Characterization and Recovery of In Situ Transesterifiable Lipids (TLs) as Potential Biofuel Feedstock from Sewage Sludge Obtained from Various Sewage Treatment Plants (STPs)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sludge Sample Preparation
2.2. Analysis of Lipid Content
2.3. In Situ Transesterification
2.4. DNA Extraction, PCR Amplification, and Pyrosequencing Analysis
2.5. Analytical Methods
2.6. Principal Component Analysis and Weighted Euclidean Distance
2.7. Statistical Analysis
3. Results
3.1. Lipid Content of Sewage Sludge
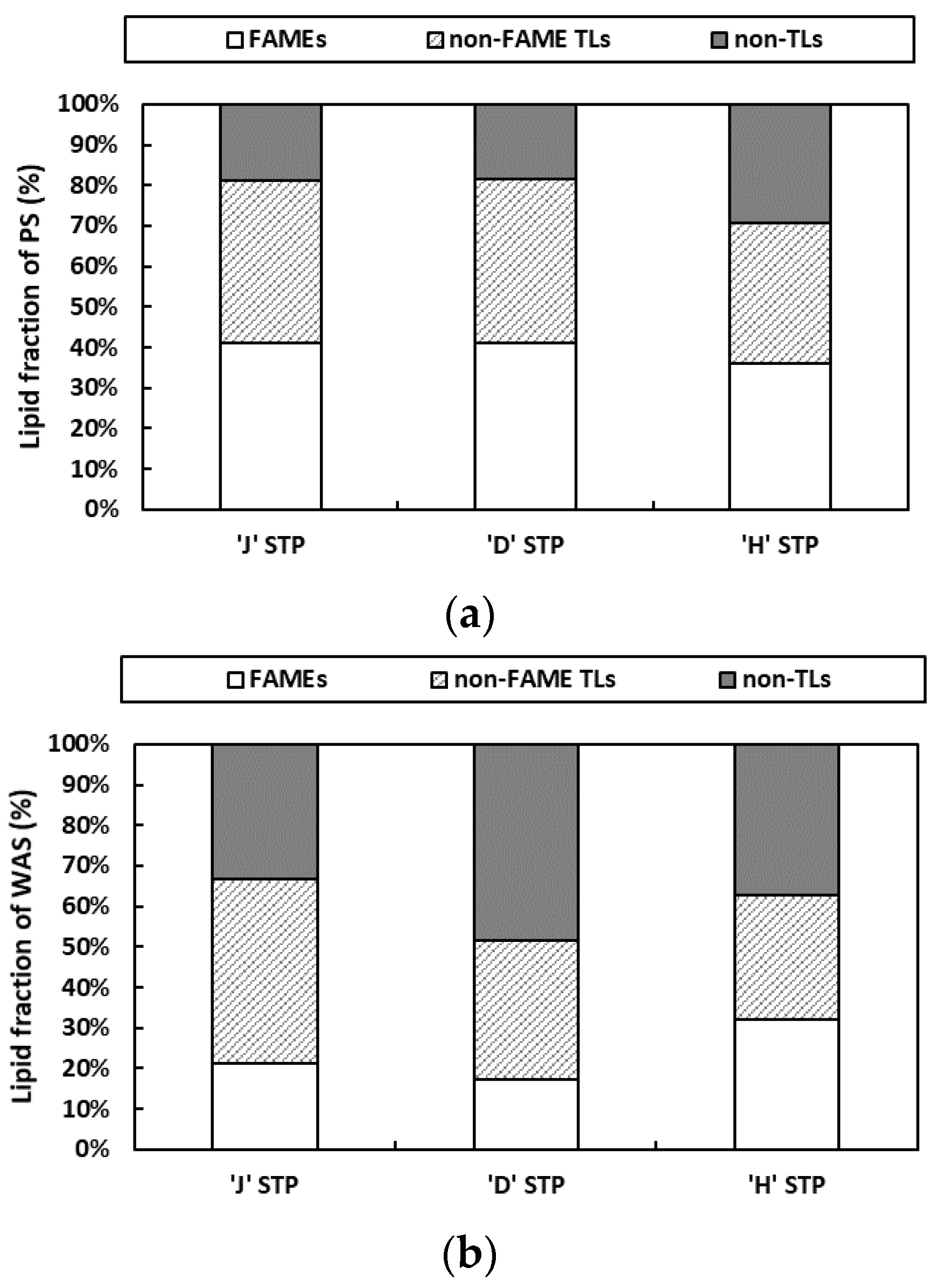
3.2. Crude Biodiesel Potential of Sewage Sludge
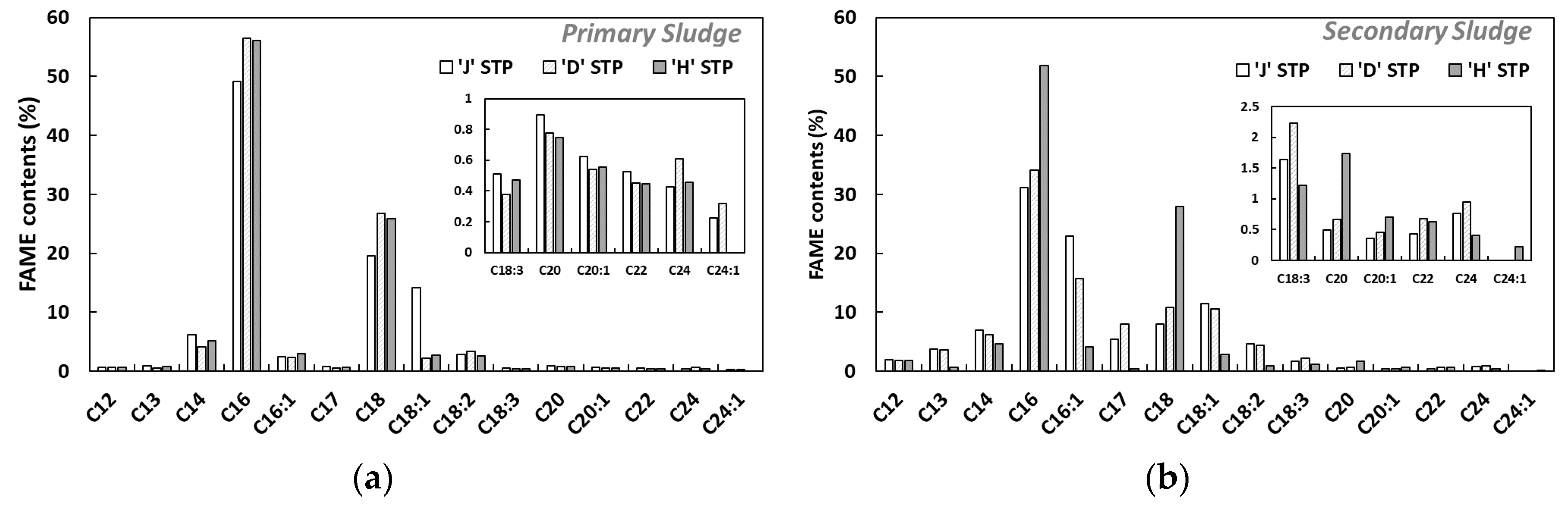
3.3. FAME Composition of Sewage Sludge Crude Biodiesel
3.4. Effect of Microbial Community Structure on FAMEs Recovered from WAS
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- North East Biosolids Residuals Association. A National Biosolids Regulation, Quality, End Use & Disposal Survey; NEBRA: Tamworth, NH, USA, 2007. [Google Scholar]
- Seiple, T.E.; Coleman, A.M.; Skaggs, R.L. Municipal wastewater sludge as a sustainable bioresource in the United States. J. Environ. Manag. 2017, 197, 673–680. [Google Scholar] [CrossRef]
- US Environmental Protection Agency. Emerging Technologies for Biosolids Management, EPA 832-R-06-005. 2006. Available online: http://nepis.epa.gov/ (accessed on 8 August 2019).
- LeBlanc, R.J.; Peter, M.; Richard, R.P. (Eds.) Global Atlas of Excreta, Wastewater Sludge and Biosolids Management: Moving Forward the Sustainable and Welcome Uses of a Global Resource; UN-HABITAT: Nairobi, Kenya, 2009. [Google Scholar]
- Ministry of Environment Korea. Statistics of Wastewater in 2016; Ministry of Environment Korea: Seoul, Korea, 2016.
- Mondala, A.; Liang, K.; Toghiani, H.; Hernandez, R.; French, T. Biodiesel production by in situ transesterification of municipal primary and secondary sludges. Bioresour. Technol. 2009, 100, 1203–1210. [Google Scholar] [CrossRef]
- Revellame, E.; Hernandez, R.; French, W.; Holmes, W.; Alley, E.; Callahan, R. Production of biodiesel from wet activated sludge. J. Chem. Technol. Biotechnol. 2011, 86, 61–68. [Google Scholar] [CrossRef]
- Choi, O.K.; Lee, K.H.; Park, K.Y.; Kim, J.K.; Lee, J.W. Pre-recovery of fatty acid methyl ester (FAME) and anaerobic digestion as a biorefinery route to valorizing waste activated sludge. Renew. Energy 2017, 108, 548–554. [Google Scholar] [CrossRef]
- Sangaletti-Gerhard, N.; Cea, M.; Risco, V.; Navia, R. In-Situ biodiesel production from greasy sewage sludge using acid and enzymatic catalysts. Bioresour. Technol. 2015, 179, 63–70. [Google Scholar] [CrossRef]
- Haas, M.J.; Foglia, T.A. Alternate feedstocks and technologies for biodiesel production. In The Biodiesel Handbook; Knothe, G., Krahl, J., Van Gerpen, J., Eds.; AOCS Press: Champaign, IL, USA, 2005; pp. 42–61. [Google Scholar]
- Kargbo, D.M. Biodiesel production from municipal sewage sludges. Energy Fuel 2010, 24, 2791–2794. [Google Scholar] [CrossRef]
- Zhu, F.; Wu, X.; Zhao, L.; Liu, X.; Qi, J.; Wang, X.; Wang, J. Lipid profiling in sewage sludge. Water Res. 2017, 116, 149–158. [Google Scholar] [CrossRef]
- Melero, J.A.; Sanchez-Vazquez, I.A.; Martinez Castillejo, F.; Bautista, L.F.; Morales, I.G.; Molina, R. Municipal sewage sludge to biodiesel by simultaneous extraction and conversion of lipids. Energy Convers. Manag. 2015, 103, 111–118. [Google Scholar] [CrossRef]
- Pokoo-Aikins, G.; Heath, A.; Mentzer, R.A.; Sam Mannan, M.; Rogers, W.J.; El-Halwagi, M.M. A multi-criteria approach to screening alternatives for convertin sewage sludge to biodiesel. J. Loss Prev. Proc. 2010, 23, 412–420. [Google Scholar] [CrossRef]
- Dufreche, S.; Hernandez, R.; French, T.; Sparks, D.; Zappi, M.; Alley, E. Extraction of lipids from municipal wastewater plant microorganisms for production of biodiesel. J. Am. Oil Chem. Soc. 2007, 84, 181–187. [Google Scholar] [CrossRef]
- Mahmudul, H.M.; Hagos, F.Y.; Mamat, R.; Abdul Adam, A.; Ishak, W.F.W.; Alenezi, R. Production, characterization and performance of biodiesel as an alternative fuel in diesel engines—A review. Renew. Sustain. Energy Rev. 2017, 72, 497–509. [Google Scholar] [CrossRef]
- Sahafi, S.M.; Ahmadibeni, A.; Talebi, A.F.; Goli, S.A.H.; Aghbashlo, M.; Tabatabaei, M. Seed oils of Sisymbrium irio and Sisymbrium sophia as a potential non-edible feedstock for biodiesel production. Biofuels 2018, 1–9. [Google Scholar] [CrossRef]
- Chen, J.; Li, J.; Dong, W.; Zhang, X.; Tyagi, R.D.; Drogui, P.; Surmpalli, R.Y. The potential of microalgae in biodiesel production. Renew. Sustain. Energy Rev. 2018, 90, 336–346. [Google Scholar] [CrossRef]
- Yi, W.; Sha, F.; Xiaojuan, B.; Jingchan, Z.; Siqing, X. Scum sludge as a potential feedstock for biodiesel production from wastewater treatment plants. Waste Manag. 2018, 47, 91–97. [Google Scholar] [CrossRef]
- Marufuzzaman, M.; Eksioglu, S.D.; Hernandez, R. Environmentally friendly supply chain planning and design for biodiesel production via wastewater sludge. Transp. Sci. 2014, 48, 555–574. [Google Scholar] [CrossRef]
- Angerbauer, C.; Siebenhofer, M.; Mittelbach, M.; Guebitz, G. Conversion of sewage sludge into lipids by Lipomyces starkeyi for biodiesel production. Bioresour. Technol. 2008, 99, 3051–3056. [Google Scholar] [CrossRef]
- Su, Y.; Mennerich, A.; Urban, B. Synergistic cooperation between wastewater-born algae and activated sludge for wastewater treatment: Influence of algae and sludge inoculation ratios. Bioresour. Technol. 2012, 105, 67–73. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhou, S.; Wang, Y.; Liu, Z.; Xu, R. Cost-effective production of Bacillus thuringiensis biopesticides by solid-state fermentation using wastewater sludge: Effects of heavy metals. Bioresour. Technol. 2011, 102, 4820–4826. [Google Scholar] [CrossRef]
- APHA; AWWA; WEF. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2015. [Google Scholar]
- Willson, R.M.; Wiesman, Z.; Brenner, A. Analyzing alternative bio-waste feedstocks for potential biodiesel production using time domain (TD)-NMR. Waste Manag. 2010, 30, 1881–1888. [Google Scholar] [CrossRef]
- Iverson, S.J.; Lang, S.L.C.; Cooper, M.H. Comparison of the Bligh and Dyer and Folch methods for total lipid determination in a broad range of marine tissue. Lipids 2001, 36, 1283–1287. [Google Scholar] [CrossRef]
- Shin, H.Y.; Ryu, J.H.; Bae, S.Y.; Crofcheck, C.; Croker, M. Lipid extraction from Scenedesmus sp. Microalgae for biodiesel production using hot compressed hexane. Fuel 2014, 130, 66–69. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.S.; Cho, Y.J.; Lee, K.; Yoon, S.H.; Kim, M.; Na, H.; Park, S.C.; Jeon, Y.S.; Lee, J.H.; Yi, H.; et al. Introducing Eztaxoneaz prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evolut. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Cha, D.K.; Oh, Y.K.; Ko, K.B.; Jin, S.H. Wastewater screening method for evaluating applicability of zero-valent iron to industrial wastewater. J. Hazard. Mater. 2010, 180, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, E.; Eddy, M.I. Wastewater Engineering, Treatment and Reuse; McGraw-Hill: New York, NY, USA, 2003. [Google Scholar]
- Siddiquee, M.N.; Rohani, S. Experimental analysis of lipid extraction and biodiesel production from wastewater sludge. Fuel Process. Technol. 2011, 92, 2241–2251. [Google Scholar] [CrossRef]
- Olkiewicz, M.; Caporgno, M.P.; Fortuny, A.; Stuber, F.; Fabregat, A.; Font, J.; Bengoa, C. Direct liquid-liquid extraction of lipid from municipal sewage sludge for biodiesel production. Fuel Process. Technol. 2014, 128, 331–338. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAO Statistics Division 2010. In Food Balance Sheets; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Akarsubasi, A.T.; Eyice, O.; Miskin, I.; Head, I.M.; Curtis, T.P. Effect of sludge age on the bacterial diversity of bench scale sequencing batch reactors. Environ. Sci. Technol. 2009, 43, 2950–2956. [Google Scholar] [CrossRef]
- Chipasa, K.; Medrzycka, K. Characterization of the fate of lipids in activated sludge. J. Environ. Sci. 2008, 20, 536–542. [Google Scholar] [CrossRef]
- Muller, E.E.; Sheik, A.R.; Wilmes, P. Lipid-based biofuel production from wastewater. Curr. Opin. Biotechnol. 2014, 30, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Wilmes, P.; Wexler, M.; Bond, P.L. Metaproteomics provides functional insight into activated sludge wastewater treatment. PLoS ONE 2008, 3, e1778. [Google Scholar] [CrossRef]
- Regmi, P.; Miller, M.W.; Holgate, B.; Bunce, R.; Park, H.; Chandran, K.; Wett, B.; Murthy, S.; Bott, C.B. Control of aeration, aerobic SRT and COD input for mainstream nitritation/denitritation. Water Res. 2014, 57, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Hellier, P.; Talibi, M.; Eveleigh, A.; Ladommatos, N. An overview of the effects of fuel molecular structure on the combustion and emissions characteristics of compression ignition engines. Proc. Inst. Mech. Eng. D 2018, 232, 90–105. [Google Scholar] [CrossRef]
- Knothe, G. Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process. Technol. 2005, 86, 1059–1070. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Sarakatsanis, C.K. Estimation of biodiesel cetane number, density, kinematic viscosity and heating values from its fatty acid weight composition. Fuel 2018, 222, 574–585. [Google Scholar] [CrossRef]
- Chhetri, A.B.; Tango, M.S.; Budge, S.M.; Watts, K.C.; Islam, M.R. Non-edible oils as new sources for biodiesel production. Int. J. Mol. Sci. 2008, 9, 169–180. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. US National Nutrient Database, Release 28; United States Department of Agriculture: Washington, DC, USA, 2016.
- Kim, D.S.; Hanifzadeh, M.; Kumar, A. Trend of biodiesel feedstock and its impact on biodiesel emission characteristics. Environ. Prog. Sustain. Energy 2018, 37, 7–19. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, D. Biodiesel production through the use of different sources and characterization of oils and their esters as the substitute of diesel: A review. Renew. Sustain. Energy Rev. 2010, 14, 200–216. [Google Scholar] [CrossRef]
- Malik, M.R.; Isaac, B.J.; Coussement, A.; Smith, P.J.; Parente, A. Principal component analysis coupled with nonlinear regression for chemistry reduction. Combust. Flame 2018, 187, 30–41. [Google Scholar] [CrossRef] [Green Version]
- Mondala, A.; Hernandez, R.; French, T.; McFarland, L.; Santo Domingo, J.W.; Meckes, M.; Ryu, H.; Iker, B. Enhanced lipid and biodiesel production from glucose-fed activated sludge: Kinetics and microbial community analysis. AICHE J. 2011, 58, 1279–1290. [Google Scholar] [CrossRef]
- Pala-Ozkok, I.; Rehman, A.; Kor-Bicakci, G.; Ural, A.; Schilhabel, M.B.; Ubay-Cokgor, E.; Jonas, D.; Orhon, D. Effect of sludge age on population dynamics and acetate utilization kinetics under aerobic conditions. Bioresour. Technol. 2013, 143, 68–75. [Google Scholar] [CrossRef]
- Moreno, E.; Stackebrandt, E.; Dorsch, M.; Wolters, J.; Busch, M.; Mayer, H. Brucella abortus 16S rRNA and lipid A reveal a phylogenetic relationship with members of the alpha-2 subdivision of the class proteobacteria. J. Bacteriol. 1990, 172, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Kruse, M.; Keuter, S.; Bakker, E.; Spieck, E.; Eggers, T.; Lipski, A. Relevance and diversity of Nitrospira populations in biofilters of brackish RAS. PLoS ONE 2013, 8, e64737. [Google Scholar] [CrossRef] [PubMed]



| STP Site | Influent Type | Sewer System (%) | Process Capacity (m3/day) | Biological Process | Solid Retention Time (days) | |
|---|---|---|---|---|---|---|
| Combined | Separated | |||||
| J | Domestic | 92 | 8 | 1,590,000 | MLE 1 | 15–20 |
| D | Domestic | 47 | 53 | 900,000 | A2/O 2 | 4–10 |
| H | Domestic | 37 | 63 | 7200 | NPR 3 | 10–15 |
| STP Site | Type | TS (%) | VS/TS (%) | Lipid Content (%) |
|---|---|---|---|---|
| J | PS | 2.07 (±0.03) | 82.51 (±0.79) | 14.01 (±0.50) |
| WAS | 2.24 (±0.08) | 81.46 (±0.98) | 11.55 (±0.79) | |
| D 1 | PS | 4.58 (±0.09) | 83.89 (±1.83) | 16.06 (±0.93) |
| WAS | 5.15 (±0.06) | 76.48 (±0.87) | 10.88 (±0.10) | |
| H | PS | 2.11 (±0.05) | 81.71 (±3.66) | 16.78 (±0.12) |
| WAS | 1.73 (±0.02) | 87.13 (±0.98) | 11.68 (±0.27) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, O.K.; Hendren, Z.; Park, K.Y.; Kim, J.-K.; Park, J.Y.; Son, A.; Lee, J.W. Characterization and Recovery of In Situ Transesterifiable Lipids (TLs) as Potential Biofuel Feedstock from Sewage Sludge Obtained from Various Sewage Treatment Plants (STPs). Energies 2019, 12, 3952. https://doi.org/10.3390/en12203952
Choi OK, Hendren Z, Park KY, Kim J-K, Park JY, Son A, Lee JW. Characterization and Recovery of In Situ Transesterifiable Lipids (TLs) as Potential Biofuel Feedstock from Sewage Sludge Obtained from Various Sewage Treatment Plants (STPs). Energies. 2019; 12(20):3952. https://doi.org/10.3390/en12203952
Chicago/Turabian StyleChoi, Oh Kyung, Zachary Hendren, Ki Young Park, Jae-Kon Kim, Jo Yong Park, Ahjeong Son, and Jae Woo Lee. 2019. "Characterization and Recovery of In Situ Transesterifiable Lipids (TLs) as Potential Biofuel Feedstock from Sewage Sludge Obtained from Various Sewage Treatment Plants (STPs)" Energies 12, no. 20: 3952. https://doi.org/10.3390/en12203952






