Optimization, Transesterification and Analytical Study of Rhus typhina Non-Edible Seed Oil as Biodiesel Production †
Abstract
:1. Introduction
2. Materials and Methods
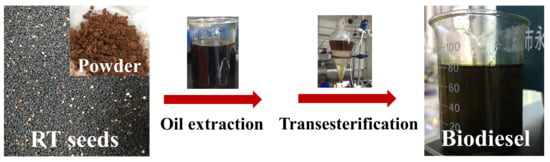
2.1. Source Collection and Preparation of Seeds for Oil Extraction
2.2. Oil Extraction
2.3. Biodiesel Production Procedure
2.4. FTIR Study
2.5. NMR Study
2.6. GCMS Study
2.7. ICP-OES and EA Study of RT Biodiesel for Elemental Analysis
3. Results and Discussion
3.1. Optimizations of Reaction Variables on Conversion Yield
3.1.1. Effect of Methanol to Oil Molar Ratio on Yield
3.1.2. Effect of Reaction Temperature on FAMEs Yield
3.1.3. Effect of the Catalyst Concentration on FAMEs Yield
3.1.4. Effect of Agitation Speed on FAMEs Yield
3.1.5. Influence of Reaction Time on FAMEs Yield
3.2. Physio-Chemical Characterization of R. typhina Methyl Ester
3.3. NMR Spectroscopy
3.3.1. 1H NMR Analysis
3.3.2. 13C NMR Analysis
3.4. FT–IR Spectroscopy Analysis
3.5. Profiling of R. typhina Oil Fatty Acid Methyl Esters Using GC–MS Analysis
3.6. ICP-OES and EA Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hoseini, S.S.; Najafi, G.; Ghobadian, B.; Mamat, R.; Ebadi, M.T.; Yusaf, T. Ailanthus altissima (tree of heaven) seed oil: Characterisation and optimisation of ultrasonication-assisted biodiesel production. Fuel 2018, 220, 621–630. [Google Scholar] [CrossRef]
- Ahmad, M.; Zafar, M.; Sadia, H.; Sultana, S.; Arshad, M.; Irfan, M. Physico-chemical characterization of sunflower oil biodiesel by using base-catalyzed transesterification. Int. J. Green Energy 2013. [Google Scholar] [CrossRef]
- Živković, S.B.; Veljković, M.V.; Banković-Ilić, I.B.; Krstić, I.M.; Konstantinović, S.S.; Ilić, S.B. Technological, technical, economic, environmental, social, human health risk, toxicological, and policy considerations of biodiesel production and use. Renew. Sustain. Energy Rev. 2017, 79, 222–247. [Google Scholar] [CrossRef]
- Bueno, A.V. Performance and emissions characteristics of castor oil biodiesel fuel blends. Appl. Therm. Eng. 2017, 125, 559–566. [Google Scholar] [CrossRef]
- Mucak, A. Performance and emission characteristics of a diesel engine fuelled with emulsified biodiesel-diesel fuel blends. Int. J. Autom. Eng. Technol. 2016, 5, 176–185. [Google Scholar] [CrossRef]
- Vahid, B.R.; Haghighi, M. Urea-nitrate combustion synthesis of MgO/MgAl2O4 nanocatalyst used in biodiesel production from sunflower oil: Influence of fuel ratio on catalytic properties and performance. Energy Convers. Manag. 2016, 126, 362–372. [Google Scholar] [CrossRef]
- Gülsen, E.; Olivetti, E.; Freire, F.; Dias, L.; Kirchain, R. Impact of feedstock diversification on the cost-effectiveness of biodiesel. Appl. Energy 2014, 126, 281–296. [Google Scholar] [CrossRef]
- Ashraful, A.M. Production and comparison of fuel properties, engine performance, and emission characteristics of biodiesel from various non-edible vegetable oils: A review. Energy Convers. Manag. 2014, 80, 202–228. [Google Scholar] [CrossRef]
- Sharma, Y.C.; Singh, B. Development of biodiesel: Current scenario. Renew. Sustain. Energy Rev. 2009, 13, 1646–1651. [Google Scholar]
- Kansedo, J.; Lee, K.T. Process optimization and kinetic study for biodiesel production from non-edible sea mango (Cerbera odollam) oil using response surface methodology. Chem. Eng. J. 2013, 214, 157–164. [Google Scholar] [CrossRef]
- Mustafa, B. Potential alternatives to edible oils for biodiesel production-a review of current work. Energy Convers. Manag. 2011, 52, 1479–1492. [Google Scholar]
- Rashed, M.M.; Kalam, M.A.; Masjuki, H.H.; Mofijur, M.; Rasul, M.G. Performance and emission characteristics of a diesel engine fueled with palm, jatropha, and moringa oil methyl ester. Ind. Crops Prod. 2016, 79, 70–76. [Google Scholar] [CrossRef]
- Fadhil, A.B. Evaluation of apricot (Prunus armeniaca L.) seed kernel as a potential feedstock for the production of liquid biofuels and activated carbons. Energy Convers. Manag. 2017, 133, 307–317. [Google Scholar] [CrossRef]
- Sinha, P.; Islam, M.A.; Negi, M.S.; Tripathi, S.B. Changes in oil content and fatty acid composition in Jatropha curcas during seed development. Ind. Crops Prod. 2015, 77, 508–510. [Google Scholar] [CrossRef]
- Foidl, N.; Foidl, G.; Sanchez, M.; Mittelbach, M.; Hackel, S. Jatropha Curcas L. as a source for the production of biofuel in Nicaragua. Bioresour. Technol. 1996, 58, 77–82. [Google Scholar] [CrossRef]
- Bhatti, H.N.; Hanif, M.A.; Qasim, M.; Ata-ur-Rehman. Biodiesel production from waste tallow. Fuel 2008, 87, 2961–2966. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Aziz, A.M.; Altamer, M.H. Biodiesel production from Silybum marianum L. seed oil with high FFA content using sulfonated carbon catalyst for esterification and base catalyst for transesterification. Energy Convers. Manag. 2016, 108, 255–265. [Google Scholar] [CrossRef]
- Aldobouni, I.A.; Fadhil, A.B.; Saied, I.K. Optimized alkali - catalyzed transesterification of wild mustard (Brassica juncea L.) seed oil. Energy Sources Part A 2016, 38, 2319–2325. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.J.; Xing, W.H.; Jaime, A.; da Silva, T. The potential of five plants growing on unproductive agriculture land as a biodiesel source. Renew. Energy 2012, 41, 191–199. [Google Scholar] [CrossRef]
- Zhang, F.; Ren, H.; Tong, G. Production of biodiesel from the extract of the sumac fruit cluster. Cellul. Chem. Technol. 2018, 52, 1275–1279. [Google Scholar]
- Kpikpi, W.M. Jatropha curcus as a vegetable source of renewable energy. ANSTI Sub Netw. Meet. Renew. Energy 2002, 13, 18–22. [Google Scholar]
- Ahmad, M.; Khan, M.A.; Zafar, M.; Sultana, S. Practical Handbook on Biodiesel Production and Properties; Taylor and Francis: London, UK, 2012; pp. 1–157. [Google Scholar]
- Antolin, G.; Tinaut, F.V.; Briceno, Y.; Castano, V.; Perez, C.; Ramrez, A.I. Optimization of biodiesel production by sunflower oil transesterification. Bioresour. Technol. 2002, 83, 111–114. [Google Scholar] [CrossRef]
- Bhandari, D.C.; Chandel, K.P.S. Status of rocket germplasm in India: Research accomplishments and priorities. Rocket Mediterr. Crop World 1996, 67, 13–14. [Google Scholar]
- Christie, W.W. Lipid Analysis, 3rd ed.; Oily Press: Bridgwater, UK, 2003. [Google Scholar]
- Encinar, J.M.; Gonzalez, J.F.; Rodriguez, R.A. Biodiesel from used frying oil. Variables affecting the yields and characteristics of the biodiesel. Ind. Eng. Chem. Res. 2005, 44, 5491–5499. [Google Scholar] [CrossRef]
- Dorado, M.P.; Ballesteros, E.; Lopez, F.J.; Mittelbach, M. Optimization of alkali catalyzed transesterification of Brassica Carinata oil for biodiesel production. Energy Fuels 2004, 18, 77–83. [Google Scholar] [CrossRef]
- Leung, D.; Guo, Y. Transesterification of neat and used frying oil: Optimization for biodiesel production. Fuel Process. Technol. 2006, 87, 883–890. [Google Scholar] [CrossRef]
- Kafuku, G.; Mbarawa, M. Alkaline catalyzed biodiesel production from Moringa oleifera oil with optimized production parameters. Appl. Energy 2010, 87, 2561–2565. [Google Scholar] [CrossRef]
- Rashid, U.; Anwar, F. Production of biodiesel through optimized alkaline-catalyzed transesterification of rapeseed oil. Fuel 2008, 87, 265–273. [Google Scholar] [CrossRef]
- Ma, F.; Clements, L.D.; Hanna, M.A. The effect of mixing on transesterification of beef tallow. Bioresour. Technol. 1999, 69, 289–293. [Google Scholar] [CrossRef]
- Peterson, C.L.; Reece, D.L.; Cruz, R.; Thompson, J. A comparison of ethyl and methyl esters of vegetable oil as diesel fuel substitutes. In Proceedings of the an Alternative Energy Conference, Nashville, TN, USA, 12–15 December 1992; pp. 99–110. [Google Scholar]
- Tomasevic, A.V.; Siler-Marinkovic, S.S. Methanolysis of Used Frying Oil. Fuel Process. Technol. 2003, 82, 1–6. [Google Scholar] [CrossRef]
- Eevera, T.; Rajendran, K.; Saradha, S. Biodiesel production process optimization and characterization to assess the suitability of the product for varied environmental conditions. Renew. Energy 2009, 34, 762–765. [Google Scholar] [CrossRef]
- Shu, Q.; Gao, J.; Nawaz, Z.; Liao, Y.; Wang, D.; Wang, J. Synthesis of biodiesel from waste vegetable oil with large amounts of free fatty acids using a carbon-based solid acid catalyst. Appl. Energy 2010, 87, 2589–2596. [Google Scholar] [CrossRef]
- Mello, V.M.; Oliveira, C.C.; Fraga, G.W.; Nascimento, C.J.D.; Suarez, P.A.Z. Determination of the content of fatty acid methyl esters (FAMEs) in biodiesel samples using 1H NMR spectroscopy. Magn. Reson. Chem. 2008, 46, 1051–1054. [Google Scholar] [CrossRef] [PubMed]
- Monterio, M.R.; Ambrozin, A.R.P.; Liao, L.M.; Ferreira, A.G. Determination of biodiesel blends levels in different diesel samples by 1H NMR. Fuel 2009, 88, 691–696. [Google Scholar] [CrossRef]
- Samios, D.; Pedrotti, F.; Nicolau, A.; Martini, D.D.; Dalcen, F.M. A transesterification double process-TDSP for biodiesel preparation from fatty acid tryglycerides. Fuel Process. Technol. 2009, 90, 599–605. [Google Scholar] [CrossRef]
- Liu, C.; Lv, P.; Yuan, Z.; Yan, F.; Luo, W. Biodiesel from different oil using fixed-bed and flow reactors. Renew. Energy 2010, 35, 1531–1536. [Google Scholar] [CrossRef]
- Gelbard, G.; Bres, O.; Vargas, R.M.; Vielfaure, F.; Schuchardt, U.F. 1H nuclear magnetic resonance determination of the yield of the transesterification of rapeseed oil with methanol. Am. Oil Chem. Soc. 1995, 72, 1239–1241. [Google Scholar] [CrossRef]
- Knothe, G. Monitoring a progressing transesterification reaction by fiber optic NIR spectroscopy with correlation to 1H NMR spectroscopy. Am. Oil Chem. Soc. 2000, 77, 489–493. [Google Scholar] [CrossRef]
- Pasto, D.; Johnson, C.; Miller, M. Experiments and Techniques in Organic Chemistry, 1st ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1992. [Google Scholar]
- Guillen, M.D.; Cabo, N. Infra-red spectroscopy in the study of edible oils and fats. Sci. Food Agric. 1997, 75, 1–11. [Google Scholar] [CrossRef]
- Safar, M.; Bertrand, D.; Robert, P.; Devaux, M.D.; Genut, C. Characterization of edible oil, butter, and margarine by Fourier Transfer Infra-Red spectroscopy with attenuated total reflectance. Am. Chem. Soc. 1994, 71, 371–377. [Google Scholar] [CrossRef]
- Ahmad, M.; Ullah, K.; Khan, M.A.; Zafar, M.; Tariq, M.; Ali, S. Physico-chemical analysis of hemp oil biodiesel: A promising non-edible new source for bioenergy. Energy Sources Part A 2011, 33, 1365–1374. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H. Biodiesel from Siberian apricot (Prunus sibirica L.) seed kernel oil. Bioresour. Technol. 2012, 112, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Schober, S.; Mittelbach, M. Influence of diesel particulate filter additives on biodiesel Quality. Eur. J. Lipid Sci. Technol. 2005, 107, 268–271. [Google Scholar] [CrossRef]
- McCormick, R.L.; Alleman, T.L.; Ratcliff, M.; Moens, L.; Lawrence, R. Survey of the Quality and Stability of Biodiesel and Biodiesel Blends in the United States in 2004; National Renewable Energy Laboratory: Golden, CO, USA, 2005.
- Song, H.; Quinton, K.S.; Peng, Z.; Zhao, H.; Ladommatos, N. Effects of the oxygen content of Fuels on combustion and emissions of diesel engines. Energies 2016, 9, 28. [Google Scholar] [CrossRef] [Green Version]
- Nakano, M.; Okawa, K. Study of oxygen-containing hydrocarbons in exhaust emission from a spark ignition combustion engine. Int. J. Eng. Res. 2014, 15, 572–580. [Google Scholar] [CrossRef]
- Mwang, J.K.; Lee, W.J.; Chang, Y.C.; Chen, C.Y.; Wang, L.C. An overview: Energy saving and pollution reduction by using green fuel blends in diesel engines. Appl. Energy 2015, 159, 214–236. [Google Scholar] [CrossRef]
- Lin, B.F.; Huang, J.H.; Huang, D.Y. Effects of Biodiesel from Palm Kernel oil on the engine performance, Exhaust emissions and combustion characteristics of a direct injection diesel engine. Energy Fuels 2008, 22, 4229–4234. [Google Scholar] [CrossRef]
- Singh, D.; Subramanian, K.A.; Juneja, M.; Singh, K.; Singh, S. Investigating the effect of fuel cetane number, oxygen content, fuel density, and engine operating variables on NOx emissions of a heavy-duty diesel engine. Environ. Prog. Sustain. Energy 2017, 36, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Demirbas, A. Combustion efficiency impacts of biofuels. Energy Sources Part A 2009, 31, 602–609. [Google Scholar] [CrossRef]
- Huber, G.W.; Iborra, S.; Corma, A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering. Chem. Rev. 2006, 106, 4044–4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korn, M.G.A.; Santos, D.S.S.; Welz, B.; Vale, M.G.R.; Teixeira, A.P.; Lima, D.D.C.; Ferreira, S.L.C. Atomic spectrometric methods for the determination of metals and metalloids in automotive fuels. A Review. Talanta 2007, 73, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Acıkalın, K.; Karaca, F.; Bolat, E. Pyrolysis of pistachio shell: Effects of pyrolysis conditions and analysis of products. Fuel 2012, 95, 169–177. [Google Scholar] [CrossRef]
- Uysal, T.; Duman, G.; Onal, Y.; Yasa, I.; Yanik, J. Production of activated carbon and fungicidal oil from peach stone by a two-stage process. J. Anal. Appl. Pyrolysis 2014, 108, 47–55. [Google Scholar] [CrossRef]
- Demiral, I.; Kul, S.C. Pyrolysis of apricot kernel shell in a fixed-bed reactor: Characterization of bio-oil and char. J. Anal. Appl. Pyrolysis 2014, 107, 17–24. [Google Scholar] [CrossRef]
- Duman, G.; Okutucu, C.; Ucar, S.; Stah, R.; Yanik, J. The slow and fast pyrolysis of cherry seed. Bioresour. Technol. 2011, 102, 1869–1878. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Singh, R.K.; Bendu, H.; Mund, R. Pyrolysis of Mahua seed (Madhuca indica) production of biofuel and its characterization. Energy Convers. Manag. 2016, 108, 529–538. [Google Scholar] [CrossRef]












| Parameter. | Descriptions |
|---|---|
| Column | QP2010SE, Shimadzu PEG-20M Length: 30 m Internal diameter: 0.32 mm Film thickness: 1 um |
| Injector temperature | 220 °C |
| Detector temperature (EI 250) | 210 °C |
| Carrier gas | Helium, flow rate = 1.2 mL min−1 |
| Injection | V = 1 uL |
| Split | Flow rate = 40:1 |
| Temperature program | Initial temperature = 100 °C Rate of progression = 10 °C min−1. Final temperature = 210 °C, 20 min. |
| Solvent | FFAs Content (wt. %) | Oil Content (wt. %) |
|---|---|---|
| Petroleum ether | 1.0 | 22 |
| Acetone | 1.5 | 16 |
| Dichloromethane | 1.8 | 14.2 |
| Ethyl acetate | 1.3 | 17.8 |
| Studied Parameters | EN 14214 | ASTM D6751 | Petro-Diesel | RT Experimental Result | ZHANG et al., 2018 [21] | Ruan et al., 2012 [20] |
|---|---|---|---|---|---|---|
| Oil content% | - | - | - | 20–22% | 12% | 9.7% |
| Density @ 15 °C (g/cm3) | 0.86–0.90 | 0.86–0.90 | 0.81–0.87 | 0.879 | 0.879 | - |
| Kinematic viscosity @ 40 °C (mm2/S) | 3.5–5.0 | 1.9–6.0 | 1.3–4.1 | 6.3 | 6.87 | - |
| Flash point, (°C) | Min 120.0 | Min 130 | ≥52 | 168 | 165 | - |
| Free fatty acid (%) | Max 0.50 | ˂1 | - | 1.0 | - | - |
| Saponification value (mg KOH/g) | - | - | - | 175.6 | - | - |
| Iodine value (g I2/100 mg) | Max. 120 | Max. 120 | - | 85 | - | - |
| Cloud point (°C) | - | - | −15–5 | 7 | - | - |
| Pour point (°C) | - | - | −2.0 | −11 | - | - |
| Fire point (°C) | - | - | - | 198 | - | - |
| Oxidation stability (110 °C, h) | Min 6 | Min. 3 | 25.8 | 18.3 | - | - |
| Ash content (g/100 g) | - | - | - | 0.3 | - | - |
| Specific gravity | - | - | - | 0.855 | - | - |
| Cold filter plugging point (CFPP, °C) | Max.19 | Max.19 | −16 | 14 | - | - |
| Sulphur content (w/w%) | <0.01 | <0.01 | - | 0.01 | - | - |
| Phosphorous content (mg/kg) | <10 | <10 | - | 4 | - | - |
| Carbon residue (w/w%) | - | - | - | 0.19 | - | - |
| S/No | Fatty Acids/Exp. Results | Retention Time | Number of Carbons and Double Bonds | Chemical Name | Chemical Structure | Weight Percentage (%) | Molecular Weight | ZHANG et al., 2018 [21] | Ruan et al., 2012 [20] |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Palmitic acid | 9.545 | C16:0 | Hexadecanoic acid, methyl ester |  | 14.0 | 270 | Methyl palmitate C16:0 | Palmitic acid C16:0 |
| 2 | Stearic acid | 14.248 | C18:0 | methyl stearate |  | 3.2 | 298 | Methyl stearate C18:0 | Stearic acid C18:0 |
| 3 | Oleic acid | 15.042 | C18:1 | 9-Octadecenoic acid (Z)-, methyl ester |  | 47.2 | 296 | Methyl oleate C18:1 | Oleic acid C18:1 |
| 4 | Linoleic acid | 16.797 | C18:2 | 9, 12-Octadecadienoic acid (Z, Z)-, methyl ester |  | 32.2 | 294 | Methyl linoleate C18:2 | Linoleic acid C18:2 |
| 5 | α-Linolenic acid | 19.565 | C18:3 | α-Linolenic acid |  | 1.1 | 292 | - | - |
| 6 | Arachidic acid | 22.587 | C20:0 | Eicosanoic acid, methyl ester |  | 0.8 | 326 | - | Arachidic acid C20:0 |
| 7 | Gondoic acid | 23.922 | C20:1 | CiS- 11- Eicosenoic acid, methyl ester |  | 0.5 | 324 | - | Arachidonic acid C20:1 |
| Ultimate Analysis | RT-BD | Pistachio Shell [58] | Peach Stones [59] | Apricot Kernel Shells [60] | Cherry Stones [61] | Mahua Seed [62] |
|---|---|---|---|---|---|---|
| C% | 74.89 | 42.41 | 45.92 | 47.33 | 52.48 | 61.24 |
| H% | 13.02 | 5.64 | 6.09 | 6.37 | 7.58 | 8.40 |
| N% | 1.97 | 0.070 | 0.580 | 0.370 | 4.54 | 4.12 |
| O% | 10.12 | 51.87 | 47.38 | 45.93 | 35.30 | 25.50 |
| HHV (MJ/kg) | 23.73 | 22.21 | 24.07 | 24.29 | 24.11 | 25.30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, I.U.; Yan, Z.; Chen, J. Optimization, Transesterification and Analytical Study of Rhus typhina Non-Edible Seed Oil as Biodiesel Production. Energies 2019, 12, 4290. https://doi.org/10.3390/en12224290
Khan IU, Yan Z, Chen J. Optimization, Transesterification and Analytical Study of Rhus typhina Non-Edible Seed Oil as Biodiesel Production. Energies. 2019; 12(22):4290. https://doi.org/10.3390/en12224290
Chicago/Turabian StyleKhan, Inam Ullah, Zhenhua Yan, and Jun Chen. 2019. "Optimization, Transesterification and Analytical Study of Rhus typhina Non-Edible Seed Oil as Biodiesel Production" Energies 12, no. 22: 4290. https://doi.org/10.3390/en12224290
APA StyleKhan, I. U., Yan, Z., & Chen, J. (2019). Optimization, Transesterification and Analytical Study of Rhus typhina Non-Edible Seed Oil as Biodiesel Production. Energies, 12(22), 4290. https://doi.org/10.3390/en12224290