Biogas and Methane Potential of Pre-Thermally Disintegrated Bio-Waste
Abstract
1. Introduction
- It is a way to recycle separately collected organic waste, which is processed into high-quality fertilizer that improves soil fertility;
- There is a possibility of energy recovery (biogas generation);
- The volume of the organic waste fraction is reduced by more than 30%, depending on the duration of the fermentation process, whereas the landfilled products can be concentrated in up to 1.3 Mg/m3, which allows optimal use of available landfill capacity;
- The fermentation process does not pose a risk of creating toxic chemical compounds, whereas organic substances present in waste can be partially transformed through the life processes of micro-organisms;
- It limits the intensity of processes that later occur in landfilled waste. Biologically processed waste consists to a large extent of an inert fraction, which is the reason that, when landfilled, it emits much smaller amounts of biogas and leachate with low pollutant concentrations.
2. Materials and Methods
3. Description of the Mesophilic Methane Fermentation Process Kinetics
- B[t] is the cumulative biogas production during the duration t of fermentation, L/kg DOM;
- Bmax is the maximum biogas yield, L/kg DOM;
- kh is the kinetic rate constant, d−1;
- t is the duration of the process, d.
- Rm is the maximum rate of biogas production, L/(kg DOM·d);
- λ is the duration of the lag phase, d.
4. Results
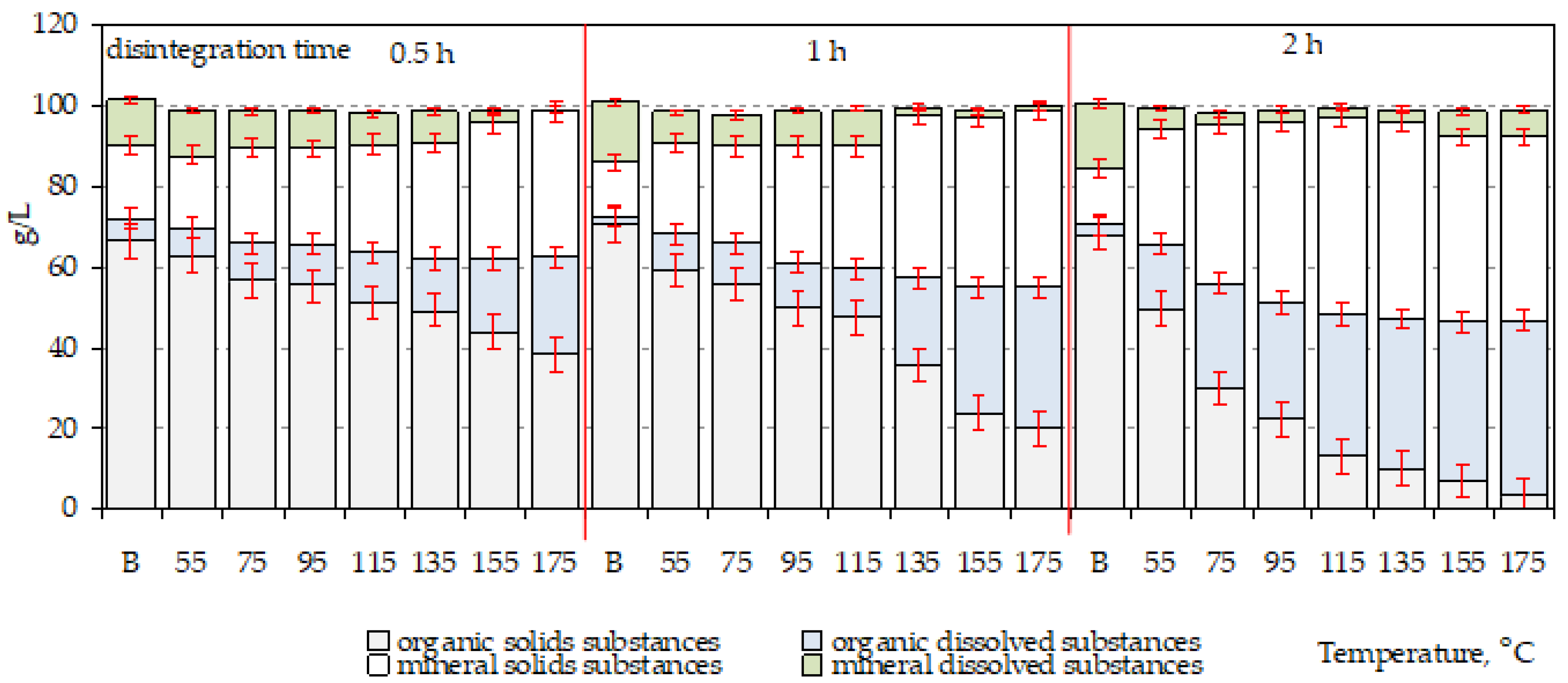
4.1. Thermal Disintegration of Bio-Waste
4.2. Biogas and Methane Potential of Raw and Thermally Disintegrated Bio-Waste
5. Discussion
6. Conclusions
Funding
Conflicts of Interest
References
- Directive 94/62/EC of the European Parliament and of the Council of 20 December 1994 on Packaging and Packaging Waste, Official Journal of the European Communities: Brussels, Belgium, 1994.
- Directive 1999/31/EC of the European Parliament and of the Council of 26 April 1999 on the Landfill of Waste, Official Journal of the European Communities: Brussels, Belgium, 1999.
- Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives, Official Journal of the European Communities: Brussels, Belgium, 2008.
- Jędrczak, A. Biologiczne Przetwarzanie Odpadów; Wydaw. Nauk. PWN: Warszawa, Poland, 2007. (In Polish) [Google Scholar]
- Florio, C.; Fiorentino, G.; Corcelli, F.; Ulgiati, S.; Dumontet, S.; Güsewell, J.; Eltrop, L. A Life Cycle Assessment of Biomethane Production from Waste Feedstock Through Different Upgrading Technologies. Energies 2019, 12, 718. [Google Scholar] [CrossRef]
- Mirtsou-Xanthopoulou, C.; Skiadas, I.V.; Gavala, H.N. On the Effect of Aqueous Ammonia Soaking Pre-Treatment on Continuous Anaerobic Digestion of Digested Swine Manure Fibers. Molecules 2019, 24, 2469. [Google Scholar] [CrossRef] [PubMed]
- Cieciura-Włoch, W.; Binczarski, M.; Tomaszewska, J.; Borowski, S.; Domański, J.; Dziugan, P.; Witońska, I. The Use of Acidic Hydrolysates after Furfural Production from Sugar Waste Biomass as a Fermentation Medium in the Biotechnological Production of Hydrogen. Energies 2019, 12, 3222. [Google Scholar] [CrossRef]
- Chulhwan, P.; Chunyeon, L.; Sangyong, K.; Yu, C.; Howard, C.H. Upgrading of Anaerobic Digestion by Incorporating Two Different Hydrolysis Processes. J. Biosci. Bioeng. 2005, 100, 164. [Google Scholar]
- Kepp, U.; Machenbach, I.; Weisz, N.; Solheim, O.E. Enhanced Stabilization of Sewage Sludge Through Thermal Hydrolysis, 3 Years of Experience with Full-scale Plant. Water Sci. Technol. 2000, 42, 89. [Google Scholar] [CrossRef]
- Barjenbruch, M.; Hoffmann, H.; Tränker, J. Minimizing of Foaming in Digesters by Pre-treatment of the Surplus Sludge. Water Sci. Technol. 1999, 42, 235. [Google Scholar] [CrossRef]
- Myszograj, S. Produkcja Biogazu z Osadów Nadmiernych i Odpadów Komunalnych Dezintegrowanych Termicznie; Instytut Inżynierii Środowiska Uniwersytetu Zielonogórskiego: Zielona Gora, Poland, 2017. (In Polish) [Google Scholar]
- Lin, L.; Koupaie, E.H.; Azizi, A.; Lakeh, A.A.B.; Dhar, B.R.; Hafez, H.; Elbeshbis, E. Comparison of Two Process Schemes Combining Hydrothermal Treatment and Acidogenic Fermentation of Source-Separated Organics. Molecules 2019, 24, 1466. [Google Scholar] [CrossRef]
- Wang, Q.; Kuninobo, M.; Kakimoto, K.; Ogawa, H.I.; Kato, Y. Upgrading of Anaerobic Digestion of WAS by Ultrasonic Pretreatment. Bioresour. Technol. 1999, 68, 309. [Google Scholar] [CrossRef]
- Climent, M.; Ferrer, I.; Baeza, M.D.; Artola, A.; Vazquez, F.; Font, X. Effects of Thermal and Mechanical Pretreatments of Secondary Sludge on Biogas Production under Thermophilic Conditions. Chem. Eng. J. 2007, 133, 335–342. [Google Scholar] [CrossRef]
- Strong, P.J.; Gapes, D.J. Thermal and Thermo-chemical Pre-treatment of Four Waste Residues and the Effect on Acetic Acid Production and Methane Synthesis. Waste Manag. 2012, 32, 1669–1677. [Google Scholar] [CrossRef]
- Lissens, G.; Thomsen, A.B.; De Baere, L.; Versraete, W.; Ahring, B. Thermal Wet Oxidation Improves Anaerobic Digestion of Raw and Digested Biowaste. Environ. Sci. Technol. 2004, 38, 3418. [Google Scholar] [CrossRef] [PubMed]
- Carrere, H.; Rafrafi, Y.; Battimelli, A.; Torrijos, M.; Delgenes, J.P.; Motte Cassini, C. Improving Methane Production During the Co-digestion of Waste-activated Sludge and Fatty Wastewater: Impact of Thermo-alkaline Pretreatment on Batch and Semi-continuous Processes. Chem. Eng. J. 2012, 10, 404. [Google Scholar] [CrossRef]
- Hendriks, A.T.; Zeeman, G. Pretreatments to Enhance the Digestibility of Lignocellulosic Biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Li, H.; Mahar, R.B.; Wang, Z.; Nie, Y. Combined Alkaline and Ultrasonic Pretreatment of Sludge before Aerobic Digestion. J. Environ. Sci. 2009, 21, 279–284. [Google Scholar] [CrossRef]
- Ramirez, I.; Motteta, A.; Carrerea, H.; Delerisb, S.; Vedrenneb, F.; Steyera, J.P. Modified ADM1 Disintegration/Hydrolysis Structures for Modeling Batch Thermophilic Anaerobic Digestion of Thermally Pretreated Waste Activated Sludge. Water Res. 2009, 43, 3479–3492. [Google Scholar] [CrossRef]
- Baserga, U. Landwirtschaftliche Co-Vergärungs-Biogasanlagen; FAT Berichte: Nr 512: Tanikon, Switzerland, 1998. [Google Scholar]
- Keymer, U.; Schilcher, A. Biogasanlagen: Berechnung der Gasausbeute von Kosubstraten; Bayrische Landesanstalt für Landwirtschaft: Freising-Weihenstephan, Germany, 2003. [Google Scholar]
- Amon, B.; Kryvoruchko, V.; Amon, T.; Zechmeister-Boltenstern, S. Methane, Nitrous Oxide and Ammonia Emissions During Storage and After Application of Dairy Cattle Slurry and Influence of Slurry Treatment. Agric. Ecosyst. Environ. 2006, 112, 153–162. [Google Scholar] [CrossRef]
- Zwietering, H.M.; Jongenburger, I.; Rombouts, F.M.; Riet, K.T. Modeling of the Bacterial Growth Curve. Appl. Environ. Microbiol. 1990, 56, 1875–1881. [Google Scholar]
- Nopharatana, A.; Pullammanappillil, P.; Clarke, W.P. Kinetics and Dynamic Modelling of Batch Anaerobic Digestion of Municipal Solid Waste in a Stirred Reactor. Waste Manag. 2007, 27, 595–603. [Google Scholar] [CrossRef]
- Altas, L. Inhibitory Effects of Heavy Metals on Methane-producing Anaerobic Granular Sludge. J. Hazard. Mat. 2009, 162, 1551–1556. [Google Scholar] [CrossRef]
- Zhu, B.; Gikas, P.; Zhang, R.; Lord, J.; Jenkins, B.; Li, X. Characteristics and Biogas Production Potential of Municipal Solid Wastes Pretreated with a Rotary Drum Reactor. Bioresour. Technol. 2009, 100, 1122–1129. [Google Scholar] [CrossRef]
- Budiyono, I.; Widiasa, I.N.; Johari, S. The Kinetic of Biogas Production Rate from Cattle Manure in Batch Mode. Int. J. Chem. Biol. Eng. 2010, 3, 39. [Google Scholar]
- Patil, J.H.; Raj, M.A.; Muralidhara, P.L.; Desai, S.M.; Raju, G.K.M. Kinetics of Anaerobic Digestion of Water Hyacinth using Poultry Litter as Inoculum. Int. J. Environ. Sci. Dev. 2012, 3, 94–98. [Google Scholar] [CrossRef]
- Gioannis, G.; Muntoni, A.; Cappai, G.; Milia, S. Landfill Gas Generation after Mechanical Biological Treatment of Municipal Solid Waste. Estimation of Gas Generation Rate Constants. Waste Manag. 2009, 29, 1026. [Google Scholar] [CrossRef] [PubMed]
- Donoso-Bravo, A.; Pérez-Elvira, S.I.; Polanco, F. Application of Simplified Models for Anaerobic Biodegradability Tests. Evaluation of Pre-treatment Processes. Chem. Eng. J. 2010, 160, 607. [Google Scholar] [CrossRef]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-state Anaerobic Digestion for Methane Production from Organic Waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Vavilin, V.A.; Fernandez, B.; Palatsi, J.; Flotats, X. Hydrolysis Kinetics in Anaerobic Degradation of Particulate Organic Material: An Overview. Waste Manag. 2008, 28, 939–951. [Google Scholar] [CrossRef]
- Batstone, D.J.; Keller, K.; Angelidaki, I.; Kalyuzhnyi, S.V.; Pavlostathis, S.G.; Rozzi, A.; Sanders, W.T.M.; Siegrist, H.; Vavilin, V.A. Anaerobic Digestion Model No. 1; Scientific and Technical Report No. 13; IWA Publishing: Cornwall, UK, 2002. [Google Scholar]
- Bougrier, C.; Delgenes, J.P.; Carrère, H. Impacts of Thermal Pre-treatments on the Semi-continuous Anaerobic Digestion of Waste Activated Sludge. Biochem. Eng. J. 2007, 34, 20–27. [Google Scholar] [CrossRef]
- Syaichurrozi, I.; Sumardiono, B.S. Predicting Kinetic Model of Biogas Production and Biodegradability Organic Materials: Biogas Production from Vinasse at Variation of COD/N ratio. Bioresour. Technol. 2013, 149, 390–397. [Google Scholar] [CrossRef]
- Adiga, S.; Ramya, R.; Shankar, B.B.; Patil, J.H.; Geetha, C.R. Kinetics of Anaerobic Digestion of Water Hyacinth, Poultry Litter, Cow Manure and Primary Sludge: A Comparative Study. Int. Conf. Biotechnol. Environ. Manag. 2012, 14, 73. [Google Scholar]
- Veeken, A.; Hamelers, B. Effect of Temperature on Hydrolysis Rates of Selected Biowaste Components. Bioresour. Technol. 1999, 69, 249–254. [Google Scholar] [CrossRef]
- Ma, J.; Duong, T.H.; Smits, M.; Verstraete, W.; Carballa, M. Enhanced Biomethanation of Kitchen Waste by Different Pre-treatments. Bioresour. Technol. 2011, 102, 592–599. [Google Scholar] [CrossRef] [PubMed]
- Polizzi, B.; Bernard, O.; Ribot, M.A. Time-space Model for the Growth of Microalgae Biofilms for Biofuel Production. J. Theor. Biol. 2017, 432, 55–79. [Google Scholar] [CrossRef] [PubMed]
- Vavilin, V.A.; Angelidaki, I. Anaerobic Degradation of Solid Material: Importance of Initiation Centers for Methanogenesis, Mixing Intensity, and 2D Distributed Model. Biotechnol. Bioeng. 2005, 89, 113. [Google Scholar] [CrossRef] [PubMed]
| Component | Percentage, % | ||
|---|---|---|---|
| 10–20 mm fraction | 26.0 | ||
| Wood | 1.0 | ||
| Potato peelings | 20-80 mm kitchen waste | 25.7 | 73.0 |
| Carrot peelings | 4.9 | ||
| Banana peel | 7.3 | ||
| Tangerine and orange peelings | 4.0 | ||
| Lemons—peelings, slices from tea | 2.5 | ||
| Onion and leek leftovers | 1.3 | ||
| Cabbage leaves | 4.6 | ||
| Meat, cold cuts | 3.2 | ||
| Fish bones and skin | 1.0 | ||
| Poultry carcasses | 1.2 | ||
| Other bones | 5.0 | ||
| Boiled pasta | 3.7 | ||
| Bread | 3.7 | ||
| Egg shells | 1.7 | ||
| Teabags | 2.7 | ||
| Sunflower husks | 0.5 | ||
| Parameter | Unit | Values | Average Value ± Dev. Stand. |
|---|---|---|---|
| Moisture | % | 54.5–61.8 | 59.8 ± 1.1 |
| Loss on ignition | % DM | 62.2–71.5 | 64.0 ± 0.5 |
| Total organic carbon | % DM | 24.4–32.9 | 29.4 ± 0.7 |
| Total Kiejdahl nitrogen | % DM | 1.00–1.40 | 1.10 ± 0.05 |
| Chemical Oxygen Demand | mg O2/g DM | 934–978 | 951 ± 16 |
| Parameter | Unit | Values | Average Value ± Dev. Stand. |
|---|---|---|---|
| pH | - | 7.2–7.4 | - |
| Dry mass | mg DM/L | 16000–18900 | 17100 ± 688 |
| Loss on ignition | mg DOM/L | 1630–5890 | 3180 ± 1790 |
| Total organic carbon | mg C/L | 83–229 | 190 ± 42 |
| Total Kiejdahl nitrogen | mg N/L | 169–211 | 186 ± 10 |
| Chemical Oxygen Demand | mg O2/L | 365–458 | 404 ± 26 |
| Volatile Fatty Acids | mg CH3COOH/L | 60–85 | 71 ± 7 |
| Time, h | Temperature, °C | Biogas Potential | Methane Potential | Methane Share in Biogas | ||
|---|---|---|---|---|---|---|
| L/kg DM | L/kg DOM | L/kg DM | L/kg DOM | % | ||
| Raw bio-waste | 146 | 206 | 69 | 97 | 47 | |
| 0.5 | 55 | 157 | 222 | 91 | 129 | 58 |
| 75 | 151 | 225 | 86 | 129 | 57 | |
| 95 | 170 | 256 | 95 | 143 | 56 | |
| 115 | 191 | 294 | 116 | 178 | 61 | |
| 135 | 194 | 308 | 123 | 195 | 63 | |
| 155 | 197 | 312 | 127 | 201 | 65 | |
| 175 | 185 | 292 | 110 | 174 | 59 | |
| 1 | 55 | 154 | 221 | 87 | 124 | 56 |
| 75 | 158 | 234 | 92 | 136 | 58 | |
| 95 | 179 | 290 | 105 | 169 | 58 | |
| 115 | 188 | 309 | 117 | 193 | 62 | |
| 135 | 200 | 347 | 130 | 224 | 65 | |
| 155 | 200 | 357 | 133 | 238 | 67 | |
| 175 | 214 | 389 | 145 | 263 | 68 | |
| 2 | 55 | 181 | 273 | 90 | 135 | 50 |
| 75 | 176 | 310 | 89 | 157 | 51 | |
| 95 | 182 | 351 | 106 | 204 | 58 | |
| 115 | 185 | 383 | 125 | 259 | 68 | |
| 135 | 217 | 456 | 147 | 308 | 68 | |
| 155 | 245 | 519 | 155 | 327 | 63 | |
| 175 | 260 | 550 | 164 | 347 | 63 | |
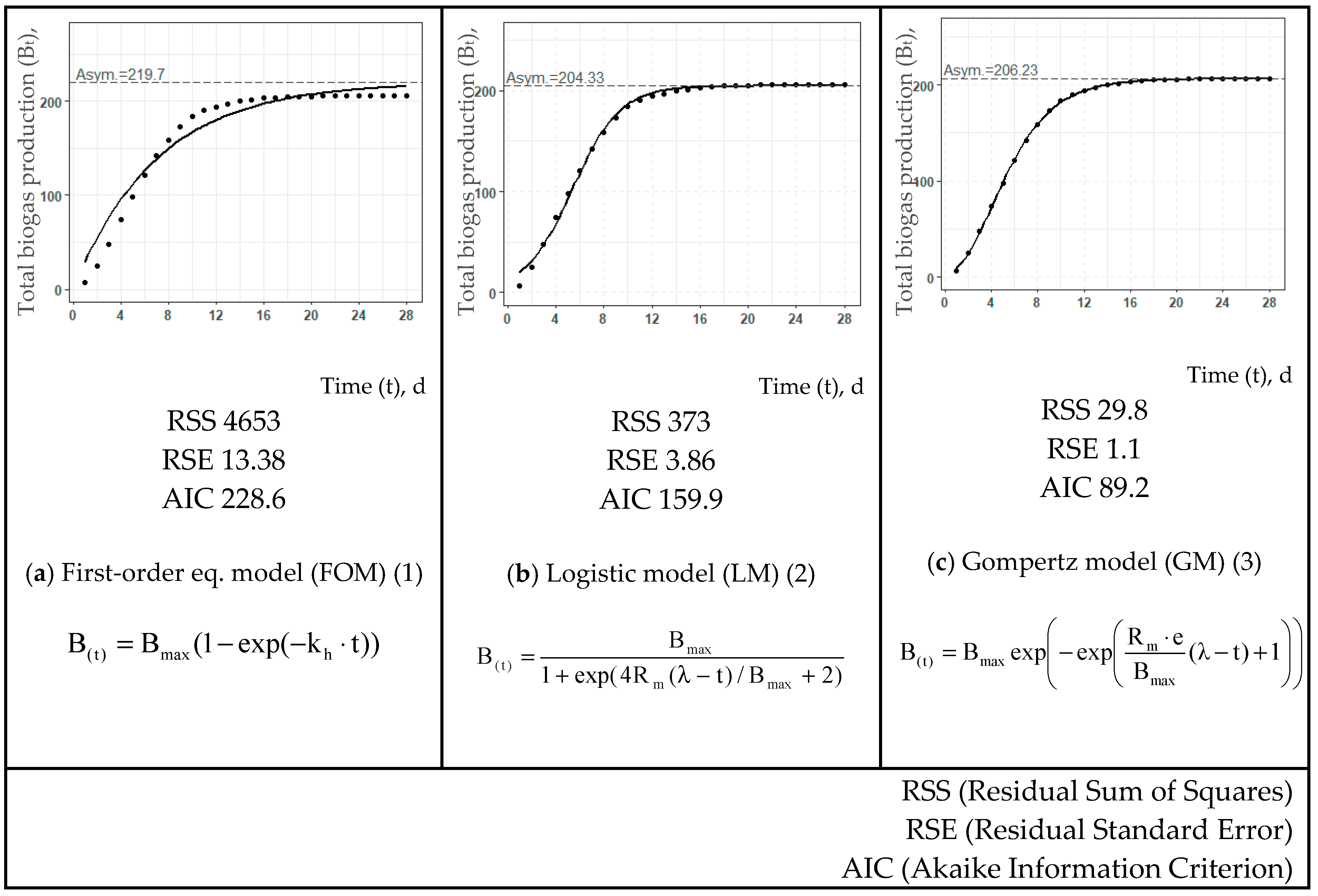
| T, °C | Estimated Values | First-Order eq. Model (FOM) (1) | Logistic Model (LM) (2) | Gompertz Model (GM) (3) | ||||||
| Raw bio-waste | ||||||||||
| Bmax, L/kg DOM | 220 | 204 | 206 | |||||||
| Rm, L/(kg DOM·d) | - | 25.8 | 26.8 | |||||||
| kh, d−1 | 0.142 | - | - | |||||||
| λ, d | - | 1.41 | 1.30 | |||||||
| Thermally disintegrated bio-waste—time of process, h | ||||||||||
| 0.5 | 1 | 2 | 0.5 | 1 | 2 | 0.5 | 1 | 2 | ||
| 55 | Bmax, L/kg DOM | 239 | 233 | 302 | 217 | 216 | 269 | 220 | 218 | 273 |
| Rm, L/(kg DOM·d) | - | - | - | 21.2 | 22.5 | 27.5 | 22.2 | 23.8 | 28.3 | |
| kh, d−1 | 0.118 | 0.133 | 0.109 | - | - | - | - | - | - | |
| λ, d | - | - | - | 0.85 | 0.69 | 1.53 | 0.82 | 0.71 | 1.37 | |
| 75 | Bmax, L/kg DOM | 240 | 250 | 331 | 222 | 231 | 307 | 224 | 239 | 318 |
| Rm, L/(kg DOM·d) | - | - | - | 25.7 | 27.2 | 32.3 | 26.7 | 28.2 | 34.1 | |
| kh, d−1 | 0.134 | 0.136 | 0.134 | - | - | - | - | - | - | |
| λ, d | - | - | - | 1.26 | 1.29 | 0.83 | 1.16 | 1.18 | 0.80 | |
| 95 | Bmax, L/kg DOM | 277 | 309 | 388 | 252 | 284 | 347 | 255 | 287 | 352 |
| Rm, L/(kg DOM·d) | - | - | - | 25.8 | 33.7 | 39.7 | 26.9 | 34.5 | 40.7 | |
| kh, d−1 | 0.120 | 0.130 | 0.113 | - | - | - | - | - | - | |
| λ, d | - | - | - | 1.09 | 1.44 | 1.94 | 1.02 | 1.28 | 1.76 | |
| 115 | Bmax, L/kg DOM | 321 | 340 | 409 | 286 | 303 | 372 | 291 | 308 | 378 |
| Rm, L/(kg DOM·d) | - | - | - | 25.2 | 26.7 | 35.3 | 26.6 | 28 | 37.2 | |
| kh, d−1 | 0.105 | 0.106 | 0.119 | - | - | - | - | - | - | |
| λ, d | - | - | - | 0.75 | 0.73 | 0.66 | 0.75 | 0.73 | 0.66 | |
| 135 | Bmax, L/kg DOM | 346 | 383 | 502 | 303 | 342 | 450 | 308 | 348 | 457 |
| Rm, L/(kg DOM·d) | - | - | - | 27.8 | 30.3 | 39.9 | 28.8 | 31.7 | 41.7 | |
| kh, d−1 | 0.098 | 0.106 | 0.107 | - | - | - | - | - | - | |
| λ, d | - | - | - | 1.34 | 0.88 | 0.83 | 1.22 | 0.82 | 0.77 | |
| 155 | Bmax, L/kg DOM | 350 | 392 | 592 | 306 | 350 | 514 | 311 | 356 | 525 |
| Rm, L/(kg DOM·d) | - | - | - | 29.0 | 34.0 | 39.4 | 30.0 | 35.3 | 41.2 | |
| kh, d−1 | 0.100 | 0.109 | 0.090 | - | - | - | - | - | - | |
| λ, d | - | - | - | 1.41 | 1.18 | 0.66 | 1.3 | 1.09 | 0.60 | |
| 175 | Bmax, L/kg DOM | 334 | 429 | 622 | 289 | 384 | 544 | 293 | 390 | 557 |
| Rm, L/(kg DOM·d) | - | - | - | 27.7 | 35.2 | 39.4 | 28.5 | 36.6 | 41.4 | |
| kh, d−1 | 0.094 | 0.108 | 0.091 | - | - | - | - | - | - | |
| λ, d | - | - | - | 1.77 | 0.96 | 0.20 | 1.60 | 0.88 | 0.20 | |
| Substrate | Bmax, L/kg DOM | Rm, L/(kg DOM.·d) | λ, d−1 | Author | |
|---|---|---|---|---|---|
| Vinasses (COD/N=600/7) | 140.1 | 16.0 | 0.21 | Syaichurrozi and Sumardiono [36] | |
| Cattle manure | 418.3 | 9.5 | 4.46 | Budiyno et al. [28] | |
| Municipal waste | 522.0 | 97.0 | 1.20 | Zhu et al. [27] | |
| Chicken manure | 390.4 | 16.5 | 8.75 | Adiga et al. [37] | |
| Raw bio-waste | 206.2 | 26.8 | 1.30 | By author | |
| Thermally disintegrated bio-waste | 0.5 h, 55 °C | 220.0 | 22.2 | 0.82 | |
| 0.5 h, 175 °C | 293.0 | 28.5 | 1.60 | ||
| 1 h, 55 °C | 218.0 | 23.8 | 0.71 | ||
| 1 h, 175 °C | 390.0 | 36.6 | 0.88 | ||
| 2h, 55 °C | 273.0 | 28.3 | 1.37 | ||
| 2h, 175 °C | 557.0 | 41.4 | 0.20 | ||
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Myszograj, S. Biogas and Methane Potential of Pre-Thermally Disintegrated Bio-Waste. Energies 2019, 12, 3880. https://doi.org/10.3390/en12203880
Myszograj S. Biogas and Methane Potential of Pre-Thermally Disintegrated Bio-Waste. Energies. 2019; 12(20):3880. https://doi.org/10.3390/en12203880
Chicago/Turabian StyleMyszograj, Sylwia. 2019. "Biogas and Methane Potential of Pre-Thermally Disintegrated Bio-Waste" Energies 12, no. 20: 3880. https://doi.org/10.3390/en12203880
APA StyleMyszograj, S. (2019). Biogas and Methane Potential of Pre-Thermally Disintegrated Bio-Waste. Energies, 12(20), 3880. https://doi.org/10.3390/en12203880





