Suppression Effects of Hydroxy Acid Modified Montmorillonite Powders on Methane Explosions
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Preparation Procedures
2.2. Powder Characterisation Methods
2.3. Powder Suppression Explosion Experiments
3. Results and Discussion
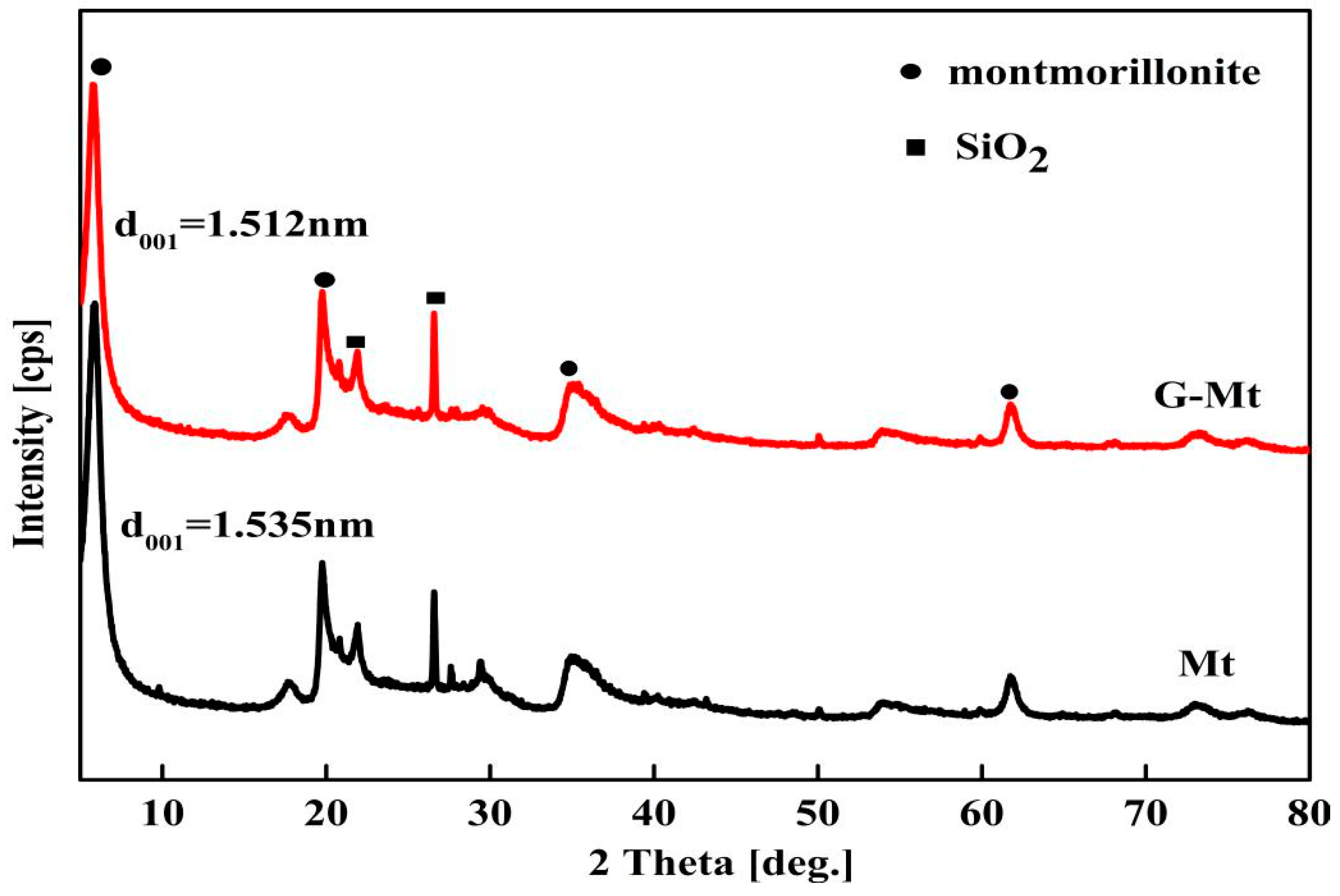
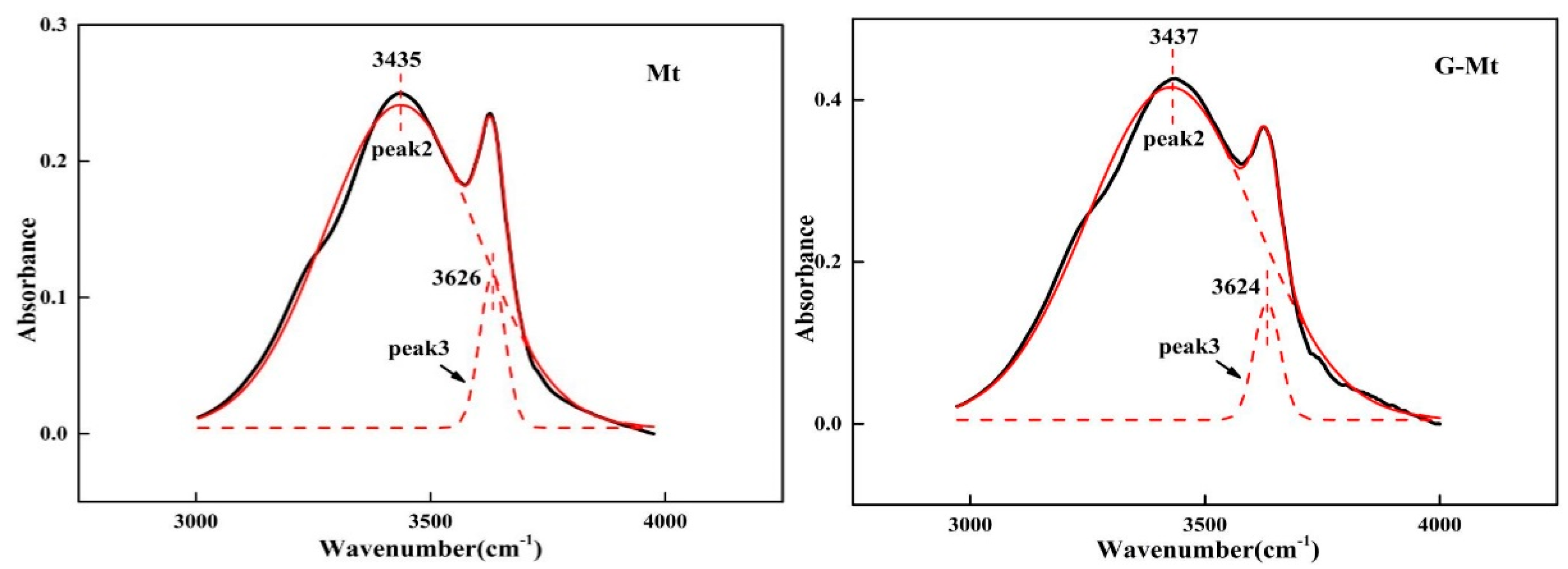
3.1. Characterisation of Mt and G-Mt
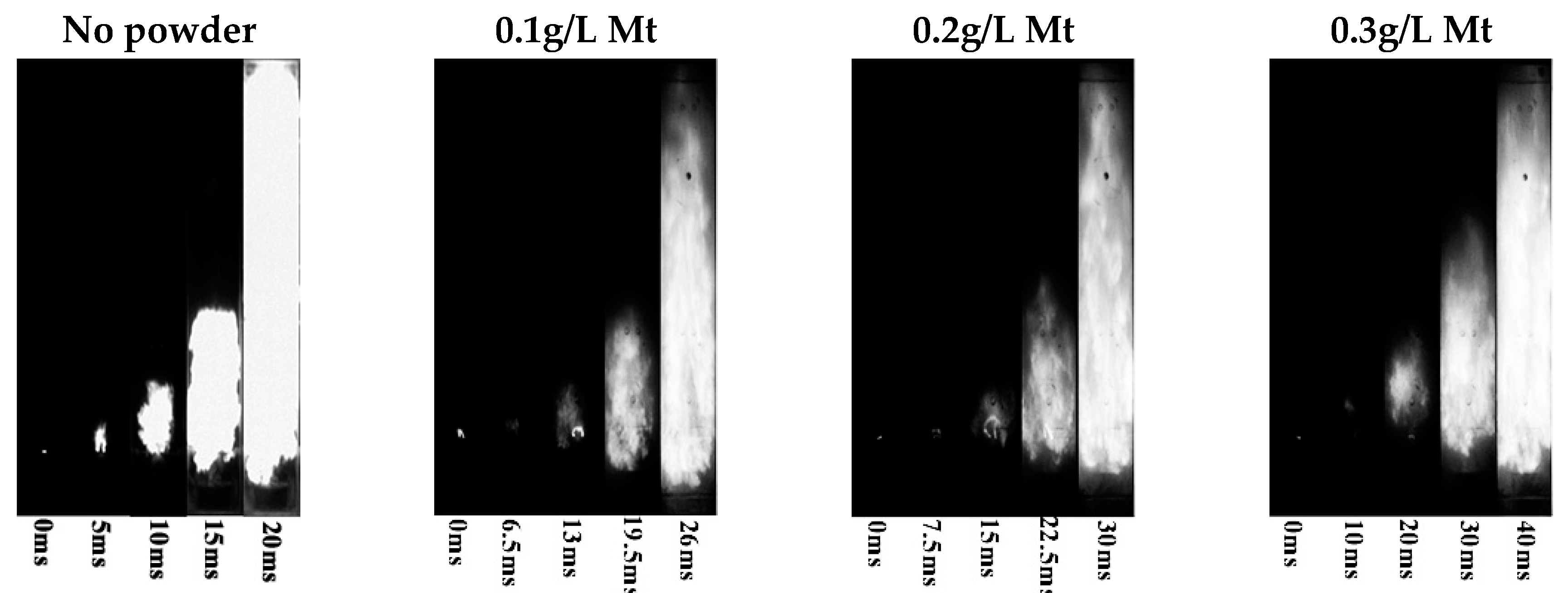
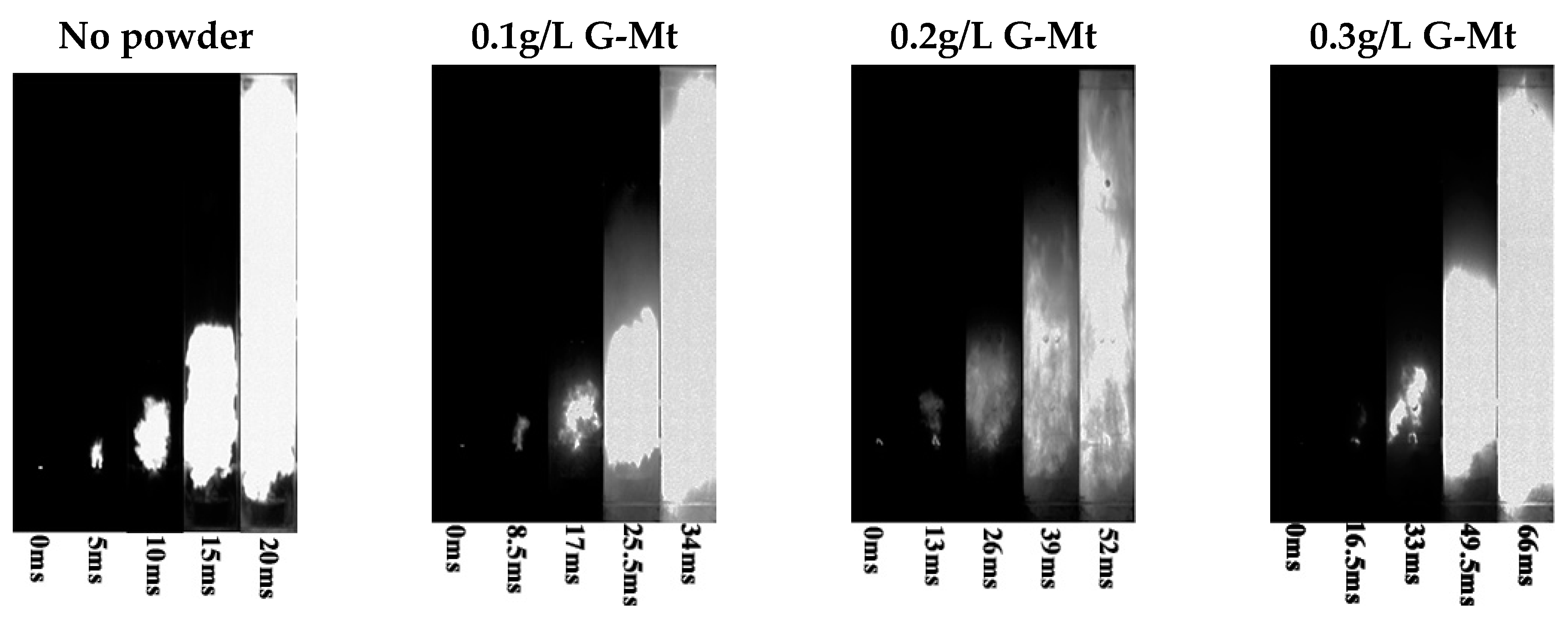
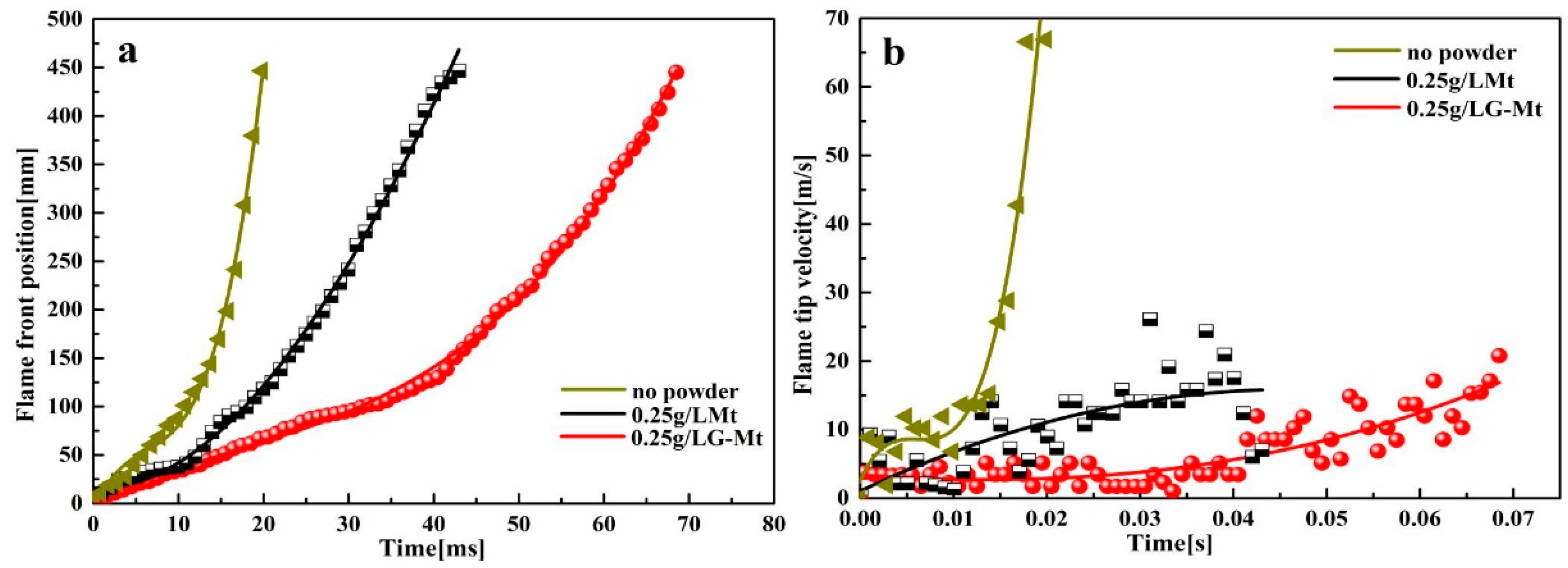
3.2. Suppression Effect of Mt and G-Mt
3.3. Suppression Mechanism of Mt and G-Mt
3.3.1. Physical Inhibition Effect
3.3.2. Chemical Inhibition Effect
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bianchini, A.; Guzzini, A.; Pellegrini, M.; Saccani, C. Natural gas distribution system: A statistical analysis of accidents data. Int. J. Press. Vessels Pip. 2018, 168, 24–38. [Google Scholar] [CrossRef]
- Cao, X.Y.; Ren, J.; Bi, M.S.; Zhou, Y.; Wang, Q. Experimental research on methane/air explosion inhibition using ultrafine water mist containing additive. J. Loss. Prev. Process Ind. 2016, 43, 352–360. [Google Scholar] [CrossRef]
- Wang, Z.R.; Ni, L.; Liu, X.; Jiang, J.C.; Wang, R. Effects of N2/CO2 on explosion characteristics of methane and air mixture. J. Loss. Prev. Process Ind. 2014, 31, 10–15. [Google Scholar] [CrossRef]
- Krasnyansky, M. Prevention and suppression of explosions in gas-air and dust-air mixtures using powder aerosol-inhibitor. J. Loss. Prev. Process Ind. 2006, 19, 729–735. [Google Scholar] [CrossRef]
- Luo, Z.; Wang, T.; Tian, Z.; Cheng, F.; Deng, J.; Zhang, Y. Experimental study on the suppression of gas explosion using the gas-solid suppressant of CO2/ABC powder. J. Loss. Prev. Process Ind. 2014, 30, 17–23. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Cammarota, F.; Di Sarli, V.; Salzano, E.; Russo, G. Effect of diluents on rapid phase transition of water induced by combustion. AIChE J. 2012, 58, 2810–2819. [Google Scholar] [CrossRef]
- Luo, Z.M.; Cheng, F.M.; Wang, T.; Deng, J.; Shu, C.M. Suppressive Effects of Silicon Dioxide and Diatomite Powder Aerosols on Coal Mine Gas Explosions in Highlands. Aerosol. Air Qual. Res. 2016, 16, 2119–2128. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Hu, Y.; Bai, C.; Chen, M. Methane/coal dust/air explosions and their suppression by solid particle suppressing agents in a large-scale experimental tube. J. Loss. Prev. Process Ind. 2013, 26, 310–316. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Zheng, L.; Yang, T.; Gao, J.; Li, Z. Effect of Pristine Palygorskite Powders on Explosion Characteristics of Methane-Air Premixed Gas. Energies 2018, 11, 12. [Google Scholar] [CrossRef]
- Sun, Y.; Yuan, B.; Chen, X.; Li, K.; Wang, L.; Yun, Y.; Fan, A. Suppression of methane/air explosion by kaolinite-based multi-component inhibitor. Powder Technol. 2019, 343, 279–286. [Google Scholar] [CrossRef]
- Ni, X.; Wang, X.; Zhang, S.; Zhao, M. Experimental study on the performance of transition metal ions modified zeolite particles in suppressing methane/air coflowing flame on cup burner. J. Fire Sci. 2014, 32, 417–430. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, Y.S.; Yu, M.G.; Li, Y.; Cao, J.L.; Zheng, L.G.; Yi, H.W. Methane explosion suppression characteristics based on the NaHCO3/red-mud composite powders with core-shell structure. J. Hazard. Mater. 2017, 335, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, H.Q.; Zong, R.W.; Lo, S.M. Study of a New Type of Fire Suppressant Powder of Mg(OH)2 Modified by DOPO-VTS. Procedia Eng. 2018, 211, 1102–1110. [Google Scholar] [CrossRef]
- Nie, B.; Yang, L.; Ge, B.; Wang, J.; Li, X. Chemical kinetic characteristics of methane/air mixture explosion and its affecting factors. J. Loss. Prev. Process Ind. 2017, 49, 675–682. [Google Scholar] [CrossRef]
- Zhang, R.M.; Nie, B.S.; He, X.Q.; Wang, C.; Zhao, C.H.; Dai, L.C.; Li, H.L. Different gas explosion mechanisms and explosion suppression techniques. Procedia Eng. 2011, 26, 1467–1472. [Google Scholar]
- De Leo, M.; Saveliev, A.; Kennedy, L.A.; Zelepouga, S.A. OH and CH luminescence in opposed flow methane oxy-flames. Combust. Flame 2007, 149, 435–447. [Google Scholar] [CrossRef]
- He, Z.; Li, X.B.; Liu, L.M.; Zhu, W. The intrinsic mechanism of methane oxidation under explosion condition: A combined ReaxFF and DFT study. Fuel 2014, 124, 85–90. [Google Scholar] [CrossRef]
- Tang, Y.; Zhuo, J.; Cui, W.; Li, S.; Yao, Q. Enhancing ignition and inhibiting extinction of methane diffusion flame by in situ fuel processing using dielectric-barrier-discharge plasma. Fuel Process. Technol. 2019, 194, 106–128. [Google Scholar] [CrossRef]
- Bee, S.L.; Abdullah, M.A.A.; Bee, S.-T.; Sin, L.T.; Rahmat, A.R. Polymer nanocomposites based on silylated-montmorillonite: A. review. Prog. Polym. Sci. 2018, 85, 57–82. [Google Scholar] [CrossRef]
- Tang, Y.B.; Wang, H. Development of a novel bentonite–acrylamide superabsorbent hydrogel for extinguishing gangue fire hazard. Powder Technol. 2018, 323, 486–494. [Google Scholar] [CrossRef]
- Komadel, P. Acid activated clays: Materials in continuous demand. Appl. Clay Sci. 2016, 131, 84–99. [Google Scholar] [CrossRef]
- Di Benedetto, A.; Russo, P.; Sanchirico, R.; Di Sarli, V. CFD simulations of turbulent fluid flow and dust dispersion in the 20 L explosion vessel. AIChE J. 2013, 59, 2485–2496. [Google Scholar] [CrossRef]
- Sanchirico, R.; Di Sarli, V.; Russo, P.; Di Benedetto, A. Effect of the nozzle type on the integrity of dust particles in standard explosion tests. Powder Technol. 2015, 279, 203–208. [Google Scholar] [CrossRef]
- Peng, K.; Fu, L.; Li, X.; Ouyang, J.; Yang, H. Stearic acid modified montmorillonite as emerging microcapsules for thermal energy storage. Appl. Clay Sci. 2017, 138, 100–106. [Google Scholar] [CrossRef]
- Cipolletti, V.; Galimberti, M.; Mauro, M.; Guerra, G. Organoclays with hexagonal rotator order for the paraffinic chains of the compensating cation. Implications on the structure of clay polymer nanocomposites. Appl. Clay Sci. 2014, 87, 178–188. [Google Scholar] [CrossRef]
- Onal, M.; Sankaya, Y. Preparation and characterization of acid-activated bentonite powders. Powder Technol. 2007, 172, 14–18. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, P.; Qin, Z.; Liu, D.; Tan, D.; Zhu, J.; He, H. Thermal degradation of organicmatter in the interlayer clay–organic complex: A TG-FTIR study on a montmorillonite/12-aminolauric acid system. Appl. Clay Sci. 2013, 80, 398–406. [Google Scholar] [CrossRef]
- Takenawa, R.; Komori, Y.; Hayashi, S.; Kawamata, J.; Kuroda, K. Intercalation of nitroanilines into kaolinite and second harmonic generation. Chem. Mater. 2001, 13, 3741–3746. [Google Scholar] [CrossRef]
- Li, X.; Ouyang, J.; Zhou, Y.; Yang, H. Assembling strategy to synthesize palladium modified kaolin nanocomposites with different morphologies. Sci. Rep. 2015, 5, 13763. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Li, Z.; Yang, Y.; Kong, B.; Wang, C. Laboratory study on the inhibitory effect of free radical scavenger on coal spontaneous combustion. Fuel Process. Technol. 2018, 171, 350–360. [Google Scholar] [CrossRef]
- Li, Q.Z.; Lin, B.Q.; Zhao, C.S.; Wu, W.F. Chemical Structure Analysis of Coal Char Surface Based on Fourier-Transform Infrared Spectrometer. Proc. CSEE 2011, 31, 46–52. [Google Scholar]
- Li, X.; Yang, H. Pd hybridizing ZnO/kaolinite nanocomposites: Synthesis, microstructure, and enhanced photocatalytic property. Appl. Clay Sci. 2014, 100, 43–49. [Google Scholar] [CrossRef]
- Klinowski, J.; Thomas, J.M.; Fyfe, C.A. A highly siliceous structural analog of zeolite Y: high-resolution solid-state silicon-29 and aluminum-27 NMR studies. Inog. Chem. 1983, 22, 63–66. [Google Scholar] [CrossRef]
- Kentgens, A.P.M.; Lemmens, J.J.M.; Geurts, F.M. Two-dimensional solid-state nutation NMR of half-integer quadrupolar nuclei. J. Magn. Reson. 1987, 71, 62–74. [Google Scholar]
- Yuan, P.; Wu, D.Q.; Lin, Z.Y.; Diao, G.Y.; Peng, J.L.; Wei, J.F. Study on the Surface Hydroxyl Species of Diatomite using DRIFT Spectroscopy. Spectrosc. Spect Anal. 2001, 21, 783–786. [Google Scholar]
- Zheng, L.; Li, G.; Wang, Y.; Zhu, X.; Pan, R.; Wang, Y. Effect of blockage ratios on the characteristics of methane/air explosion suppressed by BC powder. J. Hazard. Mater. 2018, 355, 25–33. [Google Scholar] [CrossRef]
- Li, Q.Z.; Lin, B.Q.; Jian, C.G. Investigation on the Interactions of Gas Explosion Flame and Reflected Pressure Waves in Closed Pipes. Combust. Sci. Technol. 2012, 184, 2154–2162. [Google Scholar] [CrossRef]
- Simmie, J.M. Detailed chemical kinetic models for the combustion of hydrocarbon fuels. Prog. Energy Combust. Sci. 2003, 29, 599–634. [Google Scholar] [CrossRef]
- Smith, G.P.; Golden, D.M.; Frenklach, M.; Moriarty, N.W.; Eiteneer, B.; Goldenberg, M.; Bowman, C.T.; Hanson, R.K.; Song, S.; Gardiner, W.C.; et al. GRI-Mech 3.0. Available online: http://combustion.berkeley.edu/gri-mech/version30/text30.html (accessed on 22 October 2019).
- Guo, Z.; Xing, R.; Liu, S.; Yu, H.; Wang, P.; Li, C.; Li, P. The synthesis and antioxidant activity of the Schiff bases of chitosan and carboxymethyl chitosan. Bioorg. Med. Chem. Lett. 2005, 15, 4600–4603. [Google Scholar] [CrossRef]
- Wang, J.; Hu, S.; Nie, S.; Yu, Q.; Xie, M. Reviews on Mechanisms of In Vitro Antioxidant Activity of Polysaccharides. Oxid. Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef]
- Krasnyansky, M. Studies of fundamental physical-chemical mechanisms and processes of flame extinguishing by powder aerosols. Fire Mater. 2008, 32, 27–47. [Google Scholar] [CrossRef]
- Ni, X.; Chow, W.K.; Li, Q.; Tao, C. Experimental study of new gas-solid composite particles in extinguishing cooking oil fires. J. Fire Sci. 2010, 29, 152–176. [Google Scholar]






| Sample | Peak 1 | Peak 2 | Peak 3 |
|---|---|---|---|
| Mt | 6.24 | 96.45 | 7.62 |
| G-Mt | 8.74 | 184.29 | 10.15 |
| Sample | Total Weight Loss (%) | Total Endothermic Quantity (J·g−1) |
|---|---|---|
| Mt | 16.32 | 1143 |
| G-Mt | 19.66 | 570.30 |
| Sample | Concentration (g·L−1) | Maximum Pressure (MPa) | Time of Pressure Peak (s) | Rate of Maximum-Pressure Rise (MPa·s−1) |
|---|---|---|---|---|
| No powders | 0 | 0.654 | 0.138 | 87.648 |
| Mt | 0.25 | 0.578 | 0.183 | 43.144 |
| G-Mt | 0.25 | 0.479 | 0.215 | 22.235 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Feng, H.; Zhang, Y.; Lin, C.; Zheng, L.; Ji, W.; Han, X. Suppression Effects of Hydroxy Acid Modified Montmorillonite Powders on Methane Explosions. Energies 2019, 12, 4068. https://doi.org/10.3390/en12214068
Wang Y, Feng H, Zhang Y, Lin C, Zheng L, Ji W, Han X. Suppression Effects of Hydroxy Acid Modified Montmorillonite Powders on Methane Explosions. Energies. 2019; 12(21):4068. https://doi.org/10.3390/en12214068
Chicago/Turabian StyleWang, Yan, Hao Feng, Yimin Zhang, Chendi Lin, Ligang Zheng, Wentao Ji, and Xuefeng Han. 2019. "Suppression Effects of Hydroxy Acid Modified Montmorillonite Powders on Methane Explosions" Energies 12, no. 21: 4068. https://doi.org/10.3390/en12214068






