Coal Chemical-Looping with Oxygen Uncoupling (CLOU) Using a Cu-Based Oxygen Carrier Derived from Natural Minerals
Abstract
:1. Introduction
2. Experimental
2.1. Coal Char Preparation and Characterization
2.2. Cu-Based OC (Cu-OC) Preparation and Characterization
2.3. Analysis of the Cu-OC
2.3.1. Cu-OC Oxygen Uncoupling
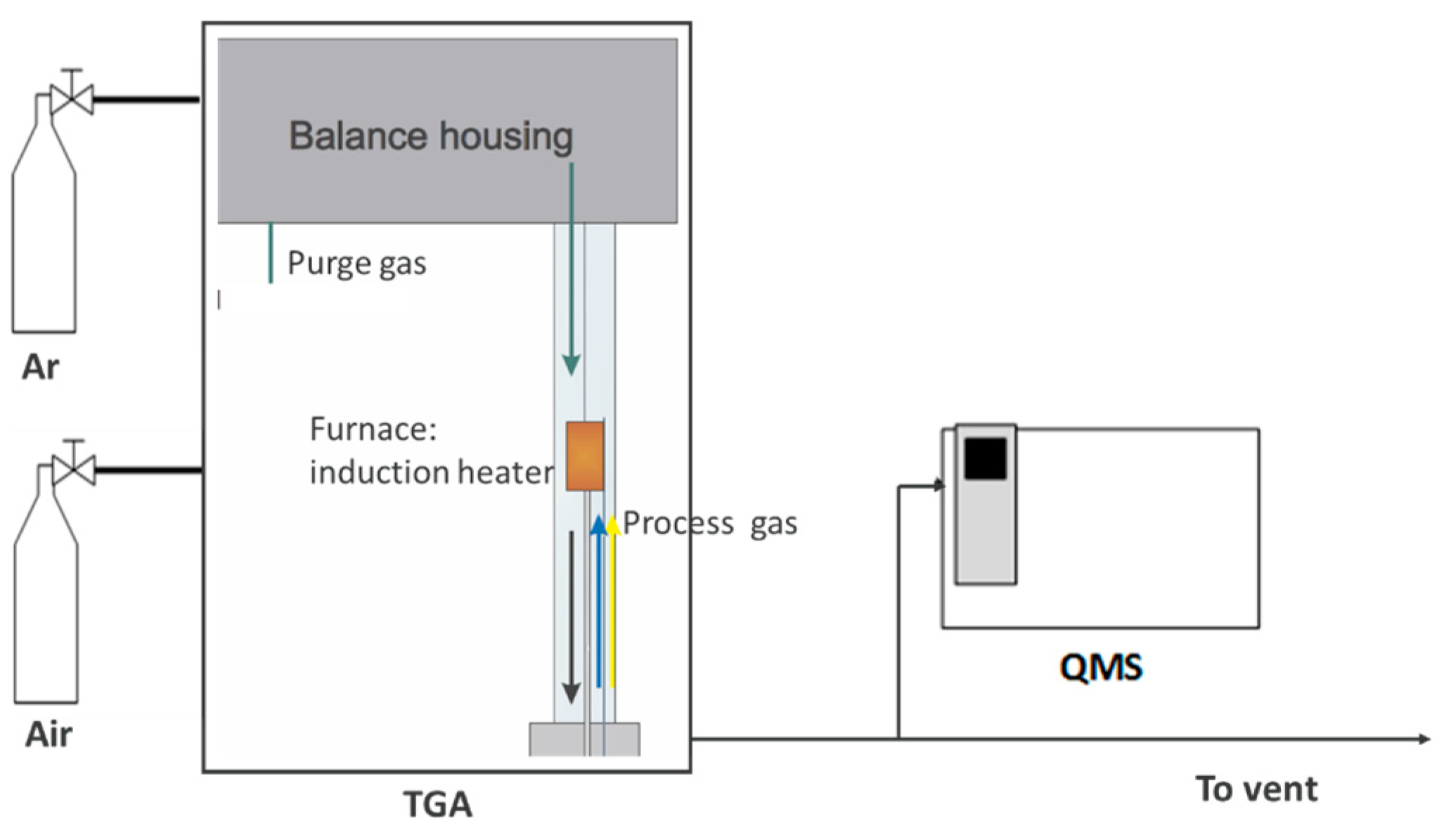
2.3.2. Recyclability of the Cu-OC with Coal Char in a TGA-QMS
2.3.3. Recyclability of the Cu-OC with Coal Char in a Fixed Bed Reactor-QMS System
2.4. Data Analysis
3. Results and Discussion
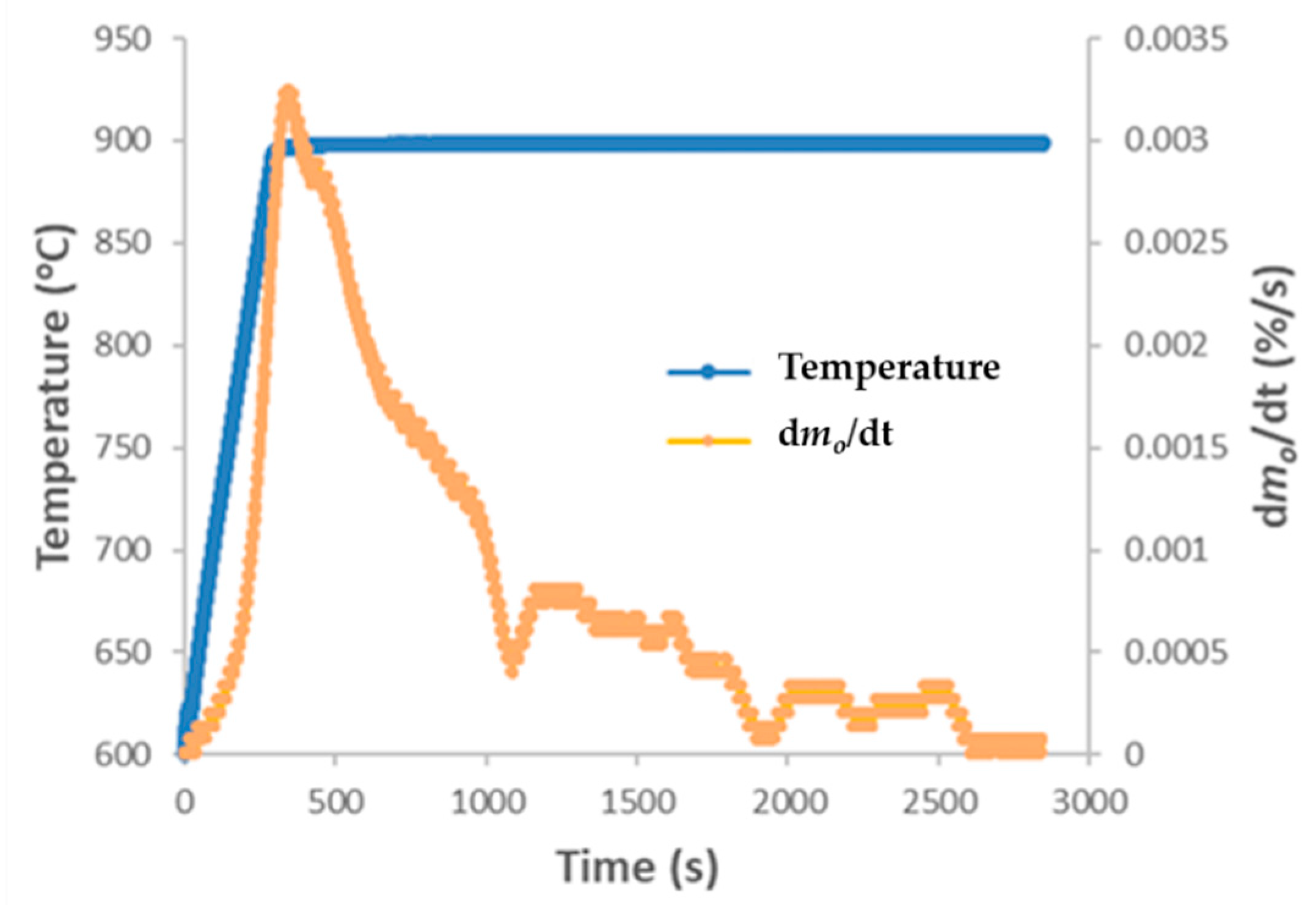
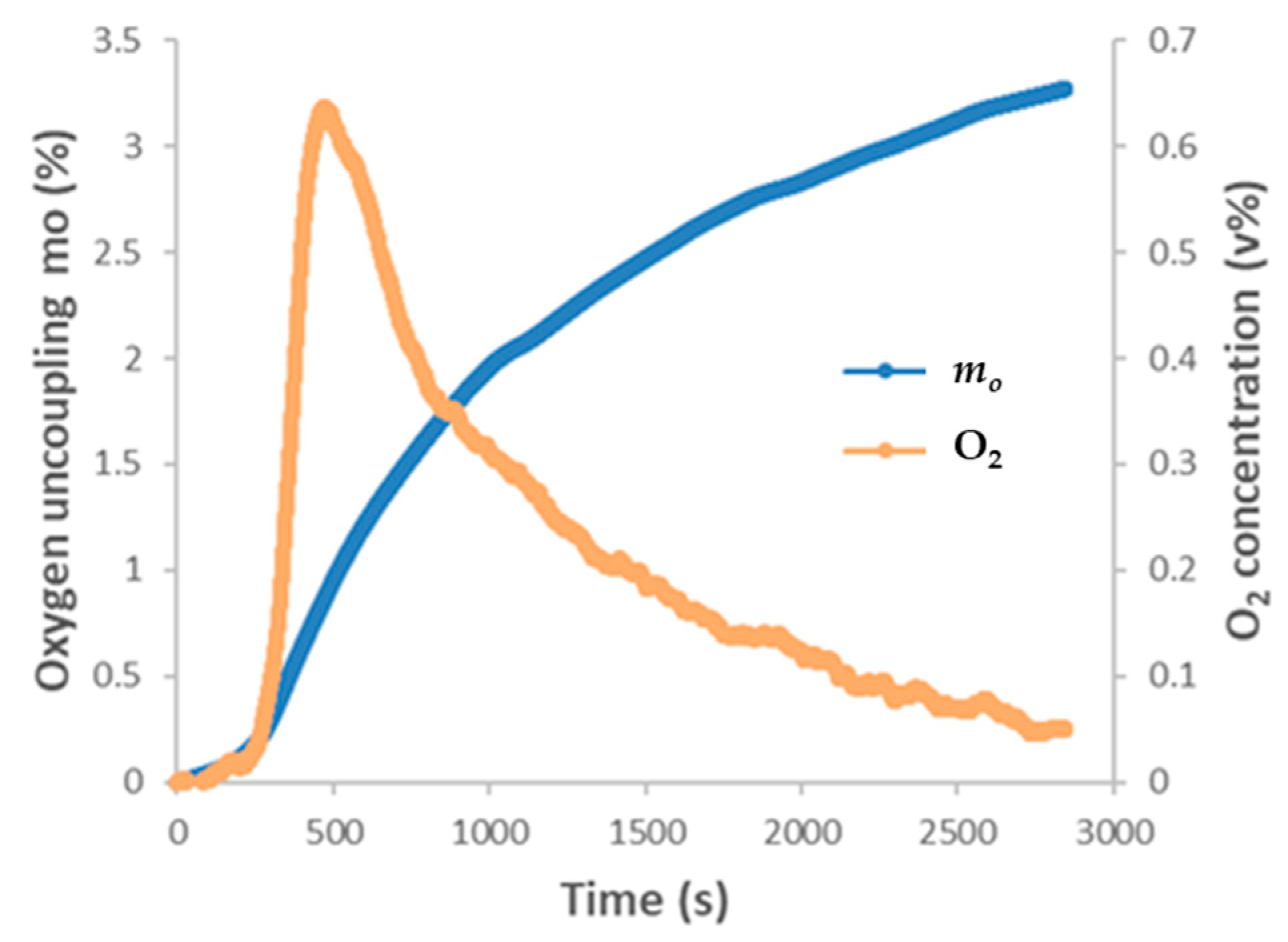
3.1. Cu-OC Oxygen Uncoupling
3.2. Reduction and Reoxidization of Cu-OC with Coal Char
3.3. Recyclability of Cu-OC in Coal Char CLOU Tested in a TGA-QMS and Fixed-Bed Reactor-QMS
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adanez, J.; Abad, A.; Mendiara, T.; Gayan, P.; de Diego, L.F.; Garcia-Labiano, F. Chemical looping combustion of solid fuels. Prog. Energy Combust. Sci. 2018, 65, 6–66. [Google Scholar] [CrossRef]
- Wang, P.; Means, N.; Shekhawat, D.; Berry, D.; Massoudi, M. Chemical-looping combustion and gasification of coals and oxygen carrier development: A brief review. Energies 2015, 8, 10605–10635. [Google Scholar] [CrossRef]
- Mattisson, T.; Lyngfelt, A.; Leion, H. Chemical-looping with oxygen uncoupling for combustion of solid fuels. Int. J. Greenh. Gas Control 2009, 3, 11–19. [Google Scholar] [CrossRef]
- Adanez, J.; Abad, A.; Garcia-Labiano, F.; Gayan, P.; de Diego, L.F. Progress in chemical-looping combustion and reforming technologies. Prog. Energy Combust. Sci. 2012, 38, 215–282. [Google Scholar] [CrossRef]
- Wang, P.; Means, N.; Howard, B.H.; Shekhawat, D.; Berry, D. The reactivity of cuo oxygen carrier and coal in chemical-looping with oxygen uncoupled (clou) and in-situ gasification chemical-looping combustion (ig-clc). Fuel 2018, 217, 642–649. [Google Scholar] [CrossRef]
- Adanez-Rubio, I.; Abad, A.; Gayan, P.; de Diego, L.F.; Garcia-Labiano, F.; Adanez, J. Performance of clou process in the combustion of different types of coal with co2 capture. Int. J. Greenh. Gas Control 2013, 12, 430–440. [Google Scholar] [CrossRef]
- Abad, A.; Adanez-Rubio, I.; Gayan, P.; Garcia-Labiano, F.; de Diego, L.F.; Adanez, J. Demonstration of chemical-looping with oxygen uncoupling (clou) process in a 1.5 kw(th) continuously operating unit using a cu-based oxygen-carrier. Int. J. Greenh. Gas Control 2012, 6, 189–200. [Google Scholar] [CrossRef]
- Adanez-Rubio, I.; Abad, A.; Gayan, P.; de Diego, L.F.; Garcia-Labiano, F.; Adanez, J. Biomass combustion with co2 capture by chemical looping with oxygen uncoupling (clou). Fuel Process. Technol. 2014, 124, 104–114. [Google Scholar] [CrossRef]
- Adanez-Rubio, I.; Arjmand, M.; Leion, H.; Gayan, P.; Abad, A.; Mattisson, T.; Lyngfelt, A. Investigation of combined supports for cu-based oxygen carriers for chemical-looping with oxygen uncoupling (clou). Energy Fuels 2013, 27, 3918–3927. [Google Scholar] [CrossRef]
- Azimi, G.; Leion, H.; Mattisson, T.; Ryden, M.; Snijkers, F.; Lyngfelt, A. Mn-fe oxides with support of mgal2o4, ceo2, zro2 and y2o3-zro2 for chemical-looping combustion and chemical-looping with oxygen uncoupling. Ind. Eng. Chem. Res. 2014, 53, 10358–10365. [Google Scholar] [CrossRef]
- Pour, N.M.; Leion, H.; Ryden, M.; Mattisson, T. Combined cu/mn oxides as an oxygen carrier in chemical looping with oxygen uncoupling (clou). Energy Fuels 2013, 27, 6031–6039. [Google Scholar] [CrossRef]
- Adanez-Rubio, I.; Gayan, P.; Abad, A.; Garcia-Labiano, F.; de Diego, L.F.; Adanez, J. Kinetic analysis of a cu-based oxygen carrier: Relevance of temperature and oxygen partial pressure on reduction and oxidation reactions rates in chemical looping with oxygen uncoupling (clou). Chem. Eng. J. 2014, 256, 69–84. [Google Scholar] [CrossRef]
- Clayton, C.K.; Whitty, K.J. Measurement and modeling of decomposition kinetics for copper oxide-based chemical looping with oxygen uncoupling. Appl. Energy 2014, 116, 416–423. [Google Scholar] [CrossRef]
- Peterson, S.B.; Konya, G.; Clayton, C.K.; Lewis, R.J.; Wilde, B.R.; Eyring, E.M.; Whitty, K.J. Characteristics and clou performance of a novel sio2-supported oxygen carrier prepared from cuo and beta-sic. Energy Fuels 2013, 27, 6040–6047. [Google Scholar] [CrossRef]
- Hu, W.T.; Donat, F.; Scott, S.A.; Dennis, J.S. Kinetics of oxygen uncoupling of a copper based oxygen carrier. Appl. Energy 2016, 161, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Matzen, M.; Pinkerton, J.; Wang, X.M.; Demirel, Y. Use of natural ores as oxygen carriers in chemical looping combustion: A review. Int. J. Greenh. Gas Control 2017, 65, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Tian, X.; Zhao, H.B. Sulfur behavior in chemical-looping combustion using a copper ore oxygen carrier. Appl. Energy 2016, 166, 84–95. [Google Scholar] [CrossRef]
- Wang, K.; Zhao, H.B.; Tian, X.; Fang, Y.F.; Ma, J.C.; Zheng, C.G. Chemical-looping with oxygen uncoupling of different coals using copper ore as an oxygen carrier. Energy Fuels 2015, 29, 6625–6635. [Google Scholar] [CrossRef]
- Zhao, H.B.; Wang, K.; Fang, Y.F.; Ma, J.C.; Mei, D.F.; Zheng, C.G. Characterization of natural copper ore as oxygen carrier in chemical-looping with oxygen uncoupling of anthracite. Int. J. Greenh. Gas Control 2014, 22, 154–164. [Google Scholar] [CrossRef]
- Tian, X.; Zhao, H.B.; Wang, K.; Ma, J.C.; Zheng, C.G. Performance of cement decorated copper ore as oxygen carrier in chemical-looping with oxygen uncoupling. Int. J. Greenh. Gas Control 2015, 41, 210–218. [Google Scholar] [CrossRef]
- Wen, Y.-Y.; Li, Z.-S.; Xu, L.; Cai, N.-S. Experimental study of natural cu ore particles as oxygen carriers in chemical looping with oxygen uncoupling (clou). Energy Fuels 2012, 26, 3919–3927. [Google Scholar] [CrossRef]
- Calcutt, V. Introduction to Copper: Mining & Extraction. Copper Development Association Inc., 2001. Available online: https://www.copper.org/publications/newsletters/innovations/2001/08/intro_mae.html (accessed on 12 October 2018).
- Baba, A.A.; Ayinla, K.I.; Adekola, F.A.; Ghosh, M.K.; Ayanda, O.S.; Bale, R.B.; Sheik, A.R.; Pradhan, S.R. A review on novel techniques for chalcopyrite ore processing. Int. J. Min. Eng. Miner. Process. 2012, 1, 1–16. [Google Scholar] [CrossRef]
- Teck-Metals-Ltd. Andacollo Copper Concentrate Safety Data Sheet. 2015. Available online: https://www.teck.com/media/Andacollo-Copper-Concentrate-SDS.pdf (accessed on 10 September 2018).
- Gayan, P.; Adanez-Rubio, I.; Abad, A.; de Diego, L.F.; Garcia-Labiano, F.; Adanez, J. Development of cu-based oxygen carriers for chemical-looping with oxygen uncoupling (clou) process. Fuel 2012, 96, 226–238. [Google Scholar] [CrossRef]
- Wang, B.; Yan, R.; Zhao, H.; Zheng, Y.; Liu, Z.; Zheng, C. Investigation of chemical looping combustion of coal with cufe2o4 oxygen carrier. Energy Fuels 2011, 25, 3344–3354. [Google Scholar] [CrossRef]
- Cao, Y.; Pan, W.-P. Investigation of chemical looping combustion by solid fuels. 1. Process analysis. Energy Fuels 2006, 20, 1836–1844. [Google Scholar] [CrossRef]
- Wang, P.; Howard, B.H.; Means, N.; Shekhawat, D. Bimetallic cu-fe Oxygen Carriers for Coal Chemical-Looping Combustion. In Proceedings of the 255th American Chemical Society National Meeting & Exposition, New Orleans, LA, USA, 18–22 March 2018. [Google Scholar]
- Fastmarkets-MB. Copper Concentrate tc/rcs Decline Further as Traders Bid Aggressively for Tonnes. 2018. Available online: https://www.metalbulletin.com/Article/3787933/Copper-concentrate-TCRCs-decline-further-as-traders-bid-aggressively-for-tonnes.html (accessed on 9 March 2019).
- Fastmarkets-MB. LIVE Futures Report 08/03: Comex Copper Price Suffers Further Losses in Final Session. 2018. Available online: https://www.metalbulletin.com/Article/3863299/LIVE-FUTURES-REPORT-0803-Comex-copper-price-suffers-further-losses-in-final-session.html (accessed on 10 March 2019).





| Sample | Proximate Analysis (% Dry Basis) | Ultimate Analysis (% Dry Basis) | ||||||
|---|---|---|---|---|---|---|---|---|
| Fixed Carbon | Volatile Matter | Ash | C | H | N | S | O (Diff.) | |
| PRB Coal | 47.66 | 45.08 | 7.26 | 65.44 | 4.39 | 0.72 | 0.48 | 21.71 |
| PRB Char | 85.60 | 1.98 | 12.42 | 85.60 | 0.23 | 1.12 | 0.53 | 0.10 |
| Chalcopyrite | Fresh Cu-OC | Uncoupled Cu-OC | Reduced Cu-OC | Reoxidized | |
|---|---|---|---|---|---|
| Chemical | wt% | with Char | Cu-OC with Char | ||
| CuFeS2 | 75 | CuO | Cu2O | Cu2O | CuO |
| CuFe2O4 (CuOFe2O3) (tetragonal) | CuFe2O4 (tetragonal) | ||||
| CuFe2O4 (cubic) | CuFe2O4 (cubic) | CuFe2O4 (cubic) | |||
| CuFeO2 (Cu2OFe2O3) | CuFeO2 | ||||
| Fe2O3 | Fe2O3 | Fe2O3 | Fe2O3 | ||
| SiO2 | 20 | SiO2 | SiO2 | SiO2 | SiO2 |
| FeCO3 | 2 | ||||
| kaolinite | 3 | ||||
| Sample | T (°C) | Oxygen Uncoupling | Carbon Conversion in Reduction | Test System | |||||
|---|---|---|---|---|---|---|---|---|---|
| momax | Tmax | dXo/dtmax | Xc | Sco2 | Tmax | dXc/dtmax | |||
| Roc (%) | (°C) | (1/s) | (°C) | (1/s) | |||||
| Cu ore OC | 900 | 3.3 | 900 | 0.0012 | TGA-QMS | ||||
| Pure CuO | 850 | 10 | 850 | 0.0015 | fixed bed-QMS | ||||
| 950 | 10 | 922 | 0.0077 | fixed bed-QMS | |||||
| Cu ore OC/char | 900 | 0.94 | 1 | 838 | 0.005 | TGA-QMS | |||
| ϕ = 75 | 900 | 0.95 | 1 | 835 | 0.006 | fixed bed-QMS | |||
| Pure CuO/char | 850 | 0.98 | 1 | 801 | 0.006 | fixed bed-QMS | |||
| ϕ = 26 | 950 | 0.99 | 1 | 833 | 0.011 | fixed bed-QMS | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Howard, B.; Means, N.; Shekhawat, D.; Berry, D. Coal Chemical-Looping with Oxygen Uncoupling (CLOU) Using a Cu-Based Oxygen Carrier Derived from Natural Minerals. Energies 2019, 12, 1453. https://doi.org/10.3390/en12081453
Wang P, Howard B, Means N, Shekhawat D, Berry D. Coal Chemical-Looping with Oxygen Uncoupling (CLOU) Using a Cu-Based Oxygen Carrier Derived from Natural Minerals. Energies. 2019; 12(8):1453. https://doi.org/10.3390/en12081453
Chicago/Turabian StyleWang, Ping, Bret Howard, Nicholas Means, Dushyant Shekhawat, and David Berry. 2019. "Coal Chemical-Looping with Oxygen Uncoupling (CLOU) Using a Cu-Based Oxygen Carrier Derived from Natural Minerals" Energies 12, no. 8: 1453. https://doi.org/10.3390/en12081453





