A Two-Dimensional Study on the Effect of Anisotropy on the Devolatilization of a Large Wood Log
Abstract
:1. Introduction
- The wood log is subject to inhomogeneous boundary conditions. This is the case when the boundaries of the wood log are affected very differently, e.g., due to different exposure to the flame, the furnace wall, blocking by other wood logs in a stack, etc.
- The wood logs have an irregular shape, i.e., they are not cylindrical or spherical. This is typically the case for realistic wood logs as used in wood stoves.
- Wood log shrinkage is considered.
- The focus of the model is to study wood logs’ internal distributions of variables.
2. Wood Log Model
2.1. Boundary Conditions
- The gas is ideal.
- Wood and char are anisotropic homogeneous porous media.
- Darcy’s law is used to derive the gas phase velocity, which is valid due to a low pore Reynolds number.
- Primary devolatilization is described by three independent competitive reactions, and secondary tar reactions are considered.
- The following solid and gaseous species are modeled: wood, char, ash, CO, CO, N, tar. No liquid species is considered since the wood log is considered to be perfectly dry.
- The solid and the gas phases are in thermal equilibrium. Therefore, it is enough to solve only one temperature equation.
- The ash content is 1 wt % (db).
2.2. Numerical Solver
2.3. Numerical Setup
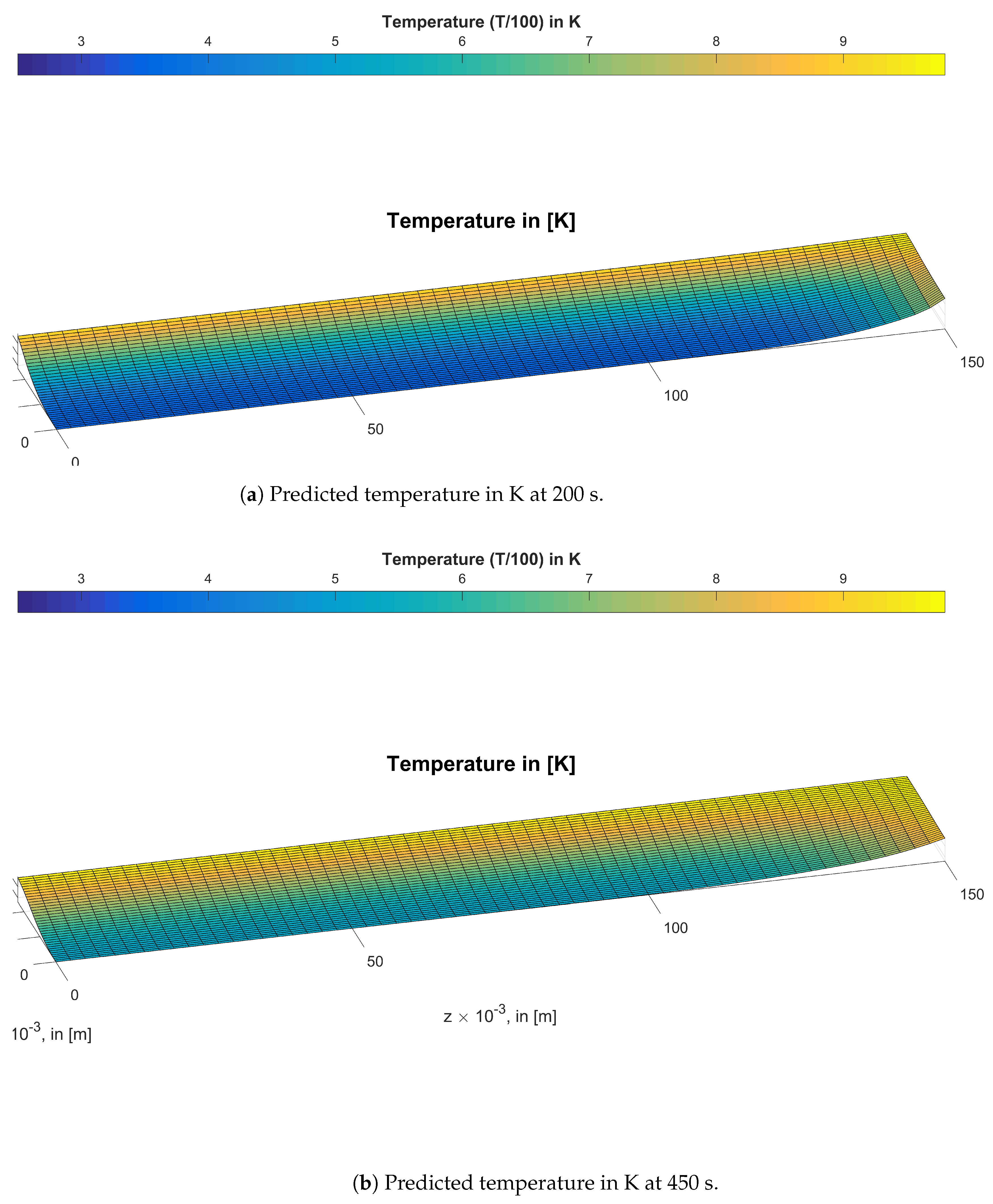
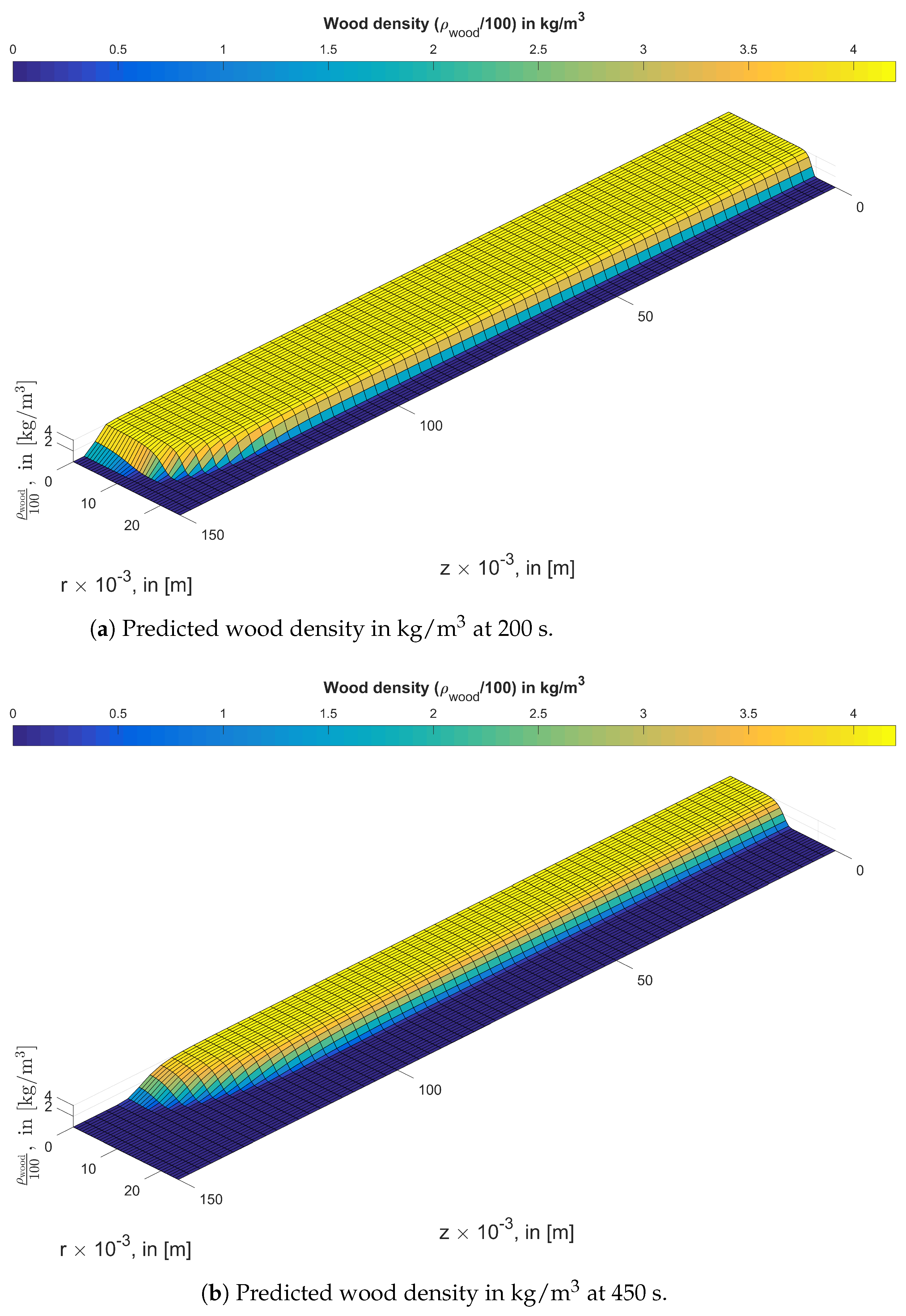
3. Results and Discussion
3.1. Study of the Anisotropy of Wood
3.2. Parametric Study: Wood and Char Permeability
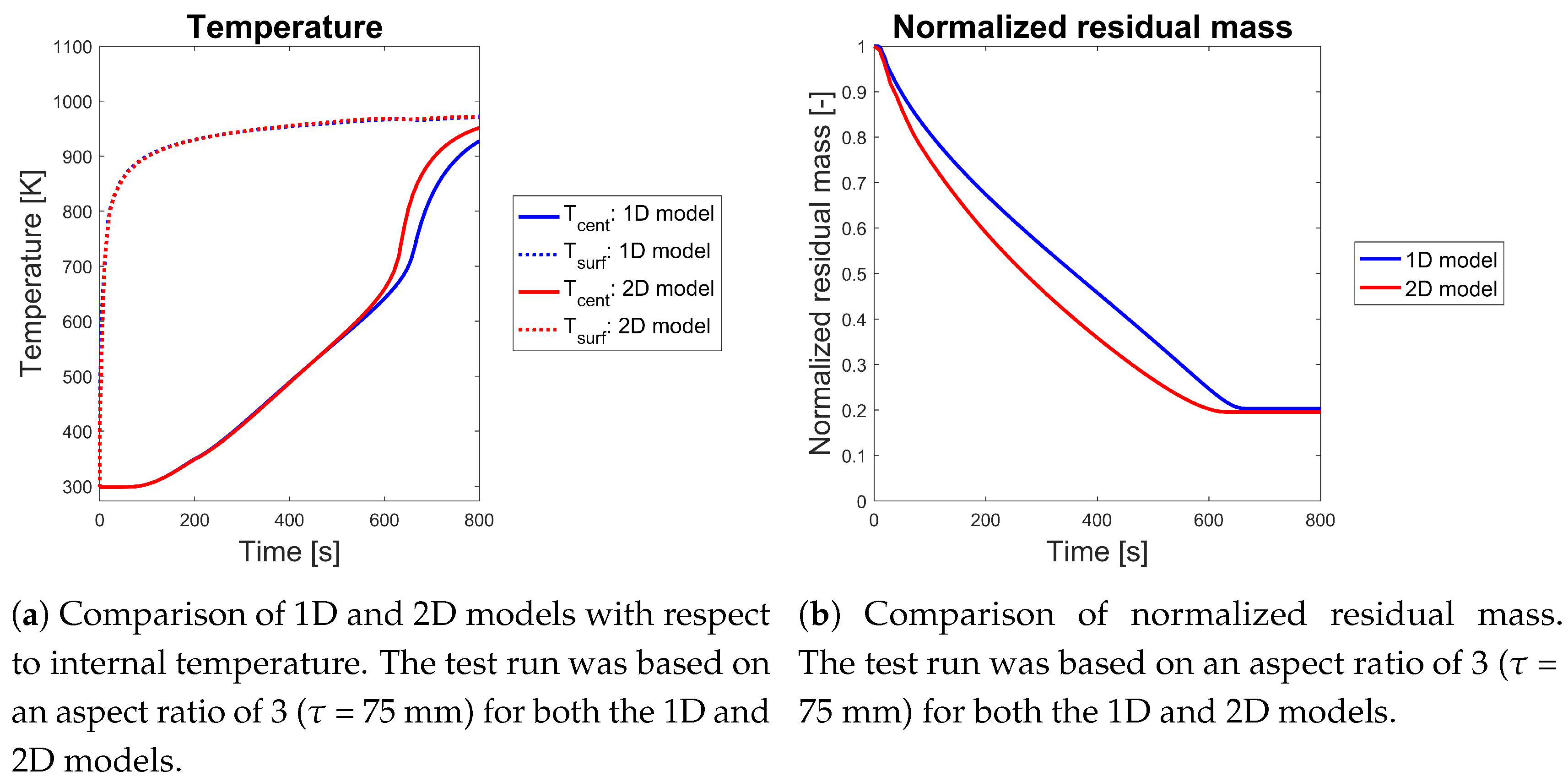
Comparison of 1D and 2D Modeling Results
4. Conclusions and Recommendations
- the wood logs have non-uniform boundary conditions (e.g., in wood stoves),
- the detailed distribution of any variable within the log is studied,
- the ratio between the longitudinal and the radial extent of the log is low,
- the log cannot be approximated as either cylindrical or spherical.
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| A | pre-exponential factor [] / [] |
| aspect ratio | |
| activation energy [] | |
| f | gas species mass fraction from primary devolatilization [-] |
| g | gas species mass fraction from secondary devolatilization [-]/gravity [m/] |
| Y | mass fraction [-] |
| T | temperature [K] |
| t | time [s] |
| surrounding gas phase temperature [K] | |
| reference temperature [K] (298 K) | |
| furnace wall temperature [K] | |
| specific heat capacity [] | |
| effective diffusivity [] | |
| Knudsen diffusion [] | |
| heat transfer coefficient [] (without Stefan flow) | |
| heat transfer coefficient [] (with Stefan flow) | |
| mass transfer coefficient [] (without Stefan flow) | |
| mass transfer coefficient [] (with Stefan flow) | |
| R | ideal gas constant [] |
| r | radius [m] |
| gas phase velocity in radial direction [] | |
| gas phase velocity in longitudinal direction [] | |
| V | gas volume [] |
| cell volume [] | |
| total mass flux of exiting gases [] | |
| n | Mol number |
| P | Pressure [Pa] |
| k | reaction rate constant [] or [] |
| reaction: wood to non-condensable gases | |
| reaction: wood to tar | |
| reaction: wood to char | |
| reaction: tar to non-condensable gases | |
| reaction: tar to char | |
| characteristic length | |
| diffusivity [] | |
| Δh | heat of reaction [] |
| z | length of the wood log [] |
| density [] | |
| porosity [-] | |
| emissivity [-] | |
| heat source term [J/(sm)] | |
| thermal conductivity [] | |
| reaction rate [] | |
| Stefan-Boltzmann constant [] | |
| tortuosity [-] | |
| permeability [] | |
| effective | |
| water vapor | |
| i | reaction |
| s | solid |
| mixed gas phase | |
| a | ash |
| w | dry wood |
| g | gas phase |
| k | gas species |
| longitudinal | |
| ‖ | parallel to fiber direction |
| ⊥ | perpendicular to fiber direction |
| c | char |
| furnace wall | |
| radiative | |
| radial | |
| primary devolatilization | |
| secondary devolatilization | |
| tar | |
| ∞ | bulk |
| g | gas phase |
References
- Pozzobon, V.; Salvador, S.; Bézian, J.J. Biomass gasification under high solar heat flux: Advanced modelling. Fuel 2018, 214, 300–313. [Google Scholar] [CrossRef]
- Pozzobon, V.; Salvador, S.; Bézian, J.J.; El-Hafi, M.; Maoult, Y.L.; Flamant, G. Radiative pyrolysis of wet wood under intermediate heat flux: Experiments and modelling. Fuel Process. Technol. 2014, 128, 319–330. [Google Scholar] [CrossRef]
- Grønli, M.G. A Theoretical and Experimental Study of the Thermal Degradation of Biomass. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 1996. [Google Scholar]
- Haberle, I.; Skreiberg, Ø.; Łazar, J.; Haugen, N.E.L. Numerical models for thermochemical degradation of thermally thick woody biomass, and their application in domestic wood heating appliances and grate furnaces. Prog. Energy Combust. Sci. 2017, 63, 204–252. [Google Scholar] [CrossRef]
- Yang, Y.B.; Sharifi, V.N.; Swithenbank, J.; Ma, L.; Darvell, L.I.; Jones, J.M.; Pourkashanian, M.; Williams, A. Combustion of a single particle of biomass. Energy Fuels 2008, 22, 306–316. [Google Scholar] [CrossRef]
- Sand, U.; Sandberg, J.; Larfeldt, J.; Fdhila, R.B. Numerical prediction of the transport and pyrolysis in the interior and surrounding of dry and wet wood log. Appl. Energy 2008, 85, 1208–1224. [Google Scholar] [CrossRef]
- Di Blasi, C. Physico-chemical processes occurring inside a degrading two-dimensional anisotropic porous medium. Int. J. Heat Mass Transf. 1998, 41, 4139–4150. [Google Scholar] [CrossRef]
- Biswas, A.K.; Umeki, K. Simplification of devolatilization models for thermally-thick particles: Differences between wood logs and pellets. Chem. Eng. J. 2015, 274, 181–191. [Google Scholar] [CrossRef]
- Larfeldt, J.; Leckner, B.; Melaaen, M. Modelling and measurements of the pyrolysis of large wood particles. Fuel 2000, 79, 1637–1643. [Google Scholar] [CrossRef]
- Larfeldt, J. Drying and Pyrolysis of Logs of Wood. Ph.D. Thesis, Chalmers University of Technology, Göteborg, Sweden, 2000. [Google Scholar]
- Haberle, I.; Haugen, N.E.L.; Skreiberg, Ø. Combustion of Thermally Thick Wood Particles: A Study on the Influence of Wood Particle Size on the Combustion Behavior. Energy Fuels 2018, 32, 6847–6862. [Google Scholar] [CrossRef]
- Haberle, I.; Haugen, N.E.L.; Skreiberg, Ø. Drying of Thermally Thick Wood Particles: A Study of the Numerical Efficiency, Accuracy, and Stability of Common Drying Models. Energy Fuels 2017, 31, 13743–13760. [Google Scholar] [CrossRef]
- Lu, H.; Robert, W.; Peirce, G.; Ripa, B.; Baxter, L.L. Comprehensive Study of Biomass Particle Combustion. Energy Fuels 2008, 22, 2826–2839. [Google Scholar] [CrossRef]
- Fatehi, H. Numerical Simulation of Combustion and Gasification of Biomass Particles. Ph.D. Thesis, LTH (Lund University), Lund, Sweden, 2014. [Google Scholar]
- Mehrabian, R.; Zahirovic, S.; Scharler, R.; Obernberger, I.; Kleditzsch, S.; Wirtz, S.; Scherer, V.; Lu, H.; Baxter, L.L. A CFD model for thermal conversion of thermally thick biomass particles. Fuel Process. Technol. 2012, 95, 96–108. [Google Scholar] [CrossRef]
- Fatehi, H.; Bai, X.S. A Comprehensive Mathematical Model for Biomass Combustion. Combust. Sci. Technol. 2014, 186, 574–593. [Google Scholar] [CrossRef]
- Bird, R.B.; Stewart, W.E.; Lightfoot, E.N. Transport Phenomena, 2nd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 2002. [Google Scholar]
- Haberle, I.; Haugen, N.E.L.; Skreiberg, Ø. Simulating Thermal Wood Particle Conversion: Ash-Layer Modeling and Parametric Studies. Energy Fuels 2018, 32, 10668–10682. [Google Scholar] [CrossRef]
- von Böckh, P.; Wetzel, T. Erzwungene Konvektion. In Wärmeübertragung: Grundlagen und Praxis; Springer: Berlin/Heidelberg, Germangy, 2014; pp. 83–125. [Google Scholar]
- Marek, R.; Nitsche, K. Praxis der Wärmeübertragung: Grundlagen-Anwendungen-Übungsaufgaben, 4th ed.; Carl Hanser Verlag GmbH & CO KG.: Munich, Germany, 2015. [Google Scholar]
- Martin, H.; Gampert, B. G5-Heat Transfer to Single Cylinders, Wires, and Fibers in Longitudinal Flow. In VDI Heat Atlas; Springer: Berlin/Heidelberg, Germany, 2010; pp. 717–722. [Google Scholar]
- National Laboratory Lawrence Livermore. SUNDIALS: SUite of Nonlinear and DIfferential/ALgebraic Equation Solvers-IDA. 2016. Available online: http://computation.llnl.gov/projects/sundials/ida (accessed on 7 April 2017).
- Di Blasi, C. Heat, momentum and mass transport through a shrinking biomass particle exposed to thermal radiation. Chem. Eng. Sci. 1996, 51, 1121–1132. [Google Scholar] [CrossRef]
- Hermansson, S.; Thunman, H. CFD modelling of bed shrinkage and channelling in fixed-bed combustion. Combust. Flame 2011, 158, 988–999. [Google Scholar] [CrossRef]
- Lu, H. Experimental and Modelling Investigation of Biomass Particle Combustion. Ph.D. Thesis, Brigham Young University, Provo, UT, USA, 2006. [Google Scholar]
- Wagenaar, B.; Prins, W.; van Swaaij, W. Flash pyrolysis kinetics of pine wood. Fuel Process. Technol. 1993, 36, 291–298. [Google Scholar] [CrossRef]
- Liden, A.; Berruti, F.; Scott, D. A kinetic model for the production of liquids from the flash pyrolysis of biomass. Chem. Eng. Commun. 1988, 65, 207–221. [Google Scholar] [CrossRef]
- Koufopanos, C.; Papayannakos, N.; Maschio, G.; Lucchesi, A. Modelling of the pyrolysis of biomass particles. Studies on kinetics, thermal and heat transfer effects. Can. J. Chem. Eng. 1991, 69, 907–915. [Google Scholar] [CrossRef]
- Di Blasi, C. Analysis of Convection and Secondary Reaction Effects Within Porous Solid Fuels Undergoing Pyrolysis. Combust. Sci. Technol. 1993, 90, 315–340. [Google Scholar] [CrossRef]
- Chan, W.C.R.; Kelbon, M.; Krieger, B.B. Modelling and experimental verification of physical and chemical processes during pyrolysis of a large biomass particle. Fuel 1985, 64, 1505–1513. [Google Scholar] [CrossRef]
- Ciuta, S.; Patuzzi, F.; Baratieri, M.; Castaldi, M.J. Biomass energy behavior study during pyrolysis process by intraparticle gas sampling. J. Anal. Appl. Pyrolysis 2014, 108, 316–322. [Google Scholar] [CrossRef]
- Ström, H.; Thunman, H. CFD simulations of biofuel bed conversion: A submodel for the drying and devolatilization of thermally thick wood particles. Combust. Flame 2013, 160, 417–431. [Google Scholar] [CrossRef]









| Equation | Equation | Ref. | |
|---|---|---|---|
| Number | |||
| Wood density (1) | [13] | ||
| Ash density | [13] | ||
| Continuity equation (3) | [7] | ||
| Species mass fraction (4) | |||
| [3] | |||
| Char density (2) | [13] | ||
| Temperature (4) | |||
| [14] | |||
| Radial gas velocity, | [13] | ||
| Longitudinal gas velocity, | [7] | ||
| Equation of state | pV = nRT | [7] | |
| Effective diffusivity | [11] |
| Property | Unit | Value | Ref. |
|---|---|---|---|
| Thermal conductivity (wood), / | [W/(mK)] | 0.419/0.158 | [3] |
| Thermal conductivity (ash–fiber), | [W/(mK)] | 1.2 | [13] |
| Thermal conductivity (char), / | [W/(mK)] | 0.1046/0.071 | [7] |
| Thermal conductivity (gases), | [W/(mK)] | 25.77 × 10 | [23] |
| Specific heat capacity (wood) (298–413 K), | [J/(kgK)] | −91.2 + 4.4T | [3] |
| Specific heat capacity (wood) (350–500 K), | [J/(kgK)] | 1500 + T | [3] |
| Specific heat capacity (ash), | [J/(kgK)] | 754 + 0.586 (T − 273) | [24] |
| Specific heat capacity (gases), | [J/(kgK)] | 770 + 0.629 T − 1.91 × 10 T | [3] |
| Specific heat capacity (char), | [J/(kgK)] | 420 + 2.09 T − 6.85 × 10 T | [3] |
| Specific heat capacity (tar), | [J/(kgK)] | −100 + 4.4 T − 1.57 × 10 T | [3] |
| Apparent wood density, (porosity, ) | [kg/m] | 410 () | [9] |
| Radiative thermal conductivity, | [W/(mK)] | [3] | |
| Binary diffusivity, D | [m/s] | 3 × 10 | [13,24] |
| Particle diameter, d | [m] | 50 × 10 | [9] |
| Aspect ratio, AR ([L/D]) | [-] | 6 | [9] |
| Moisture content, M | [% wet basis] | 0 | [9] |
| Permeability, | m | 2.96 × 10 | [3] |
| Permeability, | m | 1.29 × 10 | [3] |
| Permeability, | m | 10 | (1) |
| Permeability, | m | 10 | [9] |
| Char emissivity, | [-] | 0.95 | [13] |
| Wood emissivity, | [-] | 0.85 | [13] |
| Ash emissivity, | [-] | 0.85 | (2) |
| Ash porosity, | [-] | 0.8 | [14] |
| Char porosity, | [-] | 0.8 | [14] |
| Shrinkage factor (devolatilization) | [-] | 0.9 | [25] |
| Reaction | Reaction Rate Constant | Unit | Ref. | Heat of Reaction [kJ/kg] (2) | Ref. |
|---|---|---|---|---|---|
| Wood → Gases | [1/s] | [26] | 0 | (1) | |
| Wood → Tar | [1/s] | [26] | 0 | (1) | |
| Wood → Char | [1/s] | [26] | 0 | (1) | |
| Tar → Gases | [1/s] | [27] | 42 | [28] | |
| Tar → Char | [1/s] | [29] | 42 | [28] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haugen, N.E.L.; Skreiberg, Ø. A Two-Dimensional Study on the Effect of Anisotropy on the Devolatilization of a Large Wood Log. Energies 2019, 12, 4430. https://doi.org/10.3390/en12234430
Haugen NEL, Skreiberg Ø. A Two-Dimensional Study on the Effect of Anisotropy on the Devolatilization of a Large Wood Log. Energies. 2019; 12(23):4430. https://doi.org/10.3390/en12234430
Chicago/Turabian StyleHaugen, Nils Erland L., and Øyvind Skreiberg. 2019. "A Two-Dimensional Study on the Effect of Anisotropy on the Devolatilization of a Large Wood Log" Energies 12, no. 23: 4430. https://doi.org/10.3390/en12234430





