Effect of Bed Material on Bed Agglomeration for Palm Empty Fruit Bunch (EFB) Gasification in a Bubbling Fluidised Bed System
Abstract
:1. Introduction
2. Methodology
2.1. Biomass Properties and Preparation
2.2. Bed Materials
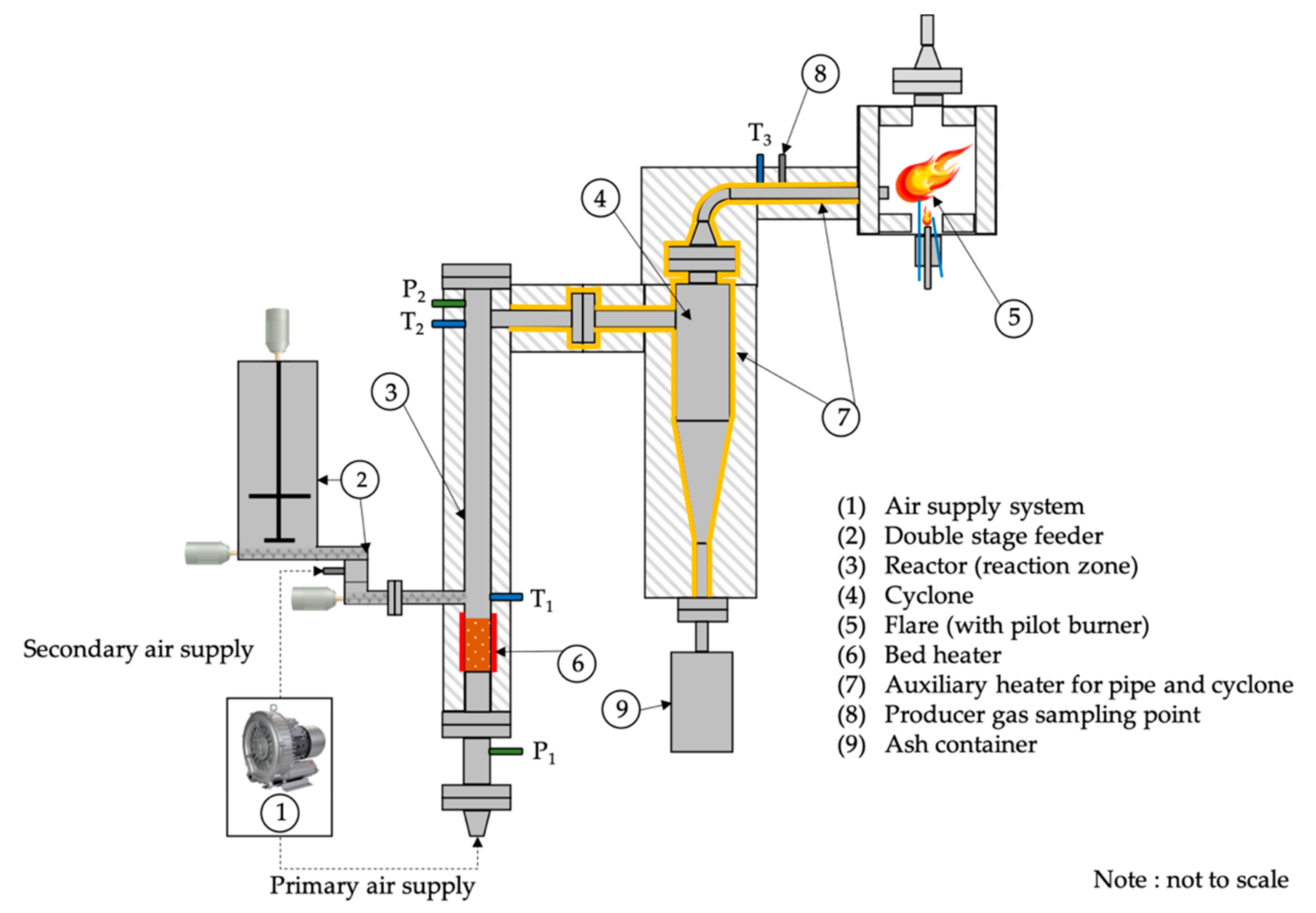
2.3. Bench Scale Fluidised Bed Reactor Gasification System
2.4. Experiment Procedure
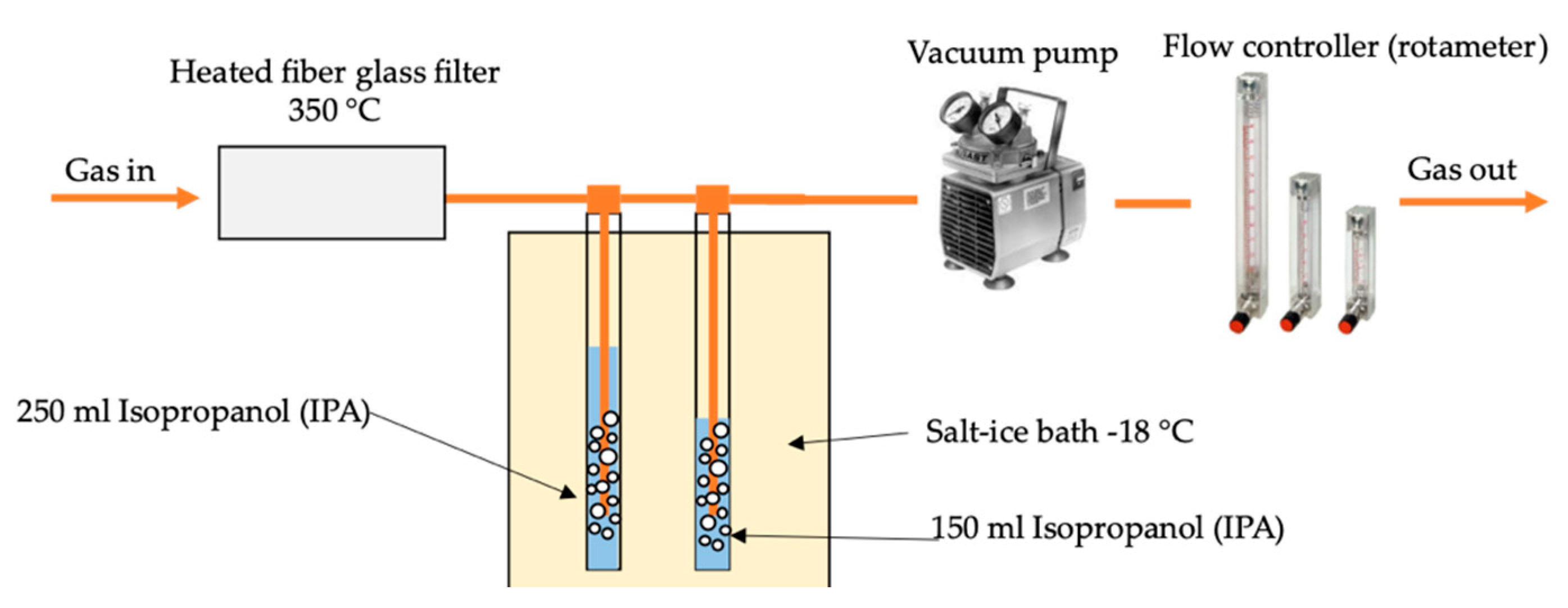
2.5. Producer Gas Sampling and Analysis Method
2.5.1. Producer Gas Composition Analysis
2.5.2. Higher Heating Value of Producer Gas
2.5.3. Dry Gas Yield
2.5.4. Carbon Conversion Efficiency
2.5.5. Cold Gasification Efficiency
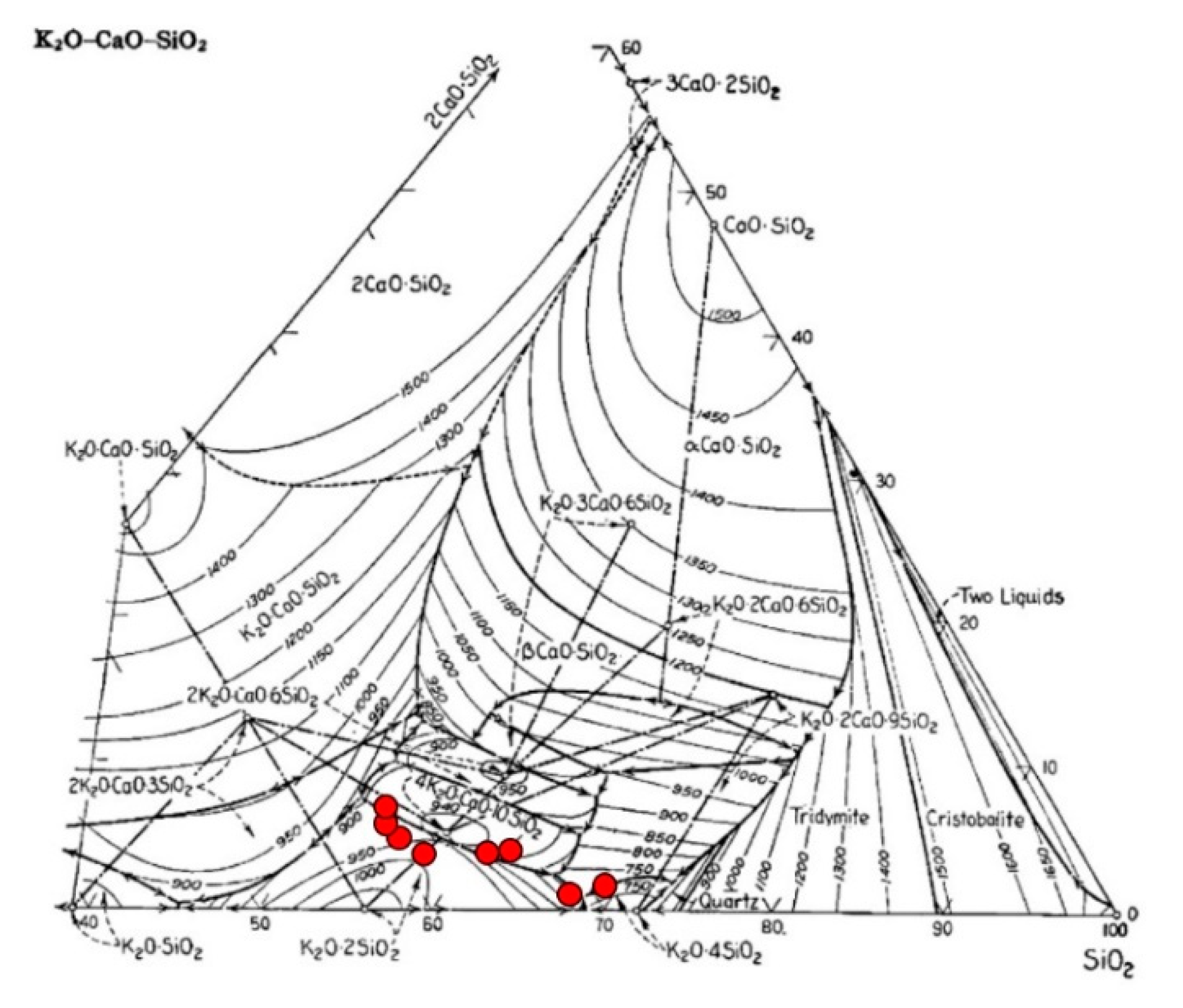
2.6. SEM-EDS for Bed Agglomeration Analysis
2.7. Error and Uncertainty
3. Results and Discussion
3.1. Fluidisation Number and Bed Temperature
3.2. Bed Behaviour
3.2.1. Using Silica Sand as Bed Material
3.2.2. Using Dolomite as Bed Material
3.2.3. Using Alumina Sand as Bed Material
3.3. Producer Gas Yield and Gasification Efficiency
3.3.1. Effect of Bed Material on Producer Gas Composition and Higher Heating Value
3.3.2. Effect of Bed Material on Cold Gasification Efficiency and Carbon Conversion Efficiency
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zainal, Z.; Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Gasification of lignocellulosic biomass in fluidized beds for renewable energy development: A review. Renew. Sustain. Energy Rev. 2019, 14, 2852–2862. [Google Scholar]
- Matas, G.B.; Sandquist, J.; Sørum, L. Gasification of Biomass to Second Generation Biofuels: A Review. J. Energy Resour. Technol. 2012, 135. [Google Scholar] [CrossRef]
- Basu, P. Biomass Gasification and Pyrolysis: Practical Design and Theory, Chapter 1: Introduction; Academic Press, Elsevier: Burlington, NJ, USA, 2010; pp. 1–25. [Google Scholar]
- Chaivatamaset, P.; Sricharoon, P.; Tia, S. Bed agglomeration characteristics of palm shell and corncob combustion in fluidized bed. Appl. Therm. Eng. 2011, 31, 2916–2927. [Google Scholar] [CrossRef]
- Visser, H.; Van, L.S.; Kiel, J. Biomass Ash-Bed Material Interactions Leading to Agglomeration in FBC. J. Energy Resour. Technol. 2008, 130. [Google Scholar] [CrossRef]
- Visvanathan, C.; Chiemchaisri, C. Management of Agricultural Wastes and Residue in Thailand: Wastes to Energy Approach. Available online: http://faculty.ait.ac.th/visu/wp-content/uploads/sites/7/2019/01/Agri-waste2energy-Thai.pdf (accessed on 30 October 2019).
- Department of Alternative Energy Development and Efficiency. Potential of Palm Empty Fruit Bunch. Available online: http://webkc.dede.go.th/testmax/node/2529 (accessed on 30 October 2019).
- Lahijani, P.; Zainal, Z.A. Gasification of palm empty fruit bunch in a bubbling fluidized bed: A performance and agglomeration study. Bioresour. Technol. 2011, 102, 2068–2076. [Google Scholar] [CrossRef] [PubMed]
- Lajijani, P.; Njafpour, G.; Zainal, Z.; Mohammadi, M. Air gasification of palm empty fruit bunch in a fluidized bed gasifier using various bed materials. In Proceedings of the World Renewable Energy Congress, Linkoping, Sweden, 8–13 May 2011; pp. 3269–3275. [Google Scholar]
- Chaivatamaset, P.; Sricharoon, P.; Tia, S.; Bilitewski, B. The characteristics of bed agglomeration/defluidization in fluidized bed firing palm empty fruit bunch and rice straw. Appl. Therm. Eng. 2014, 70, 737–747. [Google Scholar] [CrossRef]
- Kittivech, T.; Fukuda, S. Characteristic of Palm empty fruit bunch (EFB) gasification in a bubbling fluidized bed reactor. In Proceedings of the The 5th International Conference on Engineering, Energy and Environment, Bangkok, Thailand, 1–3 November 2017; pp. 307–312. [Google Scholar]
- Chen, H.; Li, B.; Yang, H.; Yang, G.; Zhang, S. Experimental Investigation of Biomass Gasification in a Fluidized bed Reactor. Energy Fuels 2008, 22, 3493–3498. [Google Scholar] [CrossRef]
- Kuprianov, V.; Ninduangdee, P.; Suheri, P. Co-firing of oil palm residues in a fuel staged fluidized-bed combustor using mixtures of alumina and silica sand as the bed material. Appl. Therm. Eng. 2018, 144, 371–382. [Google Scholar] [CrossRef]
- Ninduangdee, P.; Kuprianov, V. Combustion of an oil palm residue with elevated potassium content in a fluidized-bed combustor using alternative bed materials for preventing bed agglomeration. Bioresour. Technol. 2015, 182, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Channiwala, S.A.; Parikh, P.P. A unified correlation for estimating HHV of solid, liquid and gaseous fuels. Fuel 2002, 81, 1051–1063. [Google Scholar] [CrossRef]
- Waldheim, L.; Nilsson, T. Heating Value of Gases from Biomass Gasification; TPS-01/16; TPS Termiska Processer AB: Nykoping, Sweden, 2001. [Google Scholar]
- Chaivatamaset, P.; Tia, S. The characteristics of bed agglomeration during fluidized bed combustion of eucalyptus bark. Appl. Therm. Eng. 2015, 75, 1134–1146. [Google Scholar] [CrossRef]
- Visser, H.J.M.; Kiel, J.H.A.; Veringa, H.J. The Influence of Fuel Composition on Agglomeration Behaviour in Fluidised-Bed Combustion; ECN-C-04-054; ECN: Petten, The Netherlands, 2004. [Google Scholar]
- Roedder, E. Silicate melt systems. Phys. Chem. Earth 1959, 3, 224–297. [Google Scholar] [CrossRef]
- Lin, C.; Kou, J.; Wey, M.; Chang, S.; Wang, K. Inhibition and promotion: The effect of earth alkali metals and operating temperature on particle agglomeration/defluidization during incineration in fluidized bed. Powder Technol. 2009, 189, 57–63. [Google Scholar] [CrossRef]
- Liu, Z.; Peng, T.; Lin, C. Impact of CaO and CaCO3 addition on agglomeration/defluidization and heavy metal emission during waste combustion in fluidized bed. Fuel Process. Technol. 2014, 118, 171–179. [Google Scholar] [CrossRef]
- Kittivech, T.; Fukuda, S. Effect of bed materials on gasification performance and tar reduction in a bench-scale air blown bubbling fluidized bed reactor. In Proceedings of the The 7th International Conference on Sustainable Energy and Environment (SEE 2018), Bangkok, Thailand, 28–30 November 2018. [Google Scholar]






| Ultimate Analysis (% Dry Basis) | |
| C | 43.8 |
| H | 6.2 |
| O | 44.4 |
| N | 0.4 |
| Proximate Analysis (% Dry Basis) | |
| Volatile matter | 74.2 |
| Fix carbon | 20.6 |
| Ash | 5.2 |
| HHV (MJ/kg) 1 | 17.5 |
| Oxide Composition (% By Weight, Dry Ash Basis) | |
|---|---|
| SiO2 | 14.6 |
| CaO | 2.2 |
| MgO | 2.9 |
| Fe2O3 | 0.5 |
| K2O | 61.2 |
| SO3 | 2.0 |
| Na2O | 0.5 |
| P2O5 | 2.0 |
| Cl | 14.1 |
| Properties | Silica Sand | Dolomite | Alumina Sand |
|---|---|---|---|
| Mean particle size (µm) | 250 | 250 | 250 |
| Bulk density (kg/m3) | 1450 | 1434 | 2014 |
| Particle density (kg/m3) | 2650 | 2850 | 3920 |
| Minimum fluidisation velocity at 760 °C (Umf @ 760 °C) (m/s) | 0.06 | 0.04 | 0.08 |
| Oxide Composition (% By Weight) | |||
| SiO2 | 99.3 | 0.8 | 6.5 |
| Al2O3 | 0.7 | 0.5 | 87.8 |
| CaO | - | 27.1 | 0.5 |
| MgO | - | 16.5 | 0.3 |
| TiO2 | - | - | 4.1 |
| CO3 | - | 55.0 | - |
| Trace | - | 0.1 | 0.8 |
| Oxide Composition | % By Weight | |
|---|---|---|
| Before Use | After Use 1 | |
| SiO2 | 0.8 | 0.7 |
| Al2O3 | 0.5 | 0.2 |
| CaO | 27.1 | 35.8 |
| MgO | 16.5 | 25.5 |
| CO3 | 55.0 | 35.3 |
| K2O | - | 1.9 |
| Trace | 0.1 | 0.6 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kittivech, T.; Fukuda, S. Effect of Bed Material on Bed Agglomeration for Palm Empty Fruit Bunch (EFB) Gasification in a Bubbling Fluidised Bed System. Energies 2019, 12, 4336. https://doi.org/10.3390/en12224336
Kittivech T, Fukuda S. Effect of Bed Material on Bed Agglomeration for Palm Empty Fruit Bunch (EFB) Gasification in a Bubbling Fluidised Bed System. Energies. 2019; 12(22):4336. https://doi.org/10.3390/en12224336
Chicago/Turabian StyleKittivech, Tanakorn, and Suneerat Fukuda. 2019. "Effect of Bed Material on Bed Agglomeration for Palm Empty Fruit Bunch (EFB) Gasification in a Bubbling Fluidised Bed System" Energies 12, no. 22: 4336. https://doi.org/10.3390/en12224336




