Rodenbush et al. [58] | |
| A, B, C, D, E and F are constants | ||
| Months | WCME 1 | WCME2 | ||
|---|---|---|---|---|
| Kinematic Viscosity | Density | Kinematic Viscosity | Density | |
| 0 | 4.44 | 914.90 | 4.42 | 909.53 |
| 2 | 4.44 | 915.57 | 4.42 | 910.09 |
| 4 | 4.45 | 916.07 | 4.43 | 910.65 |
| 6 | 4.46 | 916.64 | 4.44 | 911.22 |
| 8 | 4.46 | 917.10 | 4.45 | 911.78 |
| 10 | - | - | 4.46 | 912.35 |
| 12 | - | - | 4.46 | 912.92 |
| 14 | - | - | 4.47 | 913.48 |
| 16 | - | - | 4.48 | 914.05 |
| 18 | - | - | 4.49 | 914.62 |
| 20 | - | - | 4.49 | 915.18 |
| 22 | - | - | 4.50 | 915.75 |
| 24 | - | - | 4.51 | 916.32 |
| 26 | - | - | 4.52 | 916.89 |
| 28 | - | - | 4.53 | 917.46 |
| 30 | - | - | 4.53 | 918.03 |
| Ir (8 months) | 1.007 | 1.002 | 1.007 | 1.002 |
| Ir (10 months) | - | - | 1.010 | 1.004 |
| Ir (20 months) | - | - | 1.017 | 1.006 |
| Ir (30 months) | - | - | 1.026 | 1.009 |
| Month | WCME1 | ∆T [°C] | |||||||||||||||
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 | 26 | 28 | 30 | ||
| CP (°C) | 4.8 | 4.9 | 5.0 | 5.1 | 5.2 | - | - | - | - | - | - | - | - | - | - | - | −0.4 |
| CFPP (°C) | 4.3 | 4.4 | 4.5 | 4.5 | 4.6 | - | - | - | - | - | - | - | - | - | - | - | −0.3 |
| PP (°C) | −1.9 | −1.9 | −1.9 | −2.0 | −2.0 | - | - | - | - | - | - | - | - | - | - | - | 0.1 |
| Month | WCME2 | ∆T (°C) | |||||||||||||||
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | 22 | 24 | 26 | 28 | 30 | ||
| CP (°C) | −1.4 | −1.5 | −1.5 | −1.5 | −1.6 | −1.6 | −1.6 | −1.7 | −1.7 | −1.7 | −1.8 | −1.8 | −1.9 | −1.9 | −2.0 | −2.0 | 0.6 |
| CFPP (°C) | −1.9 | −1.9 | −1.9 | −1.9 | −2.0 | −2.0 | −2.0 | −2.1 | −2.1 | −2.1 | −2.1 | −2.2 | −2.2 | −2.2 | −2.3 | −2.3 | 0.4 |
| PP (°C) | −4.5 | −4.5 | −4.6 | −4.6 | −4.7 | −4.8 | −4.8 | −4.9 | −5.0 | −5.1 | −5.1 | −5.2 | −5.3 | −5.3 | −5.4 | −5.5 | 1.0 |
| Months | IP [h] | AV [mg KOH/g] | ||
|---|---|---|---|---|
| WCME1 | WCME2 | WCME1 | WCME2 | |
| 0 | 8.0 | 12.0 | 0.35 | 0.19 |
| 2 | 7.0 | 10.8 | 0.39 | 0.21 |
| 4 | 6.2 | 9.6 | 0.42 | 0.23 |
| 6 | 5.5 | 8.6 | 0.47 | 0.25 |
| 8 | 5.0 | 7.7 | 0.51 | 0.28 |
| 10 | - | 6.8 | - | 0.31 |
| 12 | - | 6.1 | - | 0.35 |
| 14 | - | 5.4 | - | 0.39 |
| 16 | - | 4.9 | - | 0.43 |
| 18 | - | 4.3 | - | 0.48 |
| 20 | - | 3.9 | - | 0.53 |
| 22 | - | 3.5 | - | 0.59 |
| 24 | - | 3.1 | - | 0.66 |
| 26 | - | 2.8 | - | 0.73 |
| 28 | - | 2.5 | - | 0.81 |
| 30 | - | 2.2 | - | 0.90 |
| (8 months) | 0.627 | 0.636 | - | - |
| (10 months) | - | 0.567 | - | - |
| (20 months) | - | 0.322 | - | - |
| (30 months) | - | 0.183 | - | - |
| Properties | WCME 1 | WCME1-WCME2-75% | WCME1-WCME2-50% | WCME1-WCME2-25% | WCME 2 |
| CP (°C) | 5.2 | 3.4 | 1.6 | −0.2 | −2.0 |
| CFPP (°C) | 4.6 | 2.9 | 1.3 | −0.4 | −2.3 |
| PP (°C) | −2.0 | −2.46 | −3.2 | −4.2 | −5.5 |
| Properties | WCME 1 | WCME1-WCME3-75% | WCME1-WCME3-50% | WCME1-WCME3-25% | WCME 3 |
| CP (°C) | 5.2 | 5.0 | 4.8 | 4.6 | 4.5 |
| CFPP (°C) | 4.6 | 4.5 | 4.4 | 4.3 | 4.2 |
| PP (°C) | −2.0 | −0.1 | 1 | 1.3 | 1.4 |
| Properties | WCME 2 | WCME2-WCME3-75% | WCME2-WCME3-50% | WCME2-WCME3-25% | WCME 3 |
| CP (°C) | −2.0 | 0.2 | 2.0 | 3.4 | 4.5 |
| CFPP (°C) | −2.3 | −2.1 | 1.6 | 3.1 | 4.2 |
| PP (°C) | −5.5 | −4.1 | −2.5 | −0.9 | 1.4 |
| Equation Number | Blends | T [K] | Correlation parameters | R2 | ||
|---|---|---|---|---|---|---|
| A | B | C | ||||
| 5 | WCME 1 | 279–353 | 2.117 | −453.038 | 0.997 | |
| WCME 2 | 273–353 | 3.493 | −3273.49 | 0.999 | ||
| WCME 3 | 279–353 | 0.862 | −1634.08 | 5.71 | 1.000 | |
| WCME1-WCME3-75% | 279–353 | 0.168 | −1174.72 | 4.96 | 1.000 | |
| 6 | WCME 1 | 279–353 | −1.629 | 466.388 | −162.536 | 0.997 |
| WCME 2 | 273–353 | −1.483 | 434.452 | −166.933 | 0.999 | |
| WCME 3 | 279–353 | −1.931 | 567.199 | −146.216 | 1.000 | |
| WCME1-WCME3-50% | 279–353 | −1.608 | 495.710 | −151.675 | 0.994 | |
| 18 | WCME1-WCME2-50% | 275–353 | 1.400 | −248.202 | −0.007 | 0.998 |
| WCME1-WCME3-50% | 279–353 | 1.292 | −247.658 | −0.006 | 0.995 | |
| WCME2-WCME3-75% | 275–353 | 1.309 | −251.805 | −0.007 | 0.999 | |
| 20 | WCME 1 | 279–353 | 0.004 | 2207.515 | 0.994 | |
| WCME 3 | 279–353 | 0.005 | 2145.135 | 0.998 | ||
| WCME1-WCME3-25% | 279–353 | 0.005 | 2123.113 | 0.998 | ||
| 21 | WCME 1 | 279–353 | 2.00 | −4.637 | 0.896 | |
| WCME 3 | 279–353 | 1.56 | −4.597 | 0.906 | ||
| WCME1-WCME3-50% | 279–353 | 7.38 | −4.472 | 0.912 | ||
| 22 | WCME 1 | 279–353 | 9.017 | −0.024 | 0.987 | |
| WCME 3 | 279–353 | 8.781 | −0.023 | 0.991 | ||
| WCME1-WCME3-75% | 279–353 | 8.671 | −0.023 | 0.992 | ||
| Equation Number | Blends | T [K] | Correlation Parameters | R2 | |||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | ||||
| 12 | WCME1-WCME2 | 273–353 | −5.961 | 0.319 | 2332.425 | −103.074 | - | - | 0.995 |
| WCME1-WCME3 | 279–353 | −5.119 | −0.143 | 2093.396 | 47.694 | - | - | 0.994 | |
| WCME2-WCME3 | 273–353 | −5.814 | −0.127 | 2282.583 | 14,625.786 | - | - | 0.992 | |
| 23 | WCME1-WCME2 | 273–353 | 13.567 | −3.514 | 0.000 | −3.514 | 0.000 | - | 0.286 |
| WCME1-WCME3 | 279–353 | 14.096 | −4.056 | −0.012 | −4.056 | −0.012 | - | 0.212 | |
| WCME2-WCME3 | 273-353 | −9.061 | 8.07 | 0.000 | 8.070 | 0.000 | - | 0.128 | |
| 28 | WCME1-WCME2 | 273–353 | −6.019 | 0.484 | 2348.965 | 0.002 | - | - | 0.995 |
| WCME1-WCME3 | 279–353 | −5.295 | 0.000 | 2120.892 | 7.41 | - | - | 0.994 | |
| WCME2-WCME3 | 273–353 | −5.975 | 0.110 | 2328.612 | 0.000 | - | - | 0.991 | |
| 29 | WCME1-WCME2 | 273–353 | −5.930 | 0.111 | 2323.783 | −12,510.043 | - | - | 0.995 |
| WCME1-WCME3 | 279–353 | −5.173 | −0.093 | 2085.63 | 9545.73 | - | - | 0.994 | |
| WCME2-WCME3 | 273–353 | −5.814 | −0.127 | 2282.583 | 14,625.786 | - | - | 0.992 | |
| 30 | WCME1-WCME2 | 273–353 | −5.820 | 2292.567 | −12.322 | - | - | - | 0.995 |
| WCME1-WCME3 | 279–353 | −5.272 | 2114.226 | 6.337 | - | - | - | 0.994 | |
| WCME2-WCME3 | 273–353 | −5.978 | 2329.337 | 15.611 | - | - | - | 0.992 | |
| 31 | WCME1-WCME2 | 273–353 | −5.836 | 2297.325 | −3521.23 | - | - | - | 0.995 |
| WCME1-WCME3 | 279–353 | −5.261 | 2111.2 | 1860.93 | - | - | - | 0.994 | |
| WCME2-WCME3 | 273–353 | −5.948 | 2320.881 | 4466.652 | - | - | - | 0.992 | |
| 32 | WCME1-WCME2 | 273–353 | −8.426 | 2980.013 | −0.186 | −0.667 | 0.711 | 1.425 | 0.998 |
| WCME1-WCME3 | 279–353 | −7.16 | 2619.55 | −0.497 | 0.553 | 0.054 | 1.021 | 0.996 | |
| WCME2-WCME3 | 273–353 | −8.682 | 3059.044 | −0.031 | 2.881 | −2.656 | 1.091 | 0.996 | |
| Blend | Equation Number | Property | T [K] | Correlation Parameters | R2 | ||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | |||||
| WCME1-WCME2 | 32 | 273–353 | 1209.997 | −0.973 | - | - | - | 0.883 | |
| 34 | −5.579 | −0.966 | 1210.499 | - | - | 0.889 | |||
| 35 | 2358.365 | −8.396 | 0.012 | - | - | 0.935 | |||
| 36 | 4315.195 | −0.009 | 344.642 | 0.002 | - | 0.940 | |||
| 37 | −362.047 | 0.227 | 2242.487 | - | - | 0.894 | |||
| 38 | 2323.184 | −8.120 | −33.019 | 0.011 | 0.096 | 0.938 | |||
| 39 | 1267.567 | −0.001 | −5.453 | − | − | 0.893 | |||
| 40 | 1235.044 | −1.048 | −60.257 | 0.179 | − | 0.892 | |||
| WCME1−WCME3 | 32 | 279–353 | 1182.479 | −0.915 | − | − | − | 0.899 | |
| 34 | 8.482 | −0.915 | 1178.238 | − | − | 0.915 | |||
| 35 | 1963.578 | −5.928 | 0.008 | − | − | 0.925 | |||
| 36 | 946.808 | 0.000 | 166379.1 | −0.029 | − | 0.933 | |||
| 37 | −310.273 | 0.237 | 2106.391 | − | − | 0.906 | |||
| 38 | 1970.518 | −5.965 | −13.881 | 0.008 | 0.073 | 0.940 | |||
| 39 | 1230.664 | −0.001 | 8.486 | − | − | 0.917 | |||
| 40 | 1189.419 | −0.951 | −13.881 | 0.073 | − | 0.915 | |||
| WCME2-WCME3 | 32 | 273–353 | 1200.361 | −1.019 | − | − | − | 0.659 | |
| 34 | 17.024 | −0.998 | 1184.861 | − | − | 0.693 | |||
| 35 | 2543.716 | −9.700 | 0.014 | − | − | 0.707 | |||
| 36 | 3385.867 | −0.007 | 123.966 | 0.004 | − | 0.708 | |||
| 37 | 397.291 | 0.223 | 2310.942 | − | 0.668 | ||||
| 38 | 2450.237 | −12.219 | 3.081 | 0.013 | 0.048 | 0.731 | |||
| 39 | 1249.488 | −0.001 | 16.912 | − | − | 0.697 | |||
| 40 | 1194.360 | −1.762 | 0.734 | 0.053 | − | 0.690 | |||
| Blend | Equation Number | Property | T [K] | Correlation Parameters | R2 | |||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | |||||
| WCME1-WCME2 | 41 | 273–353 | −5642.06 | 548.362 | −0.062 | 89.559 | 0.981 | |
| 43 | 6.508 | 1.053 | 864.312 | - | 0.969 | |||
| 44 | 998.409 | −195.979 | − | − | 0.892 | |||
| WCME1−WCME3 | 41 | 279–353 | −4232.01 | 538.81 | −0.043 | 63.521 | 0.958 | |
| 43 | 22.646 | 0.66 | 829.195 | − | 0.942 | |||
| 44 | 986.448 | −189.335 | − | − | 0.903 | |||
| WCME2-WCME3 | 41 | 273−353 | −8805.52 | 362.959 | -0.047 | 77.419 | 0.730 | |
| 43 | 10.036 | 0.908 | 832.744 | − | 0.719 | |||
| 44 | 977.206 | −202.188 | − | − | 0.669 | |||
| Equation Number | Property | Equations |
|---|---|---|
| 52 | ||
| 53 | ||
| 54 | ||
| 55 | ||
| 56 | ||
| 57 | ||
| 58 | ||
| 59 | ||
| 60 | ||
| 61 | ||
| Kinematic viscosity of blend in mm2/s | ||
| Kinematic viscosity of pure component 1 in mm2/s | ||
| Kinematic viscosity of pure component 2 in mm2/s | ||
| T | Test temperature in K | |
| Volume fractions of pure component 1 | ||
| Volume fractions of pure component 2 | ||
| Density of blend in kg/m3 | ||
| Density of pure component 1 in kg/m3 | ||
| Density of pure component 2 in kg/m3 | ||
| A, B, C, …, P | Constant | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saeed, R.H.S.; Kassem, Y.; Çamur, H. Effect of Biodiesel Mixture Derived from Waste Frying-Corn, Frying-Canola-Corn and Canola-Corn Cooking Oils with Various Ages on Physicochemical Properties. Energies 2019, 12, 3729. https://doi.org/10.3390/en12193729
Saeed RHS, Kassem Y, Çamur H. Effect of Biodiesel Mixture Derived from Waste Frying-Corn, Frying-Canola-Corn and Canola-Corn Cooking Oils with Various Ages on Physicochemical Properties. Energies. 2019; 12(19):3729. https://doi.org/10.3390/en12193729
Chicago/Turabian StyleSaeed, Renas Hasan Saeed, Youssef Kassem, and Hüseyin Çamur. 2019. "Effect of Biodiesel Mixture Derived from Waste Frying-Corn, Frying-Canola-Corn and Canola-Corn Cooking Oils with Various Ages on Physicochemical Properties" Energies 12, no. 19: 3729. https://doi.org/10.3390/en12193729
APA StyleSaeed, R. H. S., Kassem, Y., & Çamur, H. (2019). Effect of Biodiesel Mixture Derived from Waste Frying-Corn, Frying-Canola-Corn and Canola-Corn Cooking Oils with Various Ages on Physicochemical Properties. Energies, 12(19), 3729. https://doi.org/10.3390/en12193729





