Explosion Characteristics and Flame Propagation Behavior of Mixed Dust Cloud of Coal Dust and Oil Shale Dust
Abstract
:1. Introduction
2. Experiment
2.1. Sample Preparation
2.2. Ignition Sensitivity Test and Flame Propagation Experiment
2.3. Explosibility Test
3. Results and Discussion
3.1. Ignition Sensitivity and Flame Propagation of Dust Cloud
3.2. Explosion Severity of Mixed Dust Cloud
3.3. Product Microstructure and Explosion Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lv, D.; Wang, D.; Li, Z.; Liu, H.; Li, Y. Depositional environment, sequence stratigraphy and sedimentary mineralization mechanism in the coal bed- and oil shale-bearing succession: A case from the Paleogene Huangxian Basin of China. J. Petrol. Sci. Eng. 2017, 148, 32–51. [Google Scholar] [CrossRef]
- Li, S.; Ma, X.; Liu, G.; Guo, M. A TG–FTIR investigation to the co-pyrolysis of oil shale with coal. J. Anal. Appl. Pyrol. 2016, 120, 540–548. [Google Scholar] [CrossRef]
- Miao, Z.; Wu, G.; Li, P.; Meng, X.; Zheng, Z. Investigation into co-pyrolysis characteristics of oil shale and coal. Int. J. Min. Sci. Technol. 2012, 22, 245–249. [Google Scholar] [CrossRef]
- Yuan, Z.; Khakzad, N.; Khan, F.; Amyotte, P. Dust explosions: A threat to the process industries. Process Saf. Environ. Prot. 2015, 98, 57–71. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.X.; Yuan, C.M.; Eckhoff, R.K. A catastrophic aluminium-alloy dust explosion in China. J. Loss Prev. Process Ind. 2016, 39, 121–130. [Google Scholar] [CrossRef]
- Kalejaiye, O.; Amyotte, P.R.; Pegg, M.J.; Cashdollar, K.L. Effectiveness of dust dispersion in the 20-L Siwek chamber. J. Loss Prev. Process Ind. 2010, 23, 46–59. [Google Scholar] [CrossRef]
- Miranda, J.T.; Camacho, E.M.; Latorre, C.H.; Galdo, M.I.L. A Simple Methodology Based on the Pittsburgh Coal Method for Assessing Specific Explosion Risks in Dust-Generated Explosive Atmospheres: A Case Study from Galicia (NW Spain). Dry Technol. 2015, 33, 301–314. [Google Scholar] [CrossRef]
- Cashdollar, K.L. Coal dust explosibility. J. Loss Prev. Process Ind. 1996, 9, 65–76. [Google Scholar] [CrossRef]
- Li, Q.Z.; Yuan, C.C.; Tao, Q.L.; Zheng, Y.N.; Zhao, Y. Experimental analysis on post-explosion residues for evaluating coal dust explosion severity and flame propagation behaviors. Fuel 2018, 215, 417–428. [Google Scholar] [CrossRef]
- Li, Q.Z.; Wang, K.; Zheng, Y.N.; Ruan, M.L.; Mei, X.N.; Lin, B.Q. Experimental research of particle size and size dispersity on the explosibility characteristics of coal dust. Powder Technol. 2016, 292, 290–297. [Google Scholar] [CrossRef]
- Cao, W.G.; Gao, W.; Liang, J.Y.; Xu, S.; Pan, F. Flame-propagation behavior and a dynamic model for the thermal-radiation effects in coal-dust explosions. J. Loss Prevent. Proc. 2014, 29, 65–71. [Google Scholar] [CrossRef]
- Cao, W.G.; Gao, W.; Peng, Y.H.; Liang, J.Y.; Pan, F.; Xu, S. Experimental and numerical study on flame propagation behaviors in coal dust explosions. Powder Technol. 2014, 266, 456–462. [Google Scholar] [CrossRef]
- Mishra, D.P.; Azam, S. Experimental investigation on effects of particle size, dust concentration and dust-dispersion-air pressure on minimum ignition temperature and combustion process of coal dust clouds in a G-G furnace. Fuel 2018, 227, 424–433. [Google Scholar] [CrossRef]
- Hamdan, M.A.; Qubbaj, A. Inhibition effect of inert compounds on oil shale dust explosion. Appl. Therm. Eng. 1998, 18, 221–229. [Google Scholar] [CrossRef]
- Hamdan, M.A.; Sakhrieh, A. Dust explosion of oil shale and olive cake solid fuels: A comparison study. Int. J. Energy Res. 2005, 29, 871–878. [Google Scholar] [CrossRef]
- Sweis, F.K. The effect of admixed material on the minimum explosible concentration of oil shale. J. Loss Prev. Process Ind. 2006, 19, 701–704. [Google Scholar] [CrossRef]
- Bai, C.H.; Gong, G.D.; Liu, Q.M.; Chen, Y.H.; Niu, G.T. The explosion overpressure field and flame propagation of methane/air and methane/coal dust/air mixtures. Saf. Sci. 2011, 49, 1349–1354. [Google Scholar] [CrossRef]
- Ajrash, M.J.; Zanganeh, J.; Moghtaderi, B. Methane-coal dust hybrid fuel explosion properties in a large scale cylindrical explosion chamber. J. Loss Prev. Process Ind. 2016, 40, 317–328. [Google Scholar] [CrossRef]
- Kundu, S.K.; Zanganeh, J.; Eschebach, D.; Moghtaderi, B. Explosion severity of methane-coal dust hybrid mixtures in a ducted spherical vessel. Powder Technol. 2018, 323, 95–102. [Google Scholar] [CrossRef]
- Li, Q.Z.; Lin, B.Q.; Dai, H.M.; Zhao, S. Explosion characteristics of H-2/CH4/air and CH4/coal dust/air mixtures. Powder Technol. 2012, 229, 222–228. [Google Scholar] [CrossRef]
- Song, S.X.; Cheng, Y.F.; Meng, X.R.; Ma, H.H.; Dai, H.Y.; Kan, J.T.; Shen, Z.W. Hybrid CH4/coal dust explosions in a 20-L spherical vessel. Process Saf. Environ. 2019, 122, 281–287. [Google Scholar] [CrossRef]
- Yu, L.F.; Li, G.; Liu, W.C.; Yu, J.N.; Yuan, C.M. Experimental investigations on ignition sensitivity of hybrid mixtures of oil shale dust and syngas. Fuel 2017, 210, 1–7. [Google Scholar] [CrossRef]
- Wang, J.F.; Meng, X.B.; Ma, X.S.; Xiao, Q.; Liu, B.; Zhang, G.Y. Experimental study on whether and how particle size affects the flame propagation and explosibility of oil shale dust. Process Saf. Prog. 2019. [Google Scholar] [CrossRef]
- Castellanos, D.; Carreto-Vazquez, V.H.; Mashuga, C.V.; Trottier, R.; Mejia, A.F.; Mannan, M.S. The effect of particle size polydispersity on the explosibility characteristics of aluminum dust. Powder Technol. 2014, 254, 331–337. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. Dust explosions–Cases, causes, consequences, and control. J. Hazard. Mater. 2007, 140, 7–44. [Google Scholar] [CrossRef]
- Skřínský, J.; Ochodek, T. Explosion Characteristics of Propanol Isomer–Air Mixtures. Energies 2019, 12, 1574. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, X.; Ji, W.; Pei, B.; Lin, C.; Feng, H.; Zheng, L. The Inhibition Effect of Gas–Solid Two-Phase Inhibitors on Methane Explosion. Energies 2019, 12, 398. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zheng, L.; Yang, T.; Gao, J.; Li, Z. Effect of Pristine Palygorskite Powders on Explosion Characteristics of Methane-Air Premixed Gas. Energies 2018, 11, 2496. [Google Scholar] [CrossRef]
- Liu, Z.T.; Lin, S.; Zhang, S.S.; Wang, E.Y.; Liu, G.H. Observations of microscopic characteristics of post-explosion coal dust samples. J. Loss Prev. Process Ind. 2016, 43, 378–384. [Google Scholar] [CrossRef]
- Sen, H.S.; Liu, Z.T.; Zhao, E.L.; Lin, S.; Qiu, L.M.; Qian, J.F.; Liu, H.X.; Xia, S.K. Comparison of behavior and microscopic characteristics of first and secondary explosions of coal dust. J. Loss Prev. Process Ind. 2017, 49, 382–394. [Google Scholar] [CrossRef]

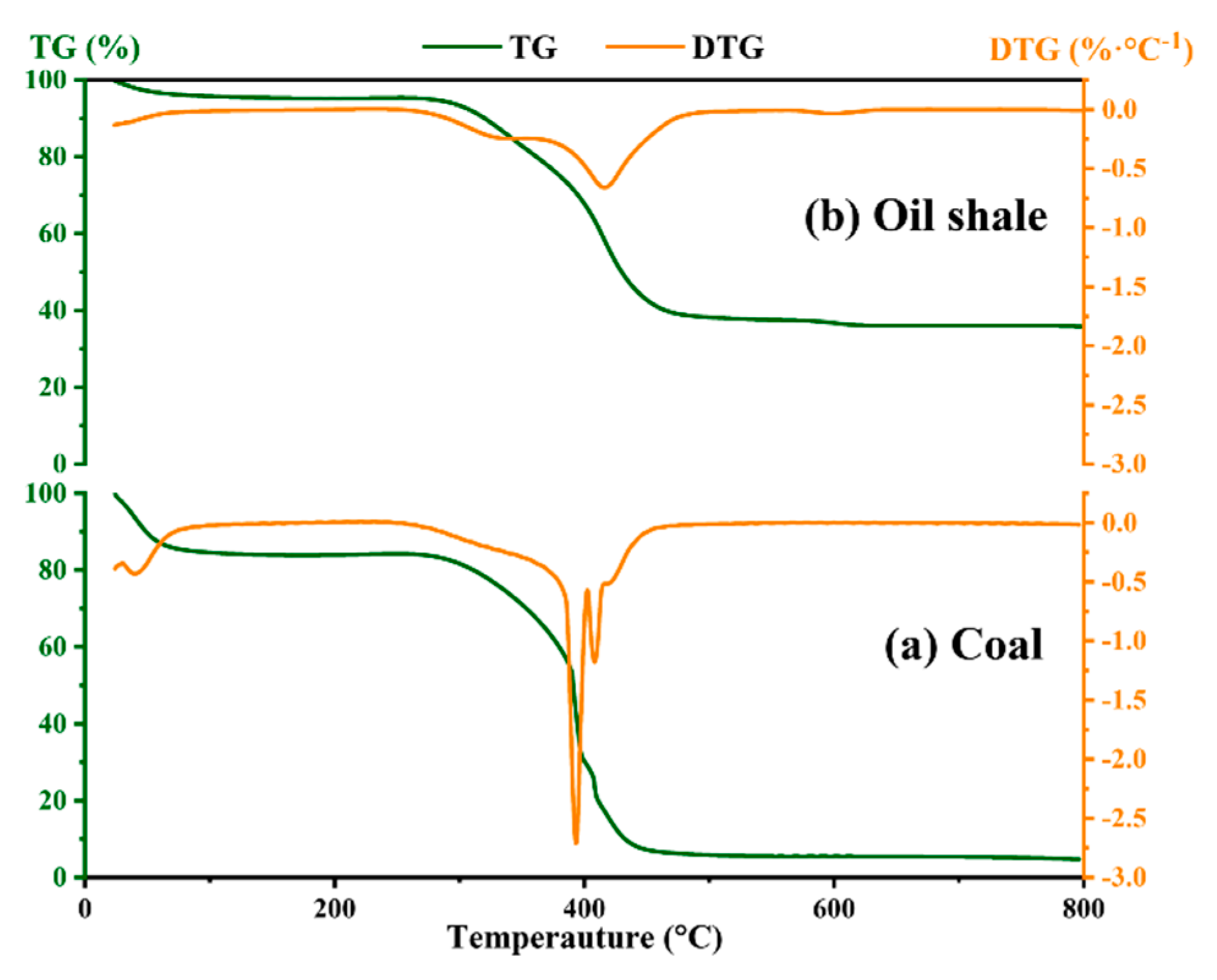


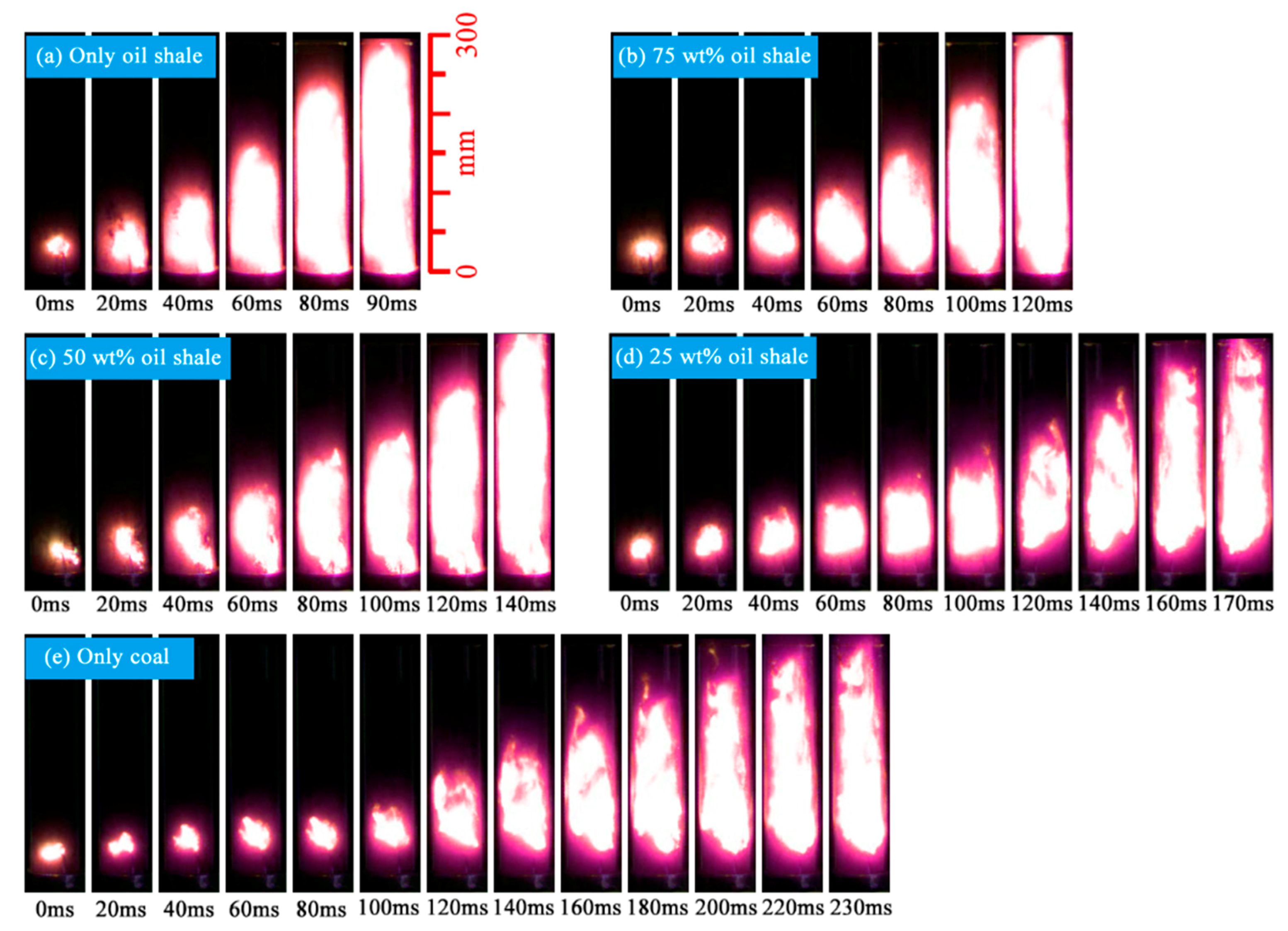



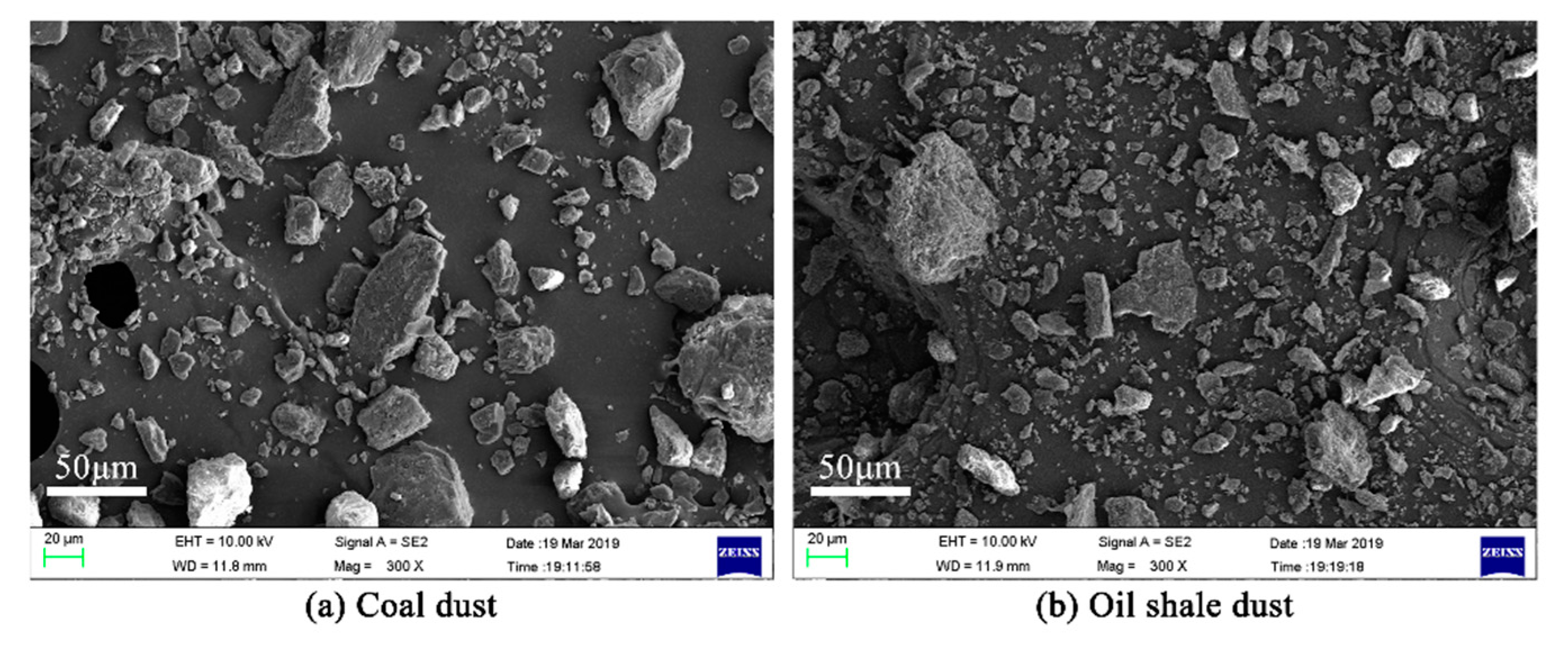
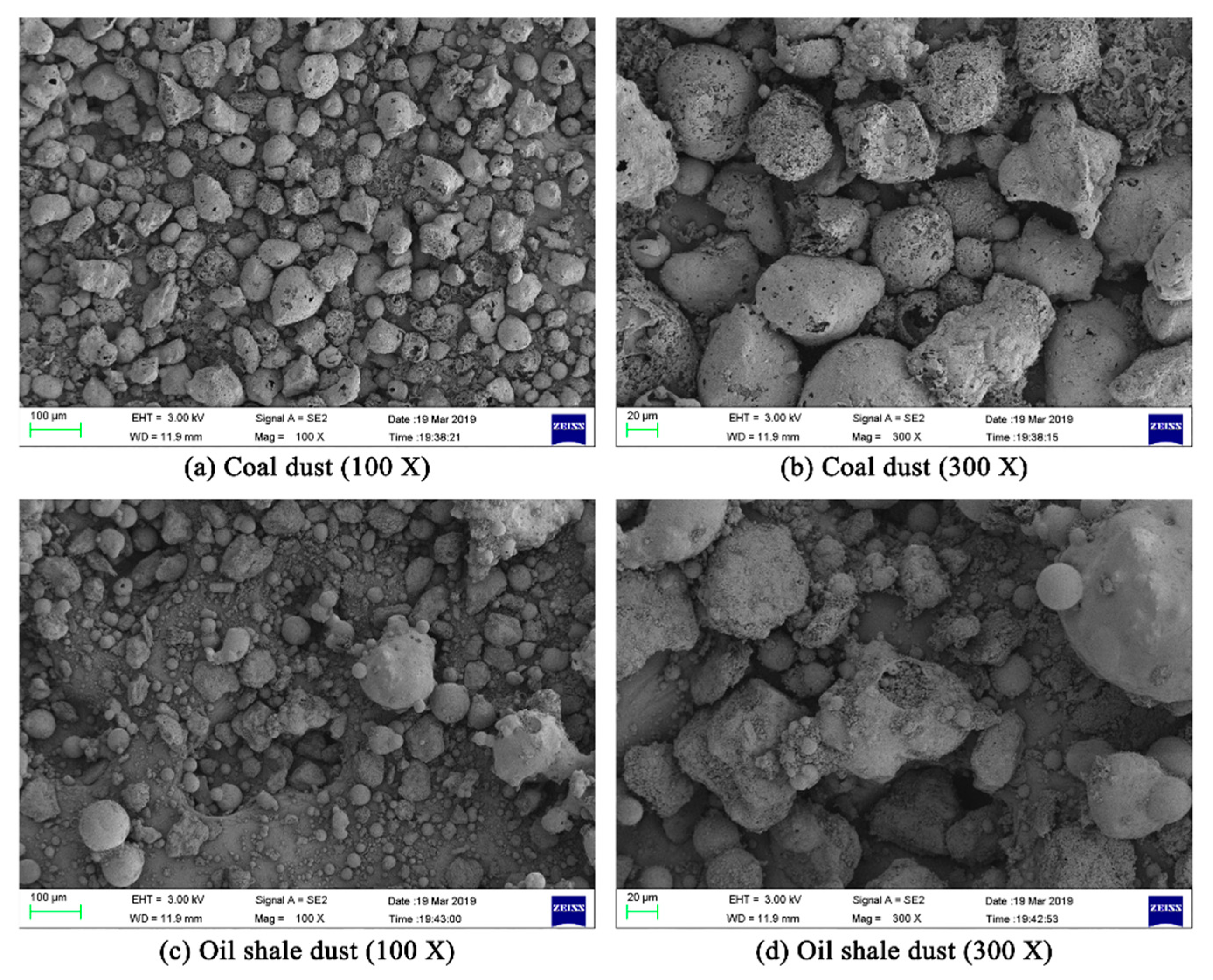


| Sample | Proximate Analyses (%) | |||
|---|---|---|---|---|
| Mad | Aad | Vad | FCad | |
| Coal | 17.12 | 5.24 | 32.7 | 44.94 |
| Oil shale | 3.53 | 37.17 | 40.86 | 18.44 |
| Sample | Only Coal | 25 wt% Oil Shale | 50 wt% Oil Shale | 75 wt% Oil Shale | Only Oil Shale |
|---|---|---|---|---|---|
| MIT (°C) | 570 | 520 | 505 | 500 | 490 |
| MIE (J) | 1.7 | 0.1 | 0.04 | 0.035 | 0.025 |
| Cdust (g/m3) | Only Coal | 25 wt% Oil Shale | 50 wt% Oil Shale | 75 wt% Oil Shale | Only Oil Shale |
|---|---|---|---|---|---|
| 125 | 0.452 | 0.438 | 0.426 | 0.414 | 0.392 |
| 250 | 0.510 | 0.489 | 0.470 | 0.453 | 0.432 |
| 375 | 0.489 | 0.535 | 0.515 | 0.497 | 0.470 |
| 500 | 0.475 | 0.515 | 0.560 | 0.542 | 0.51 |
| 750 | 0.463 | 0.492 | 0.532 | 0.591 | 0.572 |
| 1000 | 0.456 | 0.482 | 0.516 | 0.563 | 0.612 |
| 1250 | 0.45 | 0.476 | 0.506 | 0.552 | 0.592 |
| Cdust (g/m3) | Only Coal | 25 wt% Oil Shale | 50 wt% Oil Shale | 75 wt% Oil Shale | Only Oil Shale |
|---|---|---|---|---|---|
| 125 | 16.05 | 21.15 | 23.0 | 25.32 | 27.07 |
| 250 | 28.8 | 33.6 | 35.8 | 37.88 | 40.15 |
| 375 | 36.13 | 40.6 | 42.76 | 45.62 | 47.67 |
| 500 | 37.63 | 43.9 | 46.4 | 49.46 | 52.05 |
| 750 | 36.1 | 43.5 | 48.2 | 52.2 | 55.92 |
| 1000 | 34.32 | 41.06 | 46.5 | 51.8 | 57.02 |
| 1250 | 32.78 | 39.5 | 44.8 | 50.0 | 55.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Zhang, Y.; Su, H.; Chen, J.; Liu, B.; Zhang, Y. Explosion Characteristics and Flame Propagation Behavior of Mixed Dust Cloud of Coal Dust and Oil Shale Dust. Energies 2019, 12, 3807. https://doi.org/10.3390/en12203807
Wang J, Zhang Y, Su H, Chen J, Liu B, Zhang Y. Explosion Characteristics and Flame Propagation Behavior of Mixed Dust Cloud of Coal Dust and Oil Shale Dust. Energies. 2019; 12(20):3807. https://doi.org/10.3390/en12203807
Chicago/Turabian StyleWang, Junfeng, Yansong Zhang, Huifeng Su, Jinshe Chen, Bo Liu, and Yuyuan Zhang. 2019. "Explosion Characteristics and Flame Propagation Behavior of Mixed Dust Cloud of Coal Dust and Oil Shale Dust" Energies 12, no. 20: 3807. https://doi.org/10.3390/en12203807






