Effects of Water Leaching on the Ash Sintering Problems of Wheat Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Analytical Procedures for Original and Leached Samples
2.2.1. Ash Content, Volatile Matter, and Other Content Tests
2.2.2. Heating Value Test
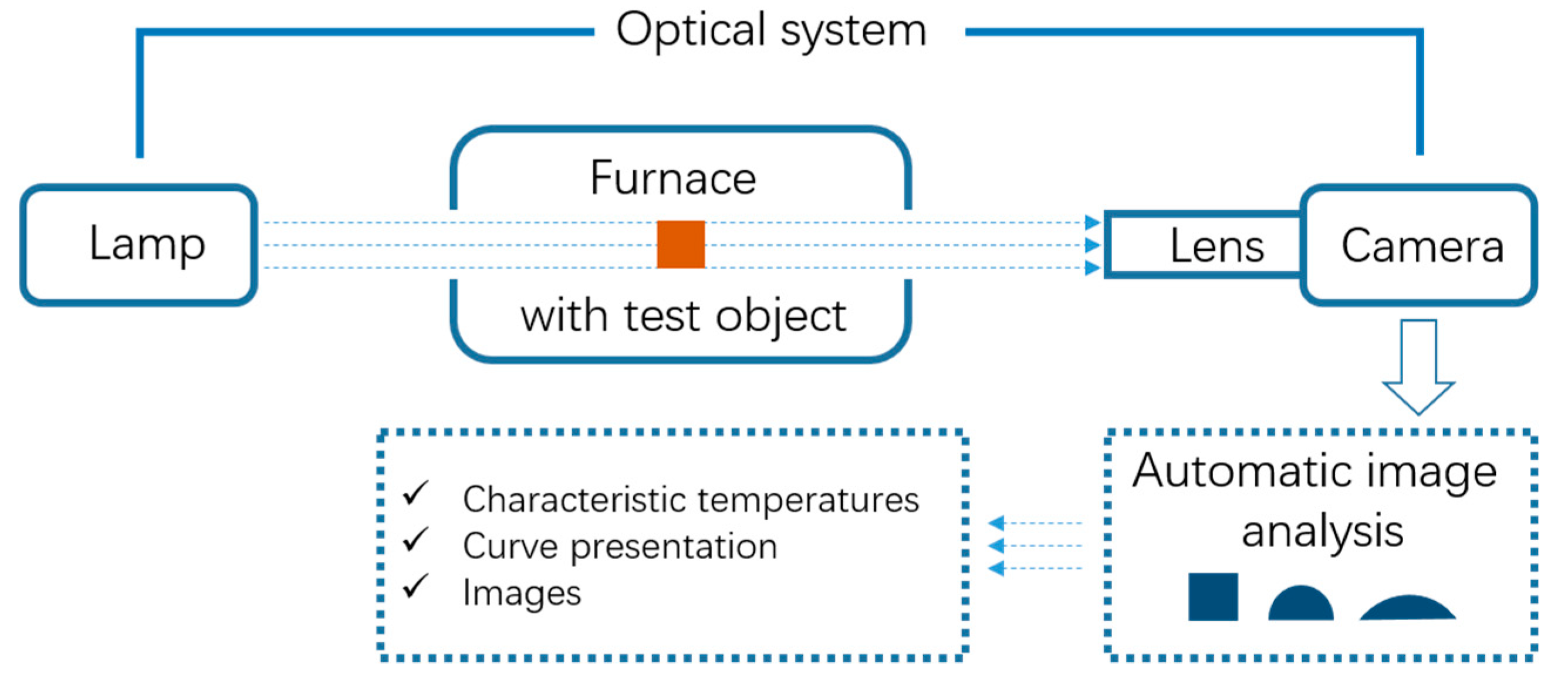
2.2.3. Ash Melting Point Test
2.2.4. Ash Sintering Degree Test
3. Results and Discussion
3.1. Proximate and Ultimate Analysis
3.2. Removal of Ash-Related Elements in Samples
3.3. HHV
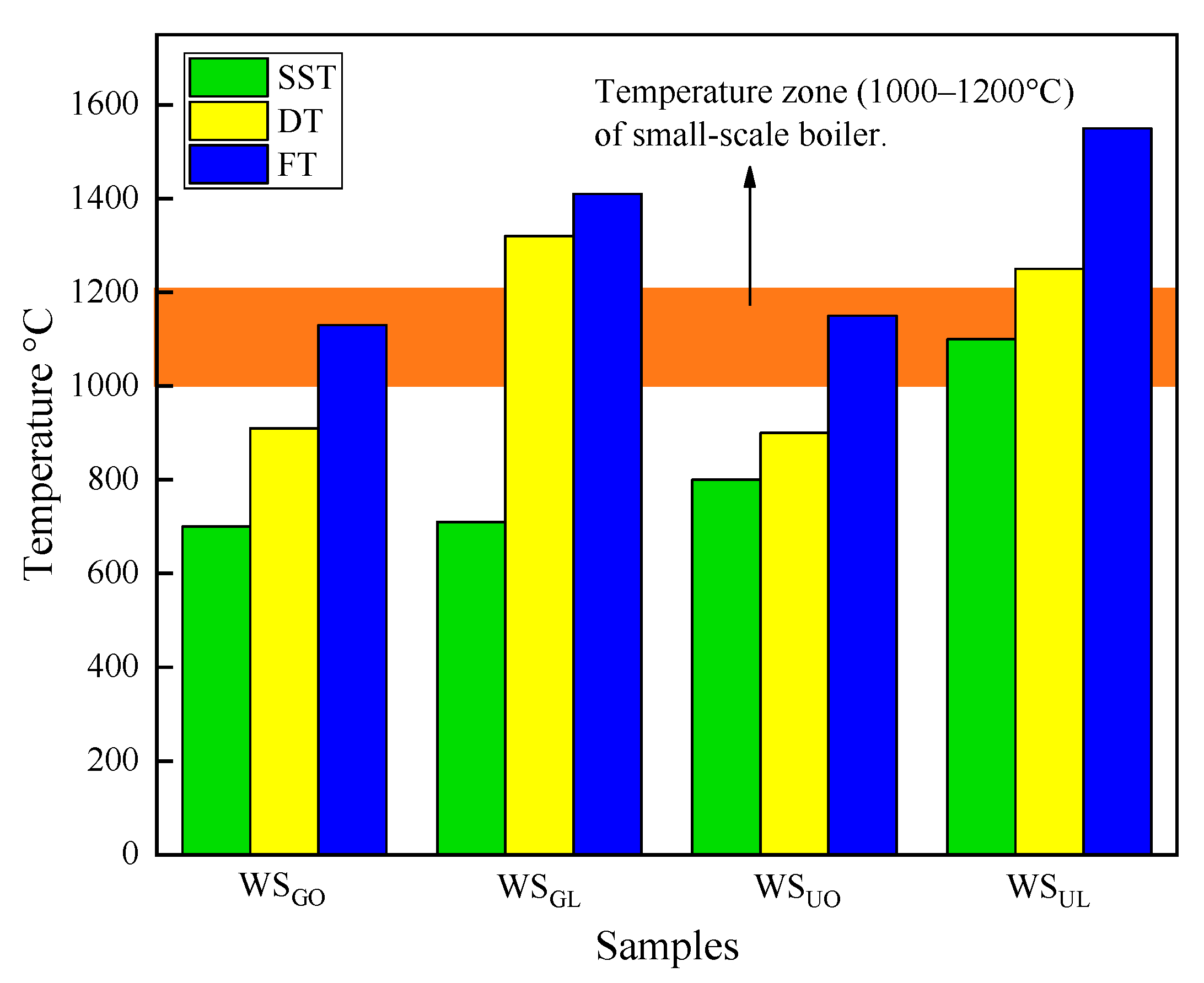
3.4. Ash Melting Test
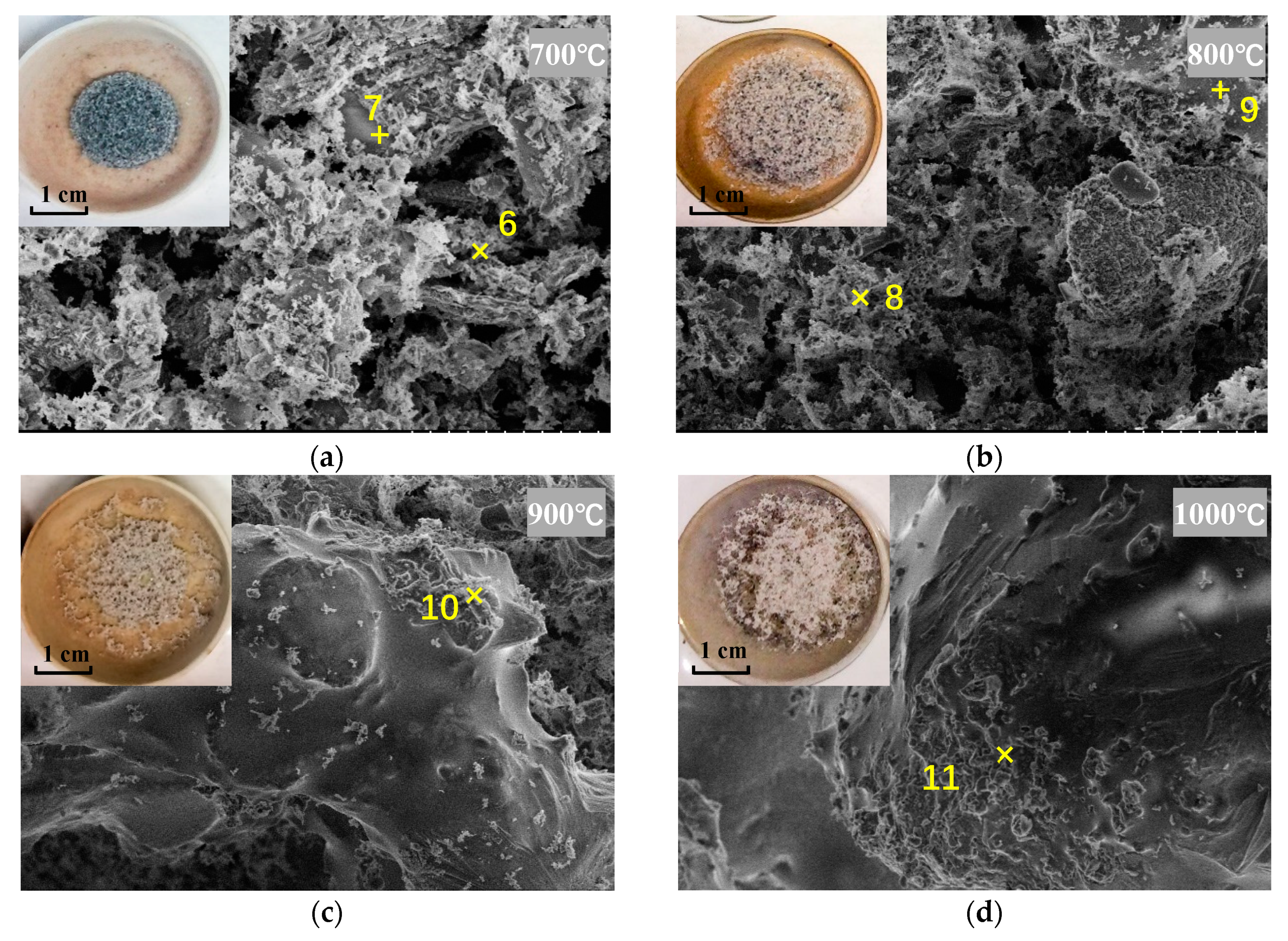
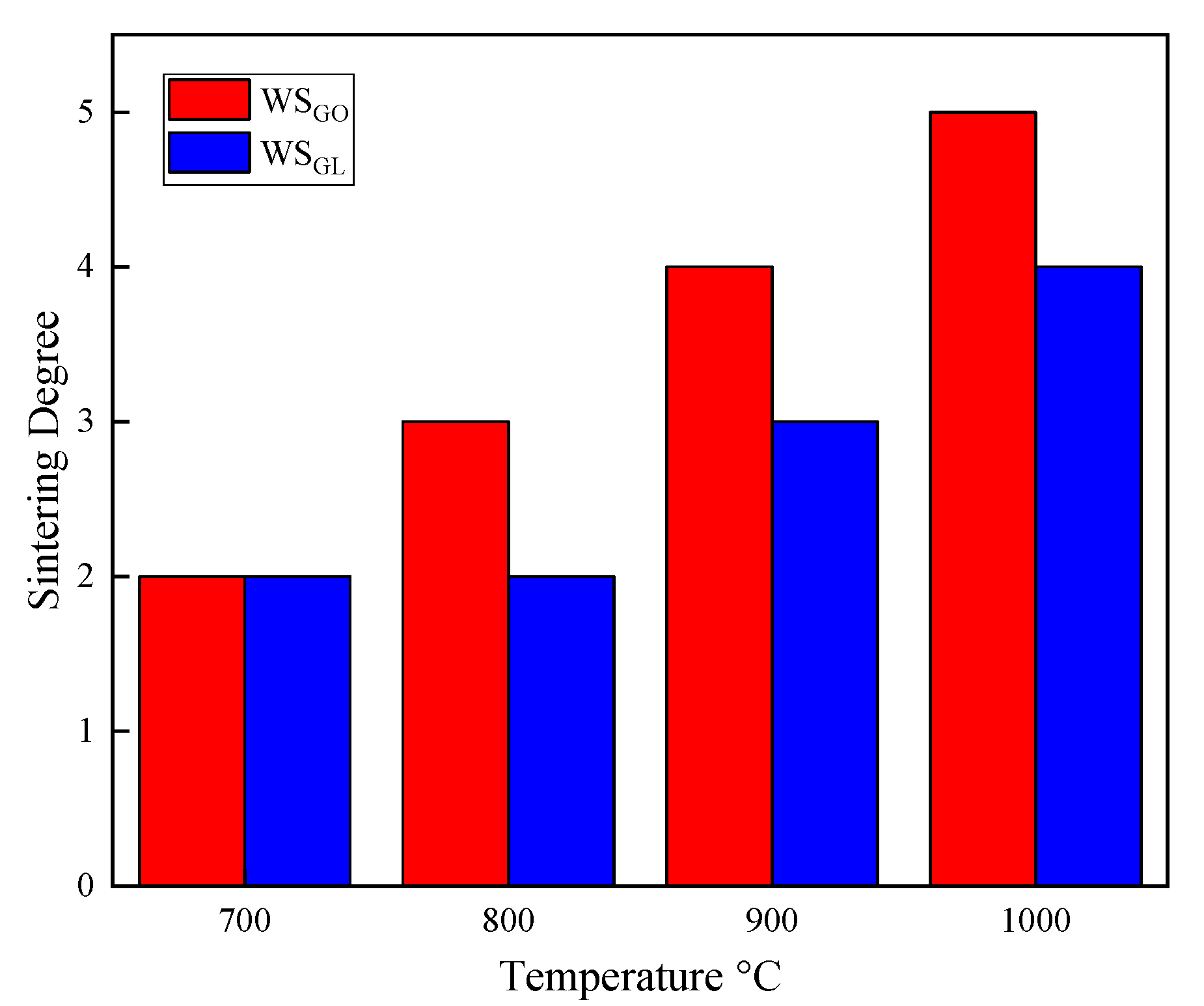
3.5. Sintering Degree Test
3.6. Predicting Model of Ash Sintering by Ash-Related Elements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Energy Agency (IEA). World Energy Outlook. Available online: http://www.iea.org/publications/freepublications/publication/world-energy-outlook-2012.html (accessed on 8 March 2018).
- Facts about Climate Change, Conserve Energy Future. Available online: https://www.conserve-energy-future.com/various-climate-change-facts-php.php (accessed on 8 March 2018).
- Boström, D.; Skoglund, N.; Grimm, A.; Boman, C.; Öhman, M.; Broström, M.; Backman, R. Ash transformation chemistry during combustion of biomass. Energy Fuels 2012, 26, 85–93. [Google Scholar] [CrossRef]
- Ghaly, A.E.; Al-Taweel, A. Physical and thermochemical properties of cereal straws. Energy Sources 1990, 12, 131–145. [Google Scholar] [CrossRef]
- Wang, L.; Skjevrak, G.; Hustad, J.E.; Skreiberg, Ø. Investigation of biomass ash sintering characteristics and the effect of additives. Energy Fuels 2014, 28, 208–218. [Google Scholar] [CrossRef]
- Werther, J.; Saenger, M.; Hartge, E.; Ogada, T.; Siagi, Z. Combustion of agricultural residues. Prog. Energy Combust. Sci. 2000, 41, 1–27. [Google Scholar] [CrossRef]
- Nunes, L.J.R.; Matias, J.C.O.; Catalão, J.P.S. Mixed biomass pellets for thermal energy production: A review of combustion models. Appl. Energy 2014, 127, 135–140. [Google Scholar] [CrossRef]
- Emil, D. Co-firing biomass with coal for power generation. In Proceedings of the Intensive Programme 2014, Pardubice, Czech Republic, 6–17 July 2014. [Google Scholar]
- Li, L.; Yu, C.; Huang, F.; Bai, J.; Fang, M.; Luo, Z. Study on the deposits derived from a biomass circulating fluidized-bed boiler. Energy Fuels 2012, 26, 6008–6014. [Google Scholar] [CrossRef]
- Niu, Y.; Du, W.; Tan, H.; Xu, W.; Liu, Y.; Xiong, Y.; Hui, S. Further study on biomass ash characteristics at elevated ashing temperatures: The evolution of K, Cl, S and the ash fusion characteristics. Bioresour. Technol. 2013, 129, 642–645. [Google Scholar] [CrossRef]
- Niu, Y.; Tan, H.; Wang, X.; Liu, Z.; Liu, H.; Liu, Y.; Xu, T. Study on fusion characteristics of biomass ash. Bioresour. Technol. 2010, 101, 9373–9381. [Google Scholar] [CrossRef]
- Nielsen, H.P.; Frandsen, F.J.; Dam-Johansen, K.; Baxter, L.L. The implications of chlorine-associated corrosion on the operation of biomass-fired boilers. Prog. Energy Combust. Sci. 2000, 26, 283–298. [Google Scholar] [CrossRef]
- Liu, H.; Feng, Y.; Wu, S.; Liu, D. The role of ash particles in the bed agglomeration during the fluidized bed combustion of rice straw. Bioresour. Technol. 2009, 100, 6505–6513. [Google Scholar] [CrossRef]
- Lindberg, D.; Backman, R.; Chartrand, P.; Hupa, M. Towards a comprehensive thermodynamic database for ash-forming elements in biomass and waste combustion—Current situation and future developments. Fuel Process. Technol. 2013, 105, 129–141. [Google Scholar] [CrossRef]
- Kassman, H.; Pettersson, J.; Steenari, B.M.; Åmand, L.E. Two strategies to reduce gaseous KCl and chlorine in deposits during biomass combustion—Injection of ammonium sulphate and co-combustion with peat. Fuel Process. Technol. 2013, 105, 170–180. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Baxter, L.L.; Miles, T.R.; Miles, T.R. Combustion properties of biomass. Fuel Process. Technol. 1998, 54, 17–46. [Google Scholar] [CrossRef]
- Liaw, S.B.; Wu, H. Leaching characteristics of organic and inorganic matter from biomass by water: Differences between batch and semi-continuous operations. Ind. Eng. Chem. Res. 2013, 52, 4280–4289. [Google Scholar] [CrossRef]
- Yu, C.; Zheng, Y.; Cheng, Y.S.; Jenkins, B.M.; Zhang, R.; VanderGheynst, J.S. Solid-liquid extraction of alkali metals and organic compounds by leaching of food industry residues. Bioresour. Technol. 2010, 101, 4331–4336. [Google Scholar] [CrossRef] [PubMed]
- Turn, S.Q.; Kinoshita, C.M.; Ishimura, D.M. Removal of inorganic constituents of biomass feedstocks by mechanical dewatering and leaching. Biomass Bioenerg. 1997, 12, 241–252. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Mannapperuma, J.D.; Bakker, R.R. Biomass leachate treatment by reverse osmosis. Fuel Process. Technol. 2003, 81, 223–246. [Google Scholar] [CrossRef]
- Bakker, R.R.; Jenkins, B.M. Feasibility of collecting naturally leached rice straw for thermal conversion. Biomass Bioenerg. 2003, 25, 597–614. [Google Scholar] [CrossRef]
- Chin, K.L.; H’ng, P.S.; Paridah, M.T.; Szymona, K.; Maminski, M.; Lee, S.H.; Lum, W.C.; Nurliyana, M.Y.; Chow, M.J.; Go, W.Z. Reducing ash related operation problems of fast growing timber species and oil palm biomass for combustion applications using leaching techniques. Energy 2015, 90, 622–630. [Google Scholar] [CrossRef]
- Said, N.; Bishara, T.; García-Maraver, A.; Zamorano, M. Effect of water washing on the thermal behavior of rice straw. Waste Manag. 2013, 33, 2250–2256. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, T.; Che, D. Effect of water washing on fuel properties, pyrolysis and combustion characteristics, and ash fusibility of biomass. Fuel Process. Technol. 2013, 106, 712–720. [Google Scholar] [CrossRef]
- Yu, C.; Thy, P.; Wang, L.; Anderson, S.N.; Vandergheynst, J.S.; Upadhyaya, S.K.; Jenkins, B.M. Influence of leaching pretreatment on fuel properties of biomass. Fuel Process. Technol. 2014, 128, 43–53. [Google Scholar] [CrossRef]
- Tonn, B.; Thumm, U.; Lewandowski, I.; Claupein, W. Leaching of biomass from semi-natural grasslands—Effects on chemical composition and ash high-temperature behaviour. Biomass Bioenerg. 2012, 36, 390–403. [Google Scholar] [CrossRef]
- Wang, L.; Becidan, M.; Skreiberg, Ø. Sintering behavior of agricultural residues ashes and effects of additives. Energy Fuels 2012, 26, 5917–5929. [Google Scholar] [CrossRef]
- Pronobis, M.; Wojnar, W. The impact of biomass co-combustion on the erosion of boiler convection surfaces. Energy Convers. Manag. 2013, 74, 462–470. [Google Scholar] [CrossRef]
- Pintana, P.; Tippayawong, N. Predicting ash deposit tendency in thermal utilization of biomass. Eng. J. 2016, 20, 15–24. [Google Scholar] [CrossRef]
- Liu, X.; Bi, X.T. Removal of inorganic constituents from pine barks and switchgrass. Fuel Process. Technol. 2011, 92, 1273–1279. [Google Scholar] [CrossRef]
- Ogden, C.A.; Ileleji, K.E.; Johnson, K.D.; Wang, Q. In-field direct combustion fuel property changes of switchgrass harvested from summer to fall. Fuel Process. Technol. 2010, 91, 266–271. [Google Scholar] [CrossRef]
- Vamvuka, D.; Zografos, D.; Alevizos, G. Control methods for mitigating biomass ash-related problems in fluidized beds. Bioresour. Technol. 2008, 99, 3534–3544. [Google Scholar] [CrossRef]
- Obernberger, I.; Thek, G. Physical characterisation and chemical composition of densified biomass fuels with regard to their combustion behaviour. Biomass Bioenerg. 2004, 27, 653–669. [Google Scholar] [CrossRef]
- Ma, T.; Fan, C.; Hao, L.; Li, S.; Song, W.; Lin, W. Fusion characterization of biomass ash. Thermochim. Acta 2016, 638, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Steenari, B.M.; Lundberg, A.; Pettersson, H.; Wilewska-Bien, M.; Andersson, D. Investigation of ash sintering during combustion of agricultural residues and the effect of additives. Energy Fuels 2009, 23, 5655–5662. [Google Scholar] [CrossRef]
- Llorente, M.J.F.; García, J.E.C. Comparing methods for predicting the sintering of biomass ash in combustion. Fuel 2005, 84, 1893–1900. [Google Scholar] [CrossRef]
- Reinmoeller, M.; Klinger, M.K.; Thieme, E.; Meyer, B. Analysis and prediction of slag-induced corrosion of chromium oxide-free refractory materials during fusion of coal and biomass ash under simulated gasification conditions. Fuel Process. Technol. 2016, 149, 218–230. [Google Scholar] [CrossRef]
- Vassilev, S.V.; Baxter, D.; Vassileva, C.G. An overview of the behaviour of biomass during combustion: Part II. Ash fusion and ash formation mechanisms of biomass types. Fuel 2014, 117, 152–183. [Google Scholar] [CrossRef]
- Xiong, Q.; Li, J.; Guo, S.; Li, G.; Zhao, J.; Fang, Y. Ash fusion characteristics during co-gasification of biomass and petroleum coke. Bioresour. Technol. 2018, 257, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Paulrud, S.; Nilsson, C.; Oehman, M. Reed canary-grass ash composition and its melting behavior during combustion. Fuel 2001, 80, 1391–1398. [Google Scholar] [CrossRef]

| Parameter | Facility | Standard |
|---|---|---|
| Moisture | Binder ED056 (Tuttlingen, Germany) | DIN EN 14774 |
| C/H/N | Leco CHN628 (Saint Joseph, USA) | DIN EN 15104 |
| Cl | Schott Titroline Alpha (Mainz, Germany) | DIN 51727 |
| S | Leco SC 144 DR (Saint Joseph, USA) | DIN 51724 |
| wt.% Dry Biomass | Original (WSGO) | Leached (WSGL) | Original (WSUO*) | Leached (WSUL*) | ||
|---|---|---|---|---|---|---|
| Mean | Standard Deviation | Mean | Standard Deviation | |||
| HHV (MJ/kg) | 19.25 | 0.15 | 19.53 | 0.35 | 17.30 | 17.25 |
| Volatile Matters | 79.12 | 0.50 | 83.33 | 0.37 | 74.00 | 80.30 |
| Ash Content | 4.14 | 0.46 | 3.06 | 0.50 | 8.28 | 6.27 |
| C | 47.62 | 0.43 | 47.83 | 0.29 | 43.50 | 44.90 |
| H | 6.20 | 0.09 | 5.99 | 0.11 | 5.70 | 5.70 |
| O | 41.53 | 0.48 | 42.84 | 0.28 | 41.90 | 42.80 |
| N | 0.43 | 0.03 | 0.26 | 0.02 | 0.47 | 0.32 |
| S | 0.06 | 0.00 | 0.02 | 0.00 | 0.11 | ND |
| Cl | 0.02 | 0.00 | ND | 0.00 | 0.85 | NA |
| Mass of ash-related elements in sample, mg/g dry biomass | ||||||
| Ca | 16.08 | 1.09 | 18.64 | 0.96 | 1.16 | 0.94 |
| K | 19.18 | 0.95 | 7.08 | 0.40 | 17.27 | 3.04 |
| Mg | 0.54 | 0.06 | 0.38 | 0.06 | 1.28 | 0.60 |
| Na | ND | NA | ND | NA | 1.92 | 0.80 |
| Si | 16.64 | 3.21 | 15.93 | 1.89 | 17.90 | 23.18 |
| Biomass | Mass of Ash-Related Elements in Ash (wt.%) | SST (°C) | Source | ||||
|---|---|---|---|---|---|---|---|
| Si | Mg | Ca | Na | K | |||
| Pine | 24.27 | 2.7 | 9.29 | 1.41 | 6.56 | 1190 | [36] |
| Eucalyptus | 19.13 | 2.52 | 12.86 | 1.41 | 7.22 | 1160 | [36] |
| Poplar | 1.31 | 2.22 | 23.57 | 0.1 | 14.94 | 1400 | [36] |
| Wheat straw | 20.53 | 1.44 | 5.79 | 0.16 | 14.94 | 850 | [36] |
| Rice straw | 23.8 | 2.1 | 6.36 | 2.08 | 13.28 | 860 | [36] |
| Barley husk | 14.83 | 0.03 | 5.44 | 0 | 23.04 | 856 | [27] |
| Barley straw | 12.83 | 1.75 | 13.88 | 0.28 | 27.91 | 960 | [27] |
| Wood | 10.5 | 3.34 | 19.92 | 0.22 | 5.58 | 1160 | [37] |
| Wood | 10.37 | 3.64 | 30.74 | 2.11 | 8.92 | 1192 | [38] |
| Herbaceous | 15.58 | 3.37 | 10.61 | 1.7 | 22.11 | 979 | [38] |
| Grasses | 21.55 | 2.41 | 8.02 | 0.93 | 20.4 | 970 | [38] |
| Straw | 20.51 | 2.8 | 10.09 | 1 | 20.32 | 857 | [38] |
| Pine sawdust | 10.75 | 4.97 | 31.01 | 1.21 | 7.68 | 1194 | [39] |
| Corn stalk | 12.14 | 7.21 | 5.68 | 1.77 | 29.8 | 1001 | [39] |
| Corn straw | 11.82 | 2.96 | 6.86 | 0 | 28.01 | 960 | [34] |
| Rice straw | 18.04 | 3.31 | 5.78 | 1.01 | 21.53 | 890 | [34] |
| Wheat straw | 19.2 | 1.12 | 4.91 | 0.22 | 20.51 | 890 | [34] |
| Peanut shell | 10.35 | 4.75 | 15.27 | 0.93 | 19.62 | 1130 | [34] |
| Rice husk | 29.65 | 2.75 | 1.46 | 0.19 | 8.86 | 1140 | [34] |
| Sawdust | 15.51 | 1.21 | 26.76 | 1.53 | 3.8 | 1240 | [34] |
| WSGO | 16.64 | 0.54 | 16.08 | 0 | 19.18 | 700 | |
| WSGL | 15.93 | 0.38 | 18.64 | 0 | 7.08 | 800 | |
| SST | Si | Mg | Ca | Na | K |
|---|---|---|---|---|---|
| Correlation | −0.366 | +0.351 | +0.005 | −0.162 | −0.617 |
| p-value | 0.164 | 0.183 | 0.985 | 0.550 | 0.011 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, S.; Chen, J.; Peng, D.; Wu, Z.; Li, Q.; Huang, T. Effects of Water Leaching on the Ash Sintering Problems of Wheat Straw. Energies 2019, 12, 387. https://doi.org/10.3390/en12030387
Wu S, Chen J, Peng D, Wu Z, Li Q, Huang T. Effects of Water Leaching on the Ash Sintering Problems of Wheat Straw. Energies. 2019; 12(3):387. https://doi.org/10.3390/en12030387
Chicago/Turabian StyleWu, Shibo, Jiannan Chen, Daoping Peng, Zheng Wu, Qin Li, and Tao Huang. 2019. "Effects of Water Leaching on the Ash Sintering Problems of Wheat Straw" Energies 12, no. 3: 387. https://doi.org/10.3390/en12030387
APA StyleWu, S., Chen, J., Peng, D., Wu, Z., Li, Q., & Huang, T. (2019). Effects of Water Leaching on the Ash Sintering Problems of Wheat Straw. Energies, 12(3), 387. https://doi.org/10.3390/en12030387





