Integrative Effects of Sonication and Particle Size on Biomethanation of Tropical Grass Pennisetum purpureum Using Superior Diverse Inocula Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Part I: Inoculum Characterization Using Specific Anaerobic Activity Assays
2.1.1. Inoculum Source
2.1.2. Napier Grass Preparation
2.1.3. Specific Anaerobic Activity Assays
2.2. Part II: Impact of Sonication and Size Reduction on Digestibility of Napier Grass Silage
2.2.1. Inoculum
2.2.2. Pretreatments of Substrate and Biochemical Methane Potential Study
2.3. Analytical Methods
2.4. Statistical Regression and Analysis
3. Results and Discussion
3.1. Anaerobic Activities of Single and Mixed Inocula
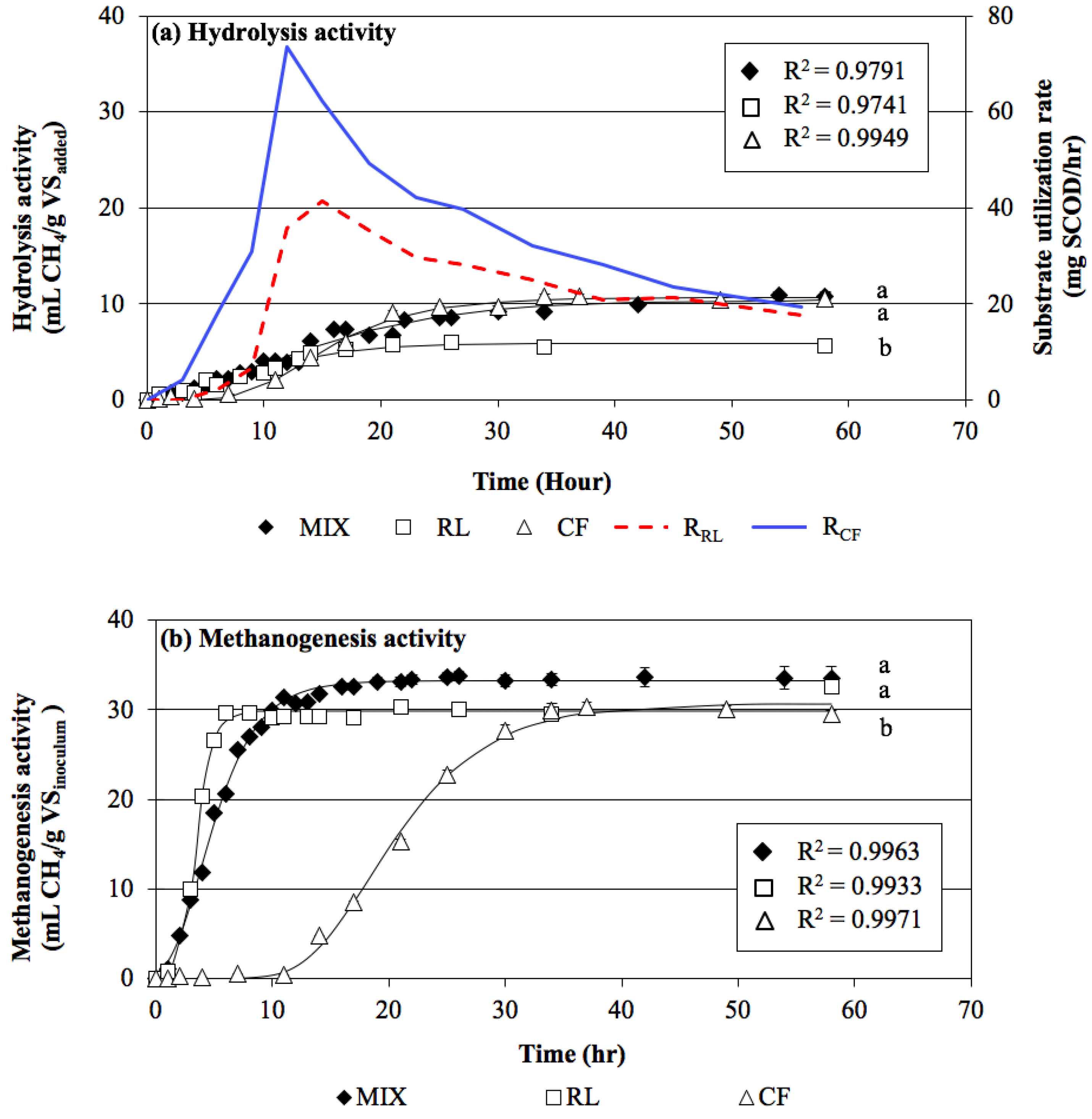
3.1.1. Hydrolysis Activity
3.1.2. Methanogenesis Activity
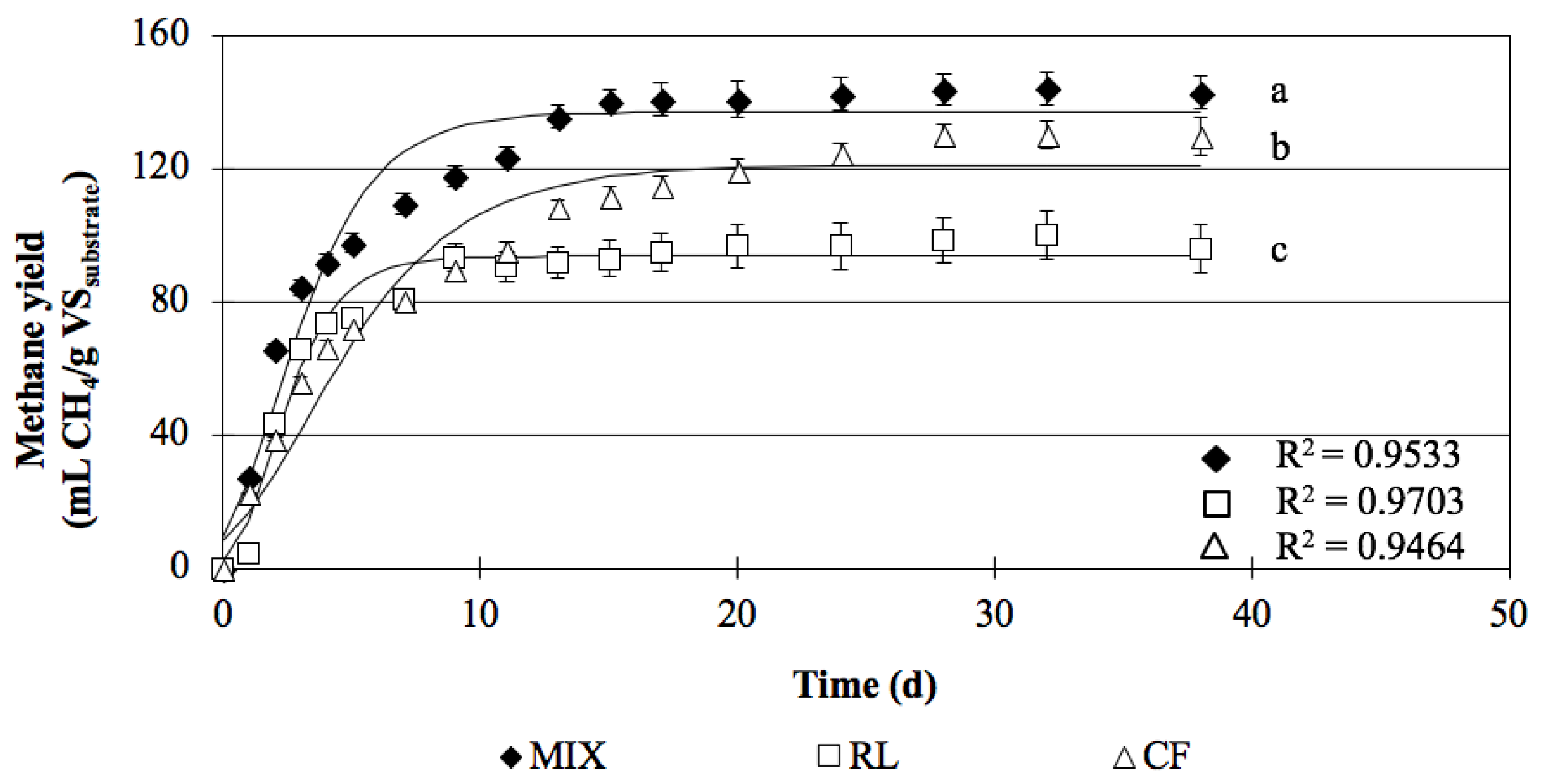
3.1.3. Verification Test with Biomass Digestion
3.2. Digestibility of Napier Grass Silage under Size Reduction and Sonication Pretreatments
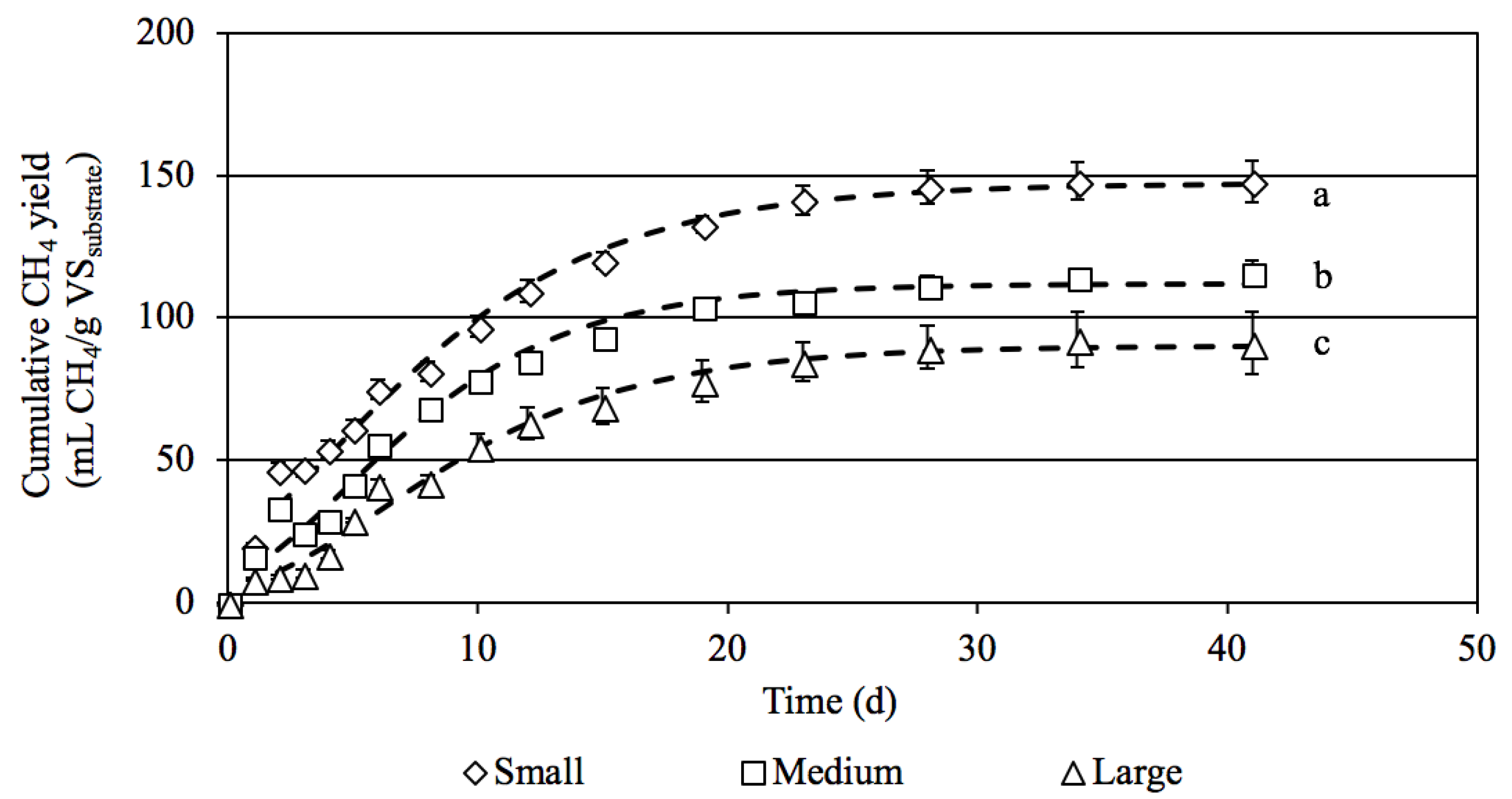
3.2.1. Effects of Particle Size of Napier Grass Silage on Methane Production
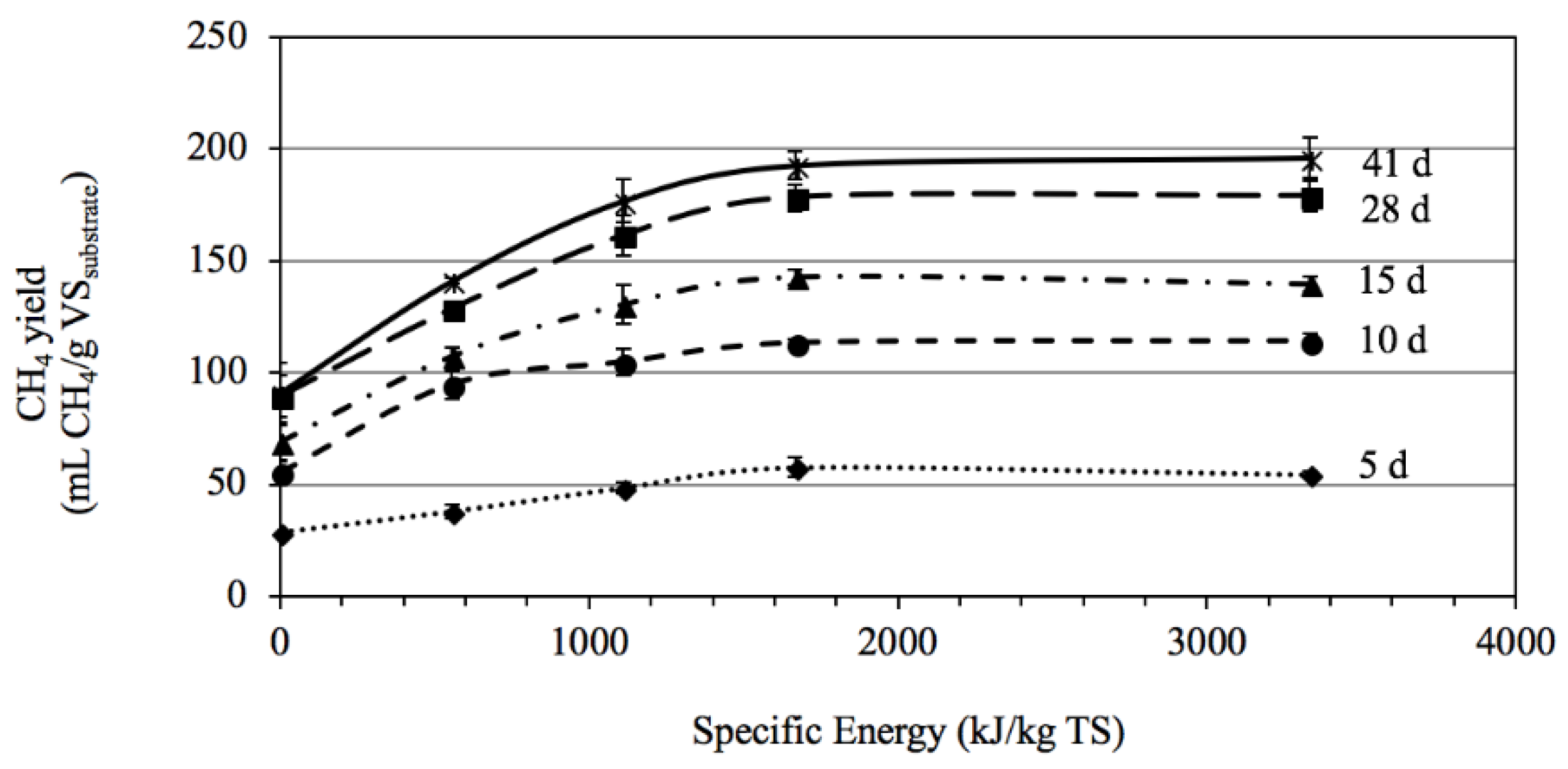
3.2.2. Effects of Sonication Pretreatment on Methane Production
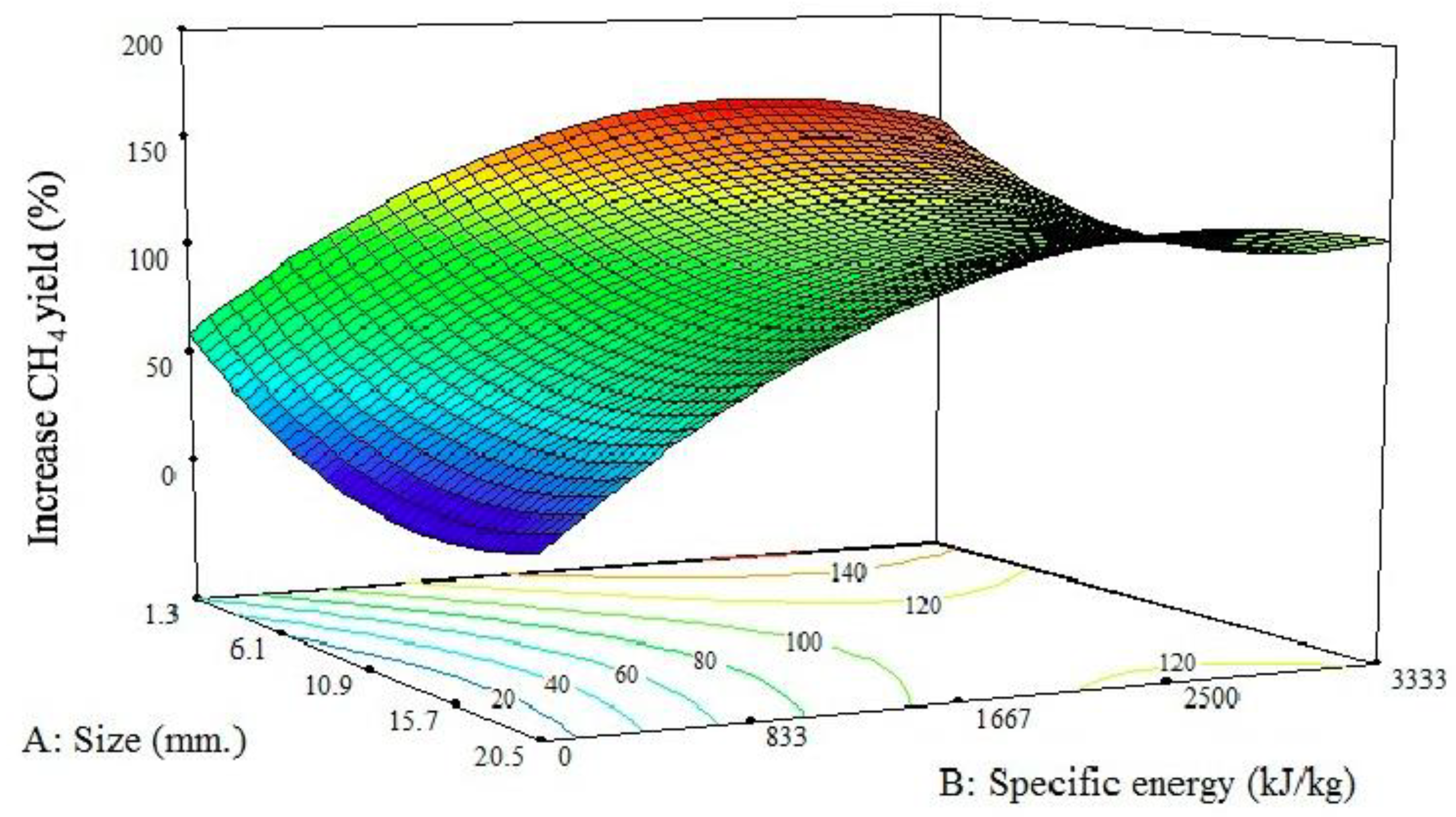
3.2.3. Interrelationship of Particle Size and Sonication Intensity on Anaerobic Digestibility
+ 0.304 × (SIZ2) − 1.673 × 10−5(SE2).
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García-Álvarez, M.T.; Moreno, B. Environmental performance assessment in the EU: A challenge for the sustainability. J. Clean. Prod. 2018, 205, 266–280. [Google Scholar] [CrossRef]
- Frigon, J.C.; Mehta, P.; Guiot, S.R. Impact of mechanical, chemical and enzymatic pre-treatments on the methane yield from the anaerobic digestion of switchgrass. Biomass Bioenergy 2012, 36, 1–11. [Google Scholar] [CrossRef]
- Nkemka, V.N.; Gilroyed, B.; Yanke, J.; Gruninger, R.; Vedres, D.; McAllister, T.; Hao, X. Bioaugmentation with an anaerobic fungus in a two-stage process for biohydrogen and biogas production using corn silage and cattail. Bioresour. Technol. 2015, 185, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Phuttaro, C.; Sawatdeenarunat, C.; Surendra, K.C.; Boonsawang, P.; Chaiprapat, S.; Khanal, S.K. Anaerobic digestion of hydrothermally-pretreated lignocellulosic biomass: Influence of pretreatment temperatures, inhibitors and soluble organics on methane yield. Bioresour. Technol. 2019, 284, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Department of Alternative Energy Development and Efficiency. Napier Grass Pakchong 1 Cultivation Handbook; Department of Alternative Energy Development and Efficiency Ministry of Energy: Bangkok, Thailand, 2013.
- Surendra, K.C.; Ogoshi, R.; Reinhardt-Hanisch, A.; Oechsner, H.; Zaleski, H.M.; Hashimoto, A.G.; Khanal, S.K. Anaerobic digestion of high-yielding tropical energy crops for biomethane production: Effects of crop types, locations and plant parts. Bioresour. Technol. 2018, 262, 194–202. [Google Scholar] [CrossRef]
- Haegele, M.T.; Arjharn, W. The effects of cultivation methods and planting season on biomass yield of Napier grass (Pennisetum purpureum Schumach.) under rainfed conditions in the northeast region of Thailand. Field Crop. Res. 2017, 214, 359–364. [Google Scholar] [CrossRef]
- Sawasdee, V.; Pisutpaisal, N. Feasibility of Biogas Production from Napier Grass. Energy Procedia 2014, 61, 1229–1233. [Google Scholar] [CrossRef]
- Strezov, V.; Evans, T.J.; Hayman, C. Thermal conversion of elephant grass (Pennisetum Purpureum Schum) to bio-gas, bio-oil and charcoal. Bioresour. Technol. 2008, 99, 8394–8399. [Google Scholar] [CrossRef]
- Mohammed, I.; Abakr, Y.; Kazi, F.; Yusup, S.; Alshareef, I.; Chin, S. Comprehensive Characterization of Napier Grass as a Feedstock for Thermochemical Conversion. Energies 2015, 8, 3403–3417. [Google Scholar] [CrossRef]
- Seidl, P.R.; Goulart, A.K. Pretreatment processes for lignocellulosic biomass conversion to biofuels and bioproducts. Curr. Opin. Green Sustain. Chem. 2016, 2, 48–53. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzym. Res. 2011, 2011, 1–17. [Google Scholar] [CrossRef] [PubMed]
- De Morandim-Giannetti, A.A.; Albuquerque, T.S.; de Carvalho, R.K.C.; Silva Araújo, R.M.; Magnabosco, R. Study of “napier grass” delignification for production of cellulosic derivatives. Carbohydr. Polym. 2013, 92, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Jeon, B.H.; Choi, J.A.; Kim, H.C.; Hwang, J.H.; Abou-Shanab, R.A.I.; Dempsey, B.A.; Regan, J.M.; Kim, J.R. Ultrasonic disintegration of microalgal biomass and consequent improvement of bioaccessibility/bioavailability in microbial fermentation. Biotechnol. Biofuels 2013, 6, 37. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops–A review. Ultrason. Sonochem. 2018, 48, 538–549. [Google Scholar] [CrossRef]
- Pilli, S.; Bhunia, P.; Yan, S.; LeBlanc, R.J.; Tyagi, R.D.; Surampalli, R.Y. Ultrasonic pretreatment of sludge: A review. Ultrason. Sonochem. 2011, 18, 1–18. [Google Scholar] [CrossRef]
- Zou, S.; Wang, X.; Chen, Y.; Wan, H.; Feng, Y. Enhancement of biogas production in anaerobic co-digestion by ultrasonic pretreatment. Energy Convers. Manag. 2016, 112, 226–235. [Google Scholar] [CrossRef]
- Rasapoor, M.; Ajabshirchi, Y.; Adl, M.; Abdi, R.; Gharibi, A. The effect of ultrasonic pretreatment on biogas generation yield from organic fraction of municipal solid waste under medium solids concentration circumstance. Energy Convers. Manag. 2016, 119, 444–452. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A.; Mohee, R. Ultrasound-assisted biological conversion of biomass and waste materials to biofuels: A review. Ultrason. Sonochem. 2018, 40, 298–313. [Google Scholar] [CrossRef]
- Dechrugsa, S.; Kantachote, D.; Chaiprapat, S. Effects of inoculum to substrate ratio, substrate mix ratio and inoculum source on batch co-digestion of grass and pig manure. Bioresour. Technol. 2013, 146, 101–108. [Google Scholar] [CrossRef]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.L.; Guwy, A.J.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier Abstract, J.B.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 595, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Khalid, H.; Zhu, Z.; Zhang, R.; Liu, G.; Chen, C.; Thorin, E. Methane production through anaerobic digestion: Participation and digestion characteristics of cellulose, hemicellulose and lignin. Appl. Energy 2018, 226, 1219–1228. [Google Scholar] [CrossRef]
- Li, Y.; Khanal, S.K. (Eds.) Bioenergy: Principles and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; ISBN 9781118568316. [Google Scholar]
- Bougrier, C.; Carrère, H.; Delgenès, J.P. Solubilisation of waste-activated sludge by ultrasonic treatment. Chem. Eng. J. 2005, 106, 163–169. [Google Scholar] [CrossRef]
- American Public Health Association (APHA). Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association/American Water Works Association/Water Environment Federation: Washington, DC, USA, 2012. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Venkiteshwaran, K.; Bocher, B.; Maki, J.; Zitomer, D. Relating Anaerobic Digestion Microbial Community and Process Function. Microbiol. Insights 2015, 8, 37–44. [Google Scholar]
- Maamri, S.; Amrani, M. Biogas Production from Waste Activated Sludge Using Cattle Dung Inoculums: Effect of Total Solid Contents and Kinetics Study. Energy Procedia 2014, 50, 352–359. [Google Scholar] [CrossRef]
- Dowd, S.E.; Callaway, T.R.; Wolcott, R.D.; Sun, Y.; McKeehan, T.; Hagevoort, R.G.; Edrington, T.S. Evaluation of the bacterial diversity in the feces of cattle using 16S rDNA bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP). BMC Microbiol. 2008, 8, 125. [Google Scholar] [CrossRef]
- Auphimai, C.; Rukruam, W.; Chaiprasert, P. Efficacies of Various Inoculum Sources on Methane Production from Agro-industrial Wastewaters. Energy Procedia 2014, 52, 167–172. [Google Scholar] [CrossRef]
- Rosman, N.H.; Nor Anuar, A.; Chelliapan, S.; Md Din, M.F.; Ujang, Z. Characteristics and performance of aerobic granular sludge treating rubber wastewater at different hydraulic retention time. Bioresour. Technol. 2014, 161, 155–161. [Google Scholar] [CrossRef]
- Satoh, H.; Miura, Y.; Tsushima, I.; Okabe, S. Layered structure of bacterial and archaeal communities and their in situ activities in anaerobic granules. Appl. Environ. Microbiol. 2007, 73, 7300–7307. [Google Scholar] [CrossRef]
- Nuchdang, S.; Khemkhao, M.; Techkarnjanaruk, S.; Phalakornkule, C. Comparative biochemical methane potential of paragrass using an unacclimated and an acclimated microbial consortium. Bioresour. Technol. 2015, 183, 111–119. [Google Scholar] [CrossRef]
- Saidu, M.; Yuzir, A.; Salim, M.R.; Azman, S.; Abdullah, N. Biological pre-treated oil palm mesocarp fibre with cattle manure for biogas production by anaerobic digestion during acclimatization phase. Int. Biodeterior. Biodegrad. 2014, 95, 189–194. [Google Scholar] [CrossRef]
- Gu, Y.; Chen, X.; Liu, Z.; Zhou, X.; Zhang, Y. Effect of inoculum sources on the anaerobic digestion of rice straw. Bioresour. Technol. 2014, 158, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Haag, N.L.; Steinbrenner, J.; Demmig, C.; Nägele, H.-J.; Oechsner, H. Influence on lactic acid content in maize silage variations by manganese supplementation. Ind. Crops Prod. 2016, 79, 146–151. [Google Scholar] [CrossRef]
- Pensri, B.; Aggarangsi, P.; Chaiyaso, T.; Chandet, N. Potential of Fermentable Sugar Production from Napier cv. Pakchong 1 Grass Residue as a Substrate to Produce Bioethanol. Energy Procedia 2016, 89, 428–436. [Google Scholar] [CrossRef]
- Surendra, K.C.; Khanal, S.K. Effects of crop maturity and size reduction on digestibility and methane yield of dedicated energy crop. Bioresour. Technol. 2015, 178, 187–193. [Google Scholar] [CrossRef]
- Menardo, S.; Airoldi, G.; Balsari, P. The effect of particle size and thermal pre-treatment on the methane yield of four agricultural by-products. Bioresour. Technol. 2012, 104, 708–714. [Google Scholar] [CrossRef]
- Tumutegyereize, P.; Muranga, F.I.; Kawongolo, J.; Nabugoomu, F. Optimization of biogas production from banana peels: Effect of particle size on methane yield. Afr. J. Biotechnol. 2011, 10, 18243–18251. [Google Scholar] [CrossRef]
- Izumi, K.; Okishio, Y.; Nagao, N.; Niwa, C.; Yamamoto, S.; Toda, T. Effects of particle size on anaerobic digestion of food waste. Int. Biodeterior. Biodegrad. 2010, 64, 601–608. [Google Scholar] [CrossRef]
- Speda, J.; Johansson, M.A.; Odnell, A.; Karlsson, M. Enhanced biomethane production rate and yield from lignocellulosic ensiled forage ley by in situ anaerobic digestion treatment with endogenous cellulolytic enzymes. Biotechnol. Biofuels 2017, 10, 129. [Google Scholar] [CrossRef]
- Li, X.; Guo, S.; Peng, Y.; He, Y.; Wang, S.; Li, L.; Zhao, M. Anaerobic digestion using ultrasound as pretreatment approach: Changes in waste activated sludge, anaerobic digestion performances and digestive microbial populations. Biochem. Eng. J. 2018, 139, 139–145. [Google Scholar] [CrossRef]
- Greenly, J.M.; Tester, J.W. Ultrasonic cavitation for disruption of microalgae. Bioresour. Technol. 2015, 184, 276–279. [Google Scholar] [CrossRef] [PubMed]
- Li, M.-F.; Sun, S.-N.; Xu, F.; Sun, R.-C. Ultrasound-enhanced extraction of lignin from bamboo (Neosinocalamus affinis): Characterization of the ethanol-soluble fractions. Ultrason. Sonochem. 2012, 19, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Fang, Z.; Smith, R.L. Ultrasound-enhanced conversion of biomass to biofuels. Prog. Energy Combust. Sci. 2014, 41, 56–93. [Google Scholar] [CrossRef]

| Parameter | Fresh | Silage |
|---|---|---|
| Proximate analysis | ||
| Moisture (%) | 86.2 | 77.8 |
| TS (g/kg wet wt.) | 138.4 | 232.5 |
| VS (g/kg dry wt.) | 918.8 | 892.5 |
| Cellulose (%) | 37.4 | 42.8 |
| Hemicellulose (%) | 29.2 | 24.6 |
| Lignin (%) | 9.6 | 11.3 |
| Ultimate analysis | ||
| C (%) | 41.8 | 39.9 |
| H (%) | 5.3 | 5.1 |
| N (%) | 1.5 | 0.7 |
| S (%) | 0.1 | 0.1 |
| O (%) | 28.4 | 32.3 |
| Ash and others (%) | 22.9 | 21.9 |
| Parameters | Specific Energy (kJ/kg TS) | ||||
|---|---|---|---|---|---|
| 0 | 556 | 1111 | 1667 | 3333 | |
| SCOD (mg COD/L) * | |||||
| Small | 121.00 ± 12.47 a | 610.00 ± 8.16 b | 813.33 ± 10.27 c | 831.67 ± 2.36 c | 891.67 ± 10.80 d |
| Medium | 128.33 ± 21.60 a | 623.33 ± 25.50 b | 711.67 ± 11.79 c | 723.33 ± 13.12 c | 725.00 ± 13.12 c |
| Large | 23.33 ± 8.16 a | 141.67 ± 8.16 b | 216.67 ± 6.24 c | 286.67 ± 10.27 d | 288.33 ± 8.50 d |
| Digestibility at 41 days (%) | |||||
| Small | 34.06 ± 1.69 a | 39.53 ± 1.43 ab | 48.59 ± 3.22 bc | 51.85 ± 2.30 c | 53.51 ± 2.06 c |
| Medium | 26.64 ± 1.00 a | 36.90 ± 1.57 b | 40.69 ± 2.28 bc | 43.77 ± 1.46 bc | 43.63 ± 1.80 c |
| Large | 20.97 ± 2.52 a | 32.48 ± 0.20 b | 40.75 ± 1.8 c | 44.41 ± 1.12 c | 45.19 ± 1.12 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phuttaro, C.; Reungsang, A.; Boonsawang, P.; Chaiprapat, S. Integrative Effects of Sonication and Particle Size on Biomethanation of Tropical Grass Pennisetum purpureum Using Superior Diverse Inocula Cultures. Energies 2019, 12, 4226. https://doi.org/10.3390/en12224226
Phuttaro C, Reungsang A, Boonsawang P, Chaiprapat S. Integrative Effects of Sonication and Particle Size on Biomethanation of Tropical Grass Pennisetum purpureum Using Superior Diverse Inocula Cultures. Energies. 2019; 12(22):4226. https://doi.org/10.3390/en12224226
Chicago/Turabian StylePhuttaro, Chettaphong, Alissara Reungsang, Piyarat Boonsawang, and Sumate Chaiprapat. 2019. "Integrative Effects of Sonication and Particle Size on Biomethanation of Tropical Grass Pennisetum purpureum Using Superior Diverse Inocula Cultures" Energies 12, no. 22: 4226. https://doi.org/10.3390/en12224226
APA StylePhuttaro, C., Reungsang, A., Boonsawang, P., & Chaiprapat, S. (2019). Integrative Effects of Sonication and Particle Size on Biomethanation of Tropical Grass Pennisetum purpureum Using Superior Diverse Inocula Cultures. Energies, 12(22), 4226. https://doi.org/10.3390/en12224226







