Formation Damage Avoidance by Reducing Invasion with Sodium Silicate-Modified Water-Based Drilling Fluid
Abstract
:1. Introduction
1.1. Drilling Fluid Rheological Properties
1.2. Drill-in Fluids for Unconventional Reservoirs
2. Experimental Study
2.1. Water-Based Drilling Fluid
2.2. Experimental Procedure
- The drilling fluid was prepared and mixed using a different percentage of sodium silicate (0, 0.05, 0.075, and 0.1 wt.%).
- The fluid density and pH were measured at ambient conditions (77 °F and 14.7 psi).
- The rheological properties were measured at different temperature (77, 120, 170, and 300 °F).
- The initial permeability of tight sandstone core (2 inch length and 2.5 inch thickness) was determined using the modified high-pressure-high temperature cell.
- The filtration test was performed at 300 °F and 300 psi differential pressure and the formed filter cake thickness was measured after using a different concentration of SS.
- The formed filter cake was removed mechanically and the damage core permeability was obtained.
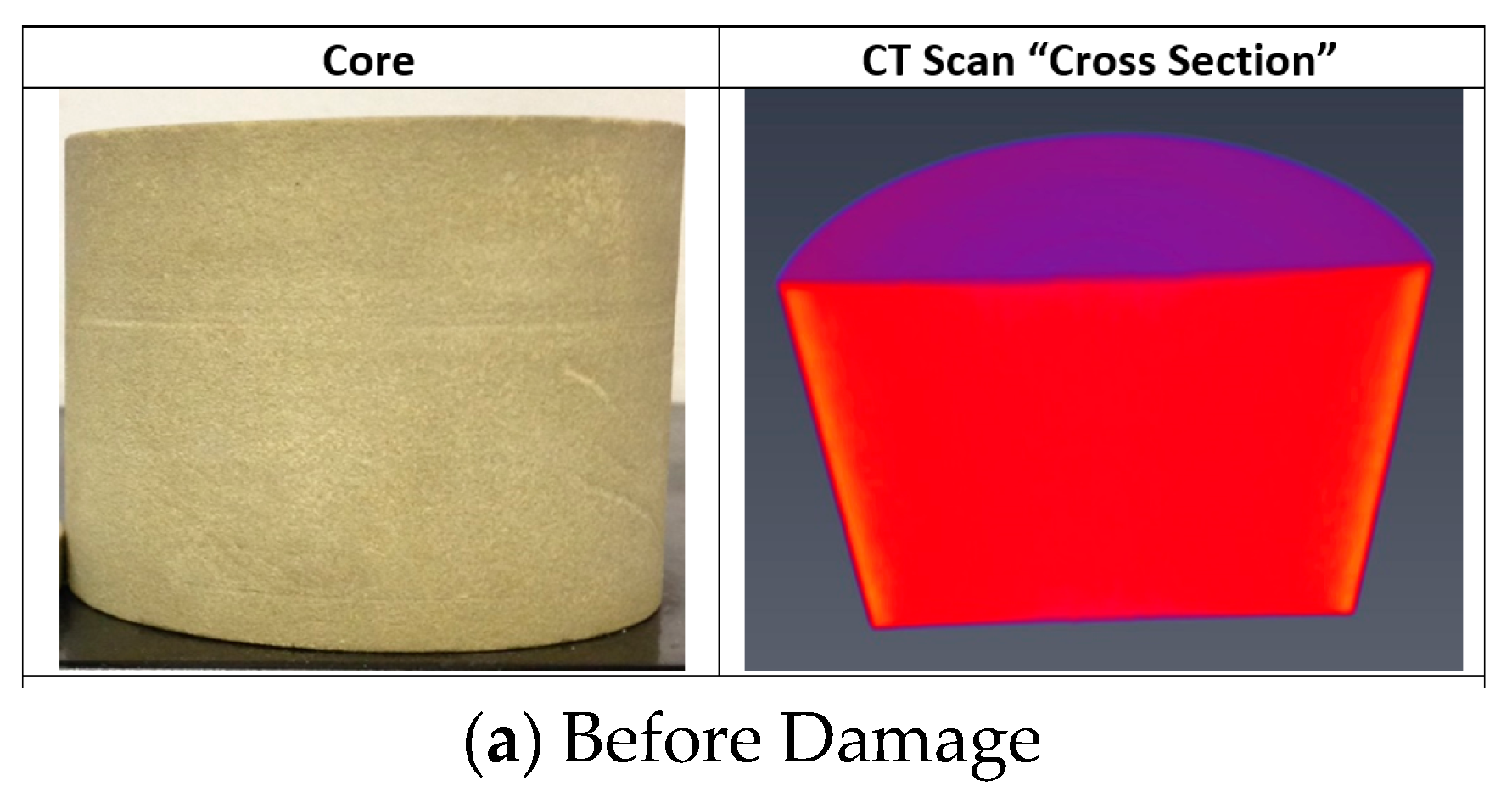
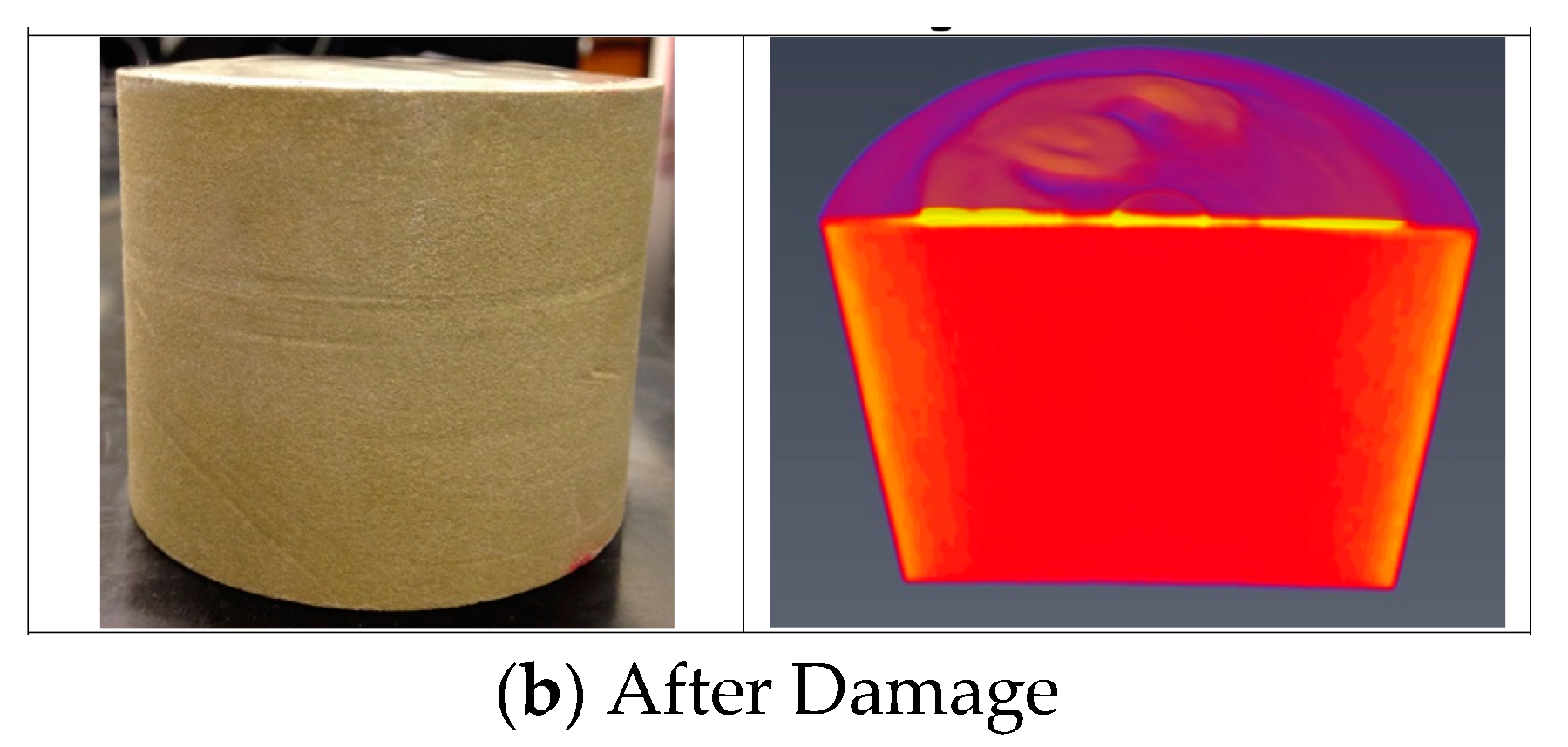
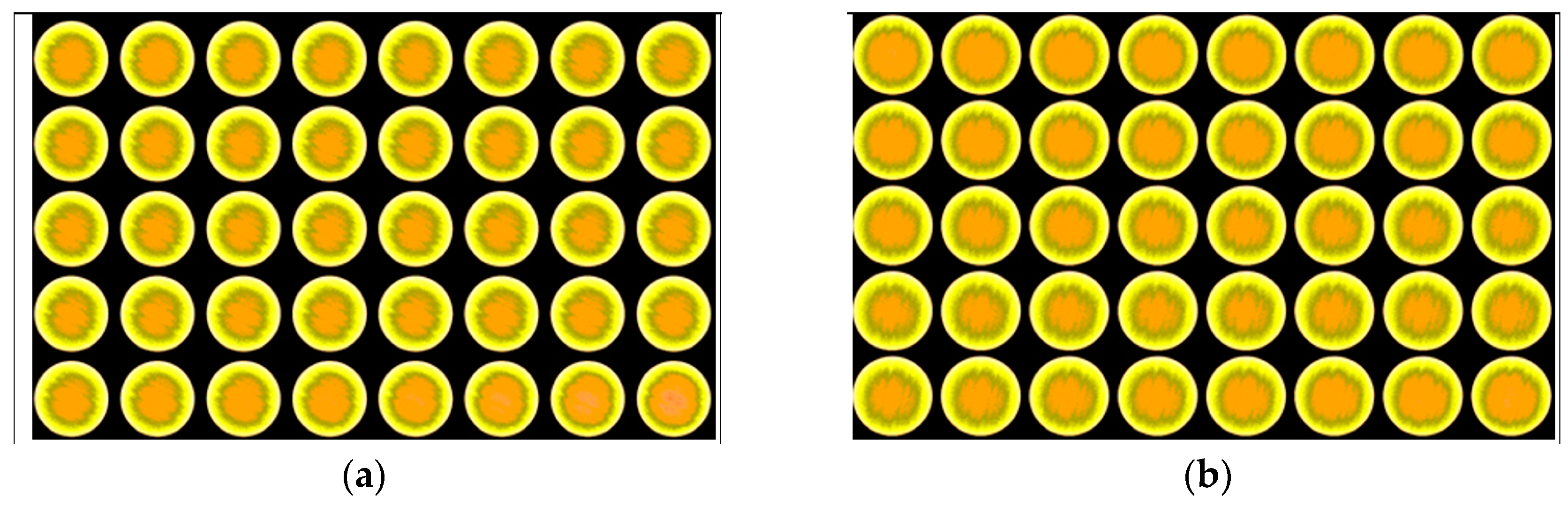
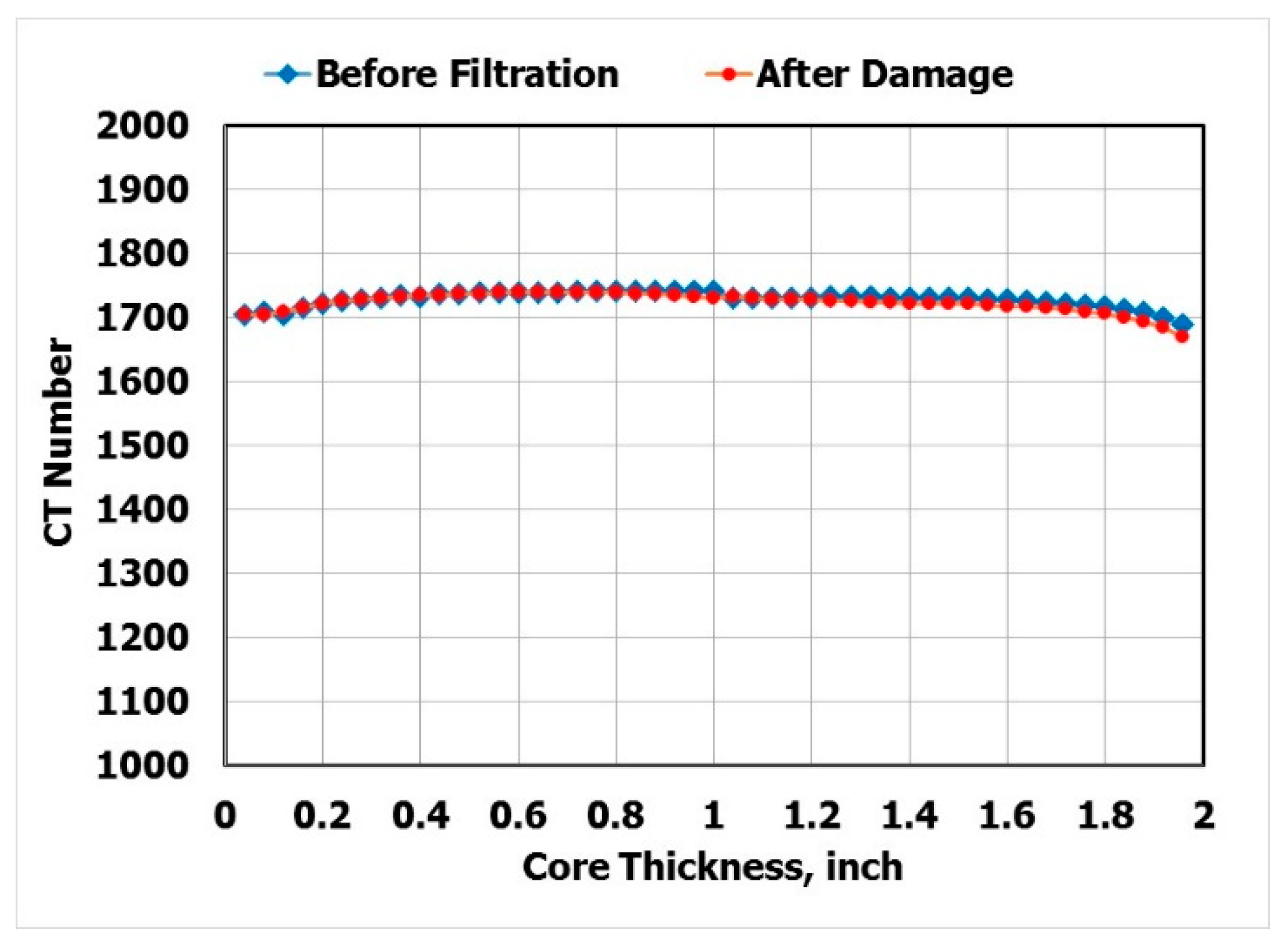
- CT scan was performed for the core before the filtration and the mechanical removal of the filter cake.
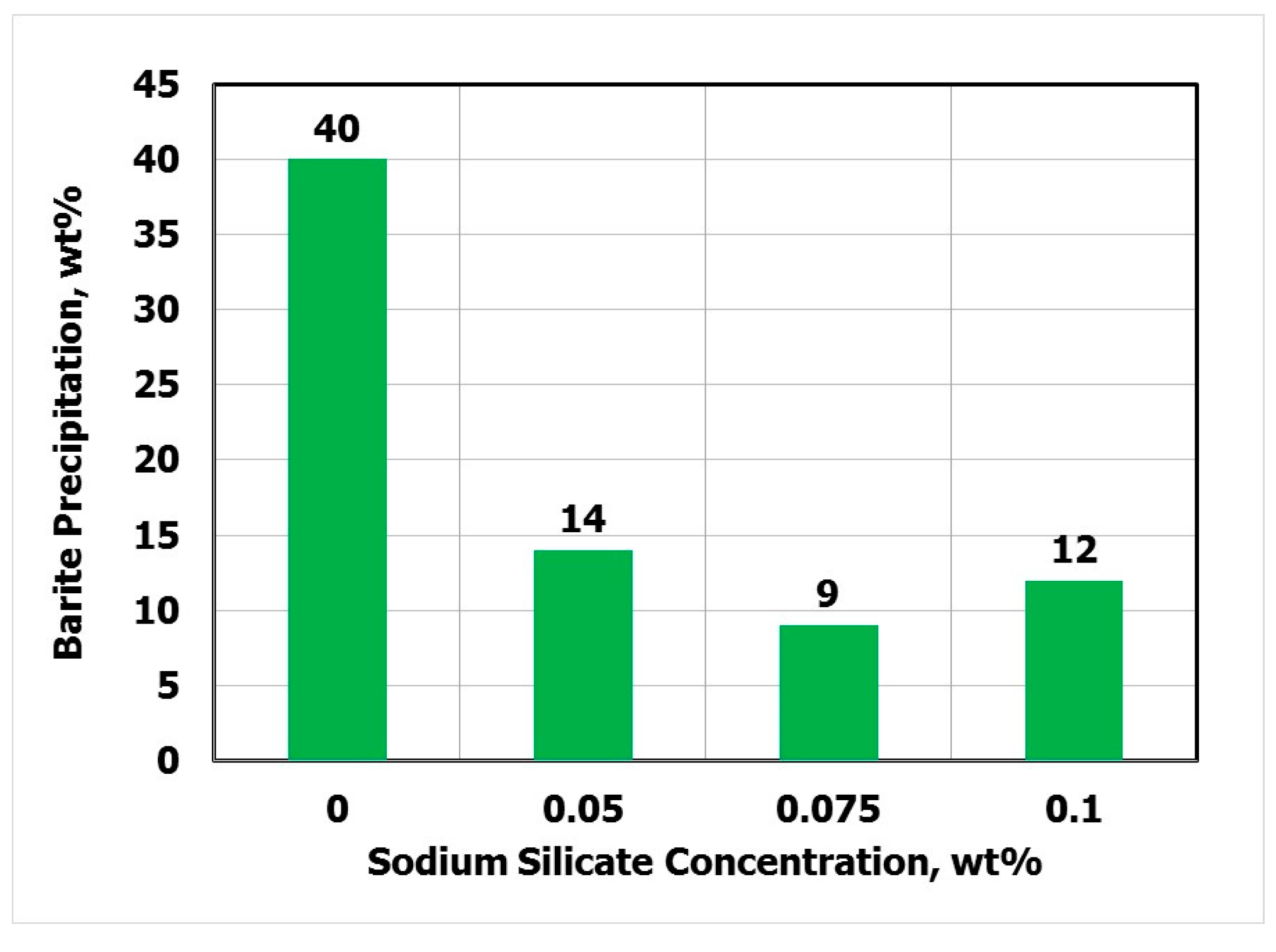
- The effect of sodium silicate on barite solubility was performed at 200 °F.
3. Results and Discussion
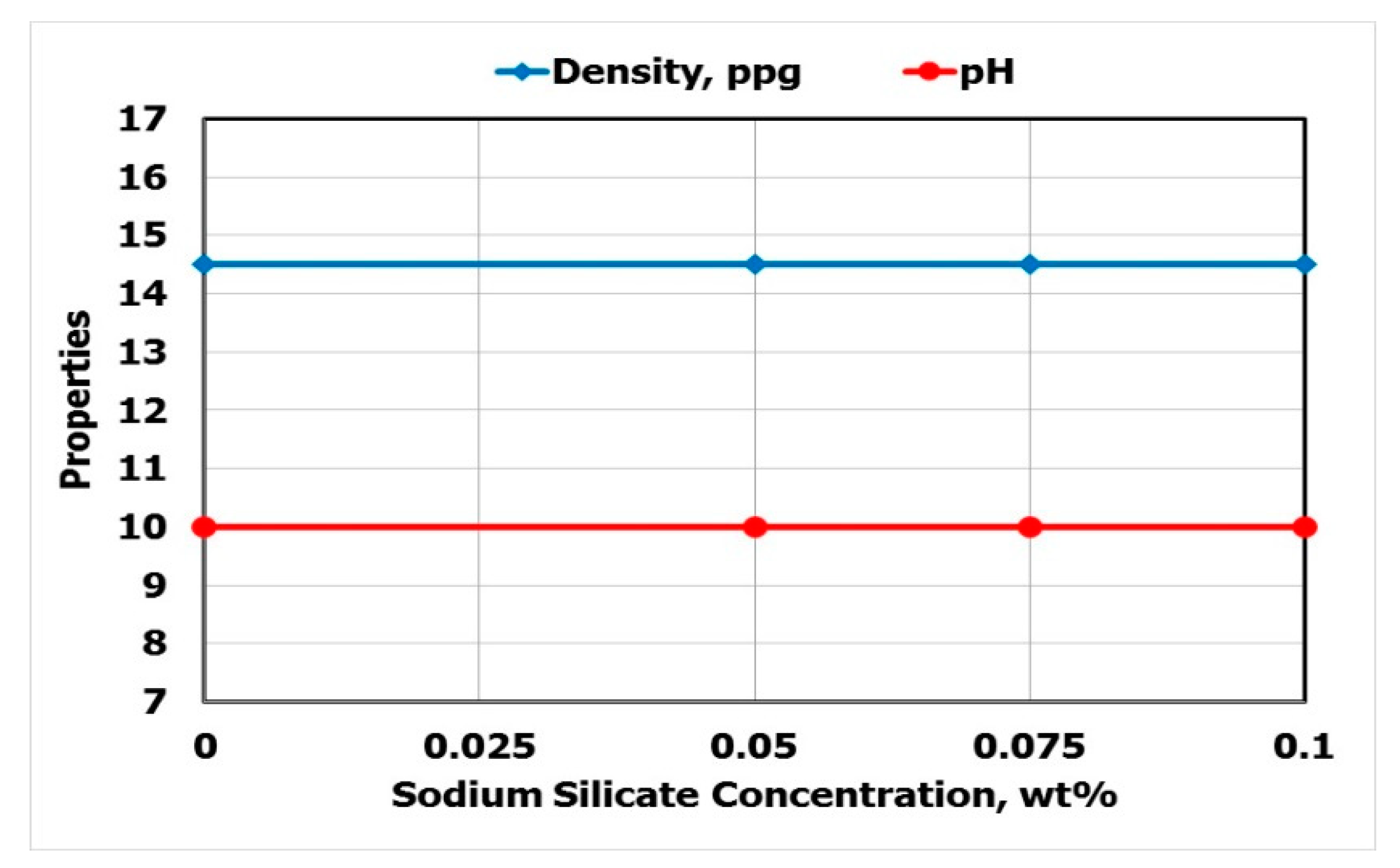
3.1. Effect of Adding Sodium Silicate
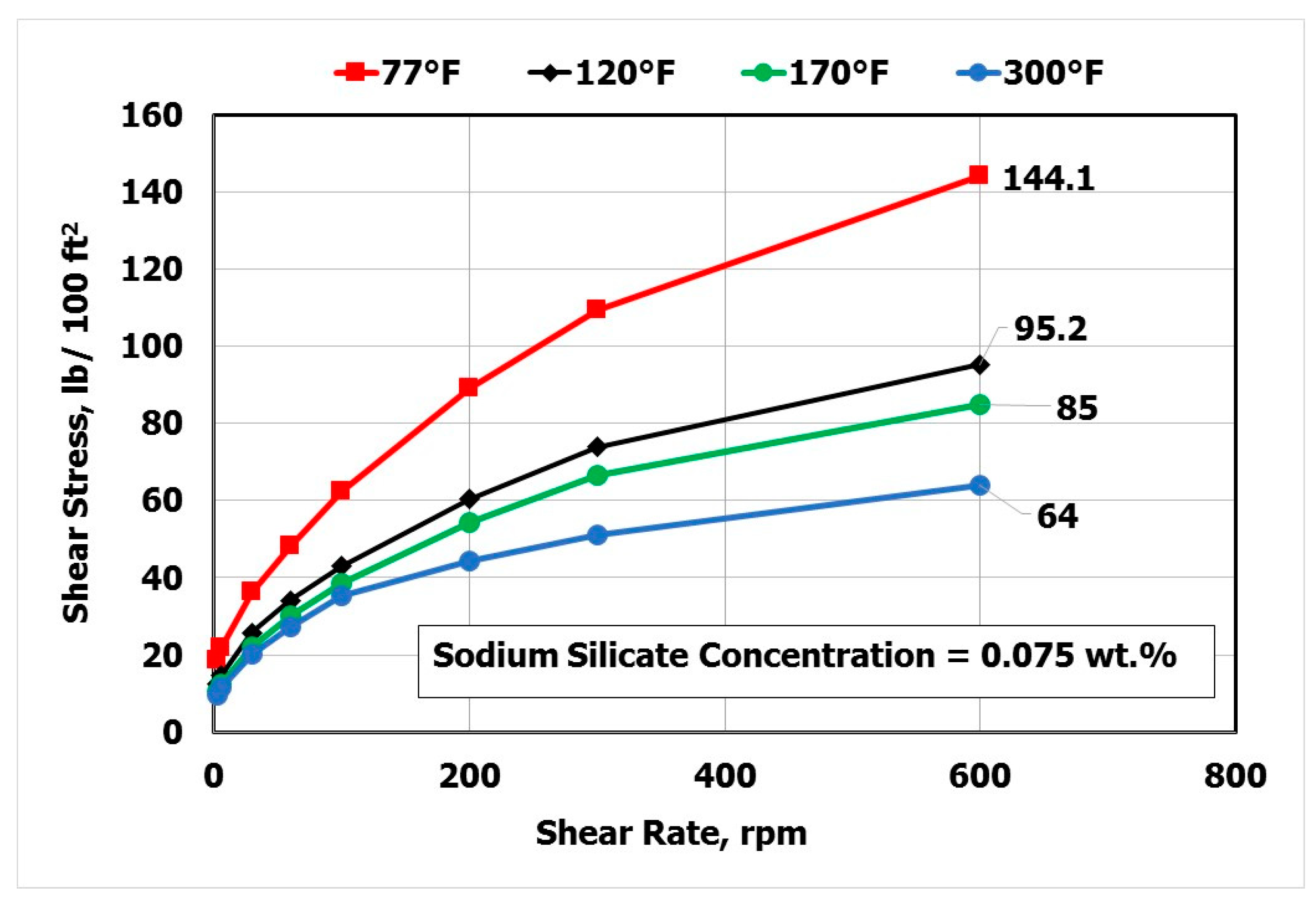
3.2. Effect of Temperature and Sodium Silicate (SS) Concentration
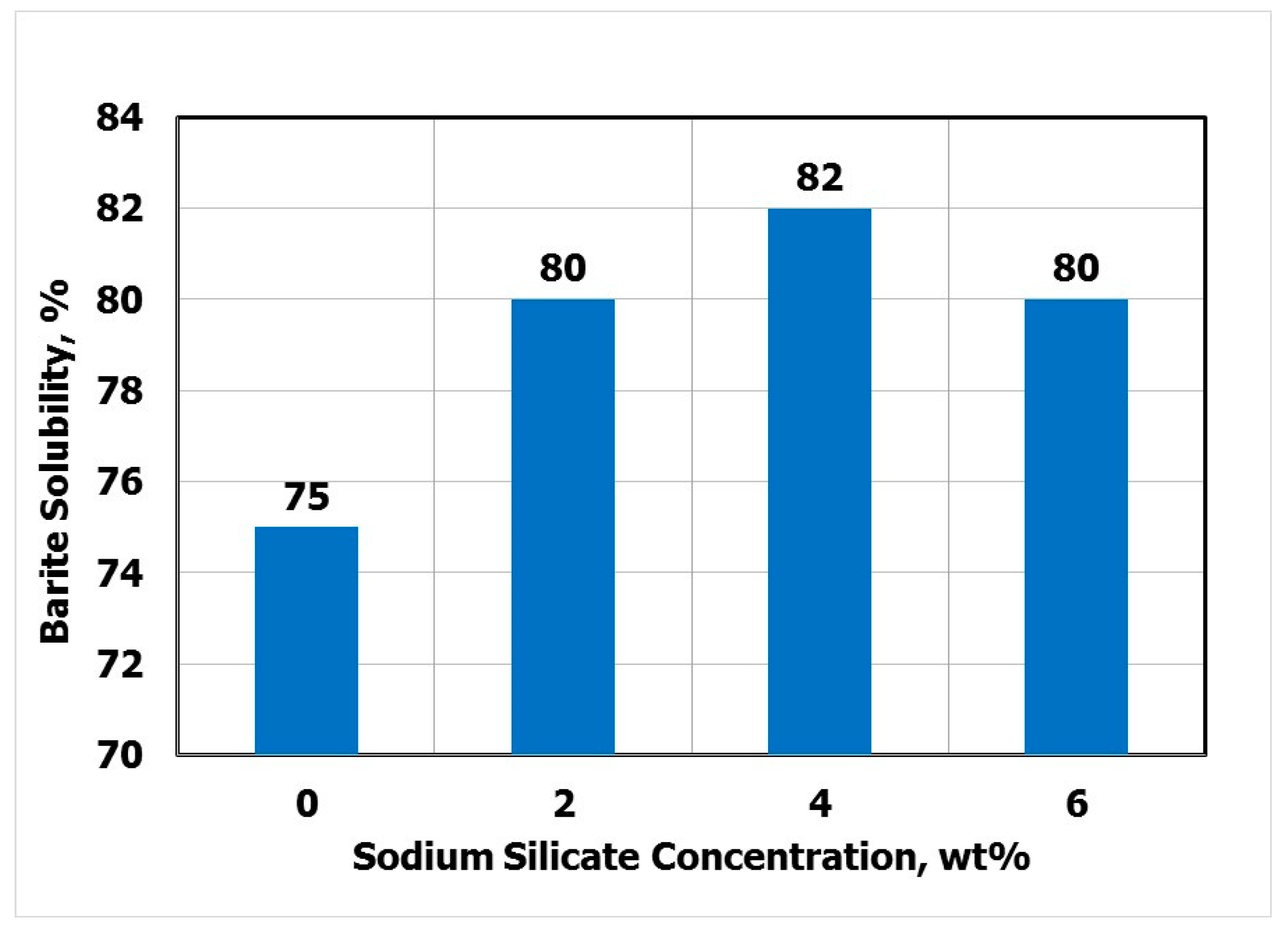
3.3. Effect of Sodium Silicate on Barite Solubility
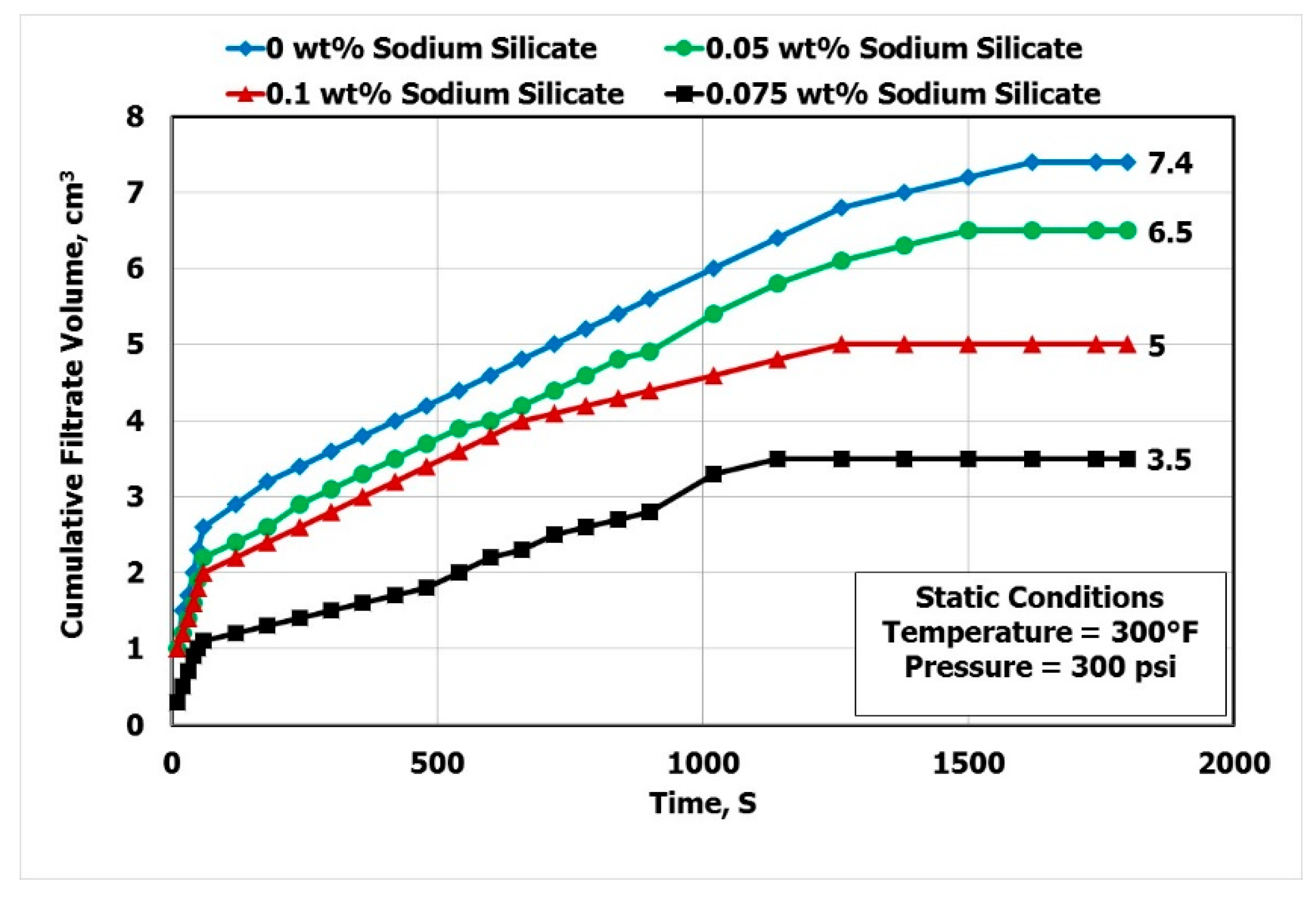
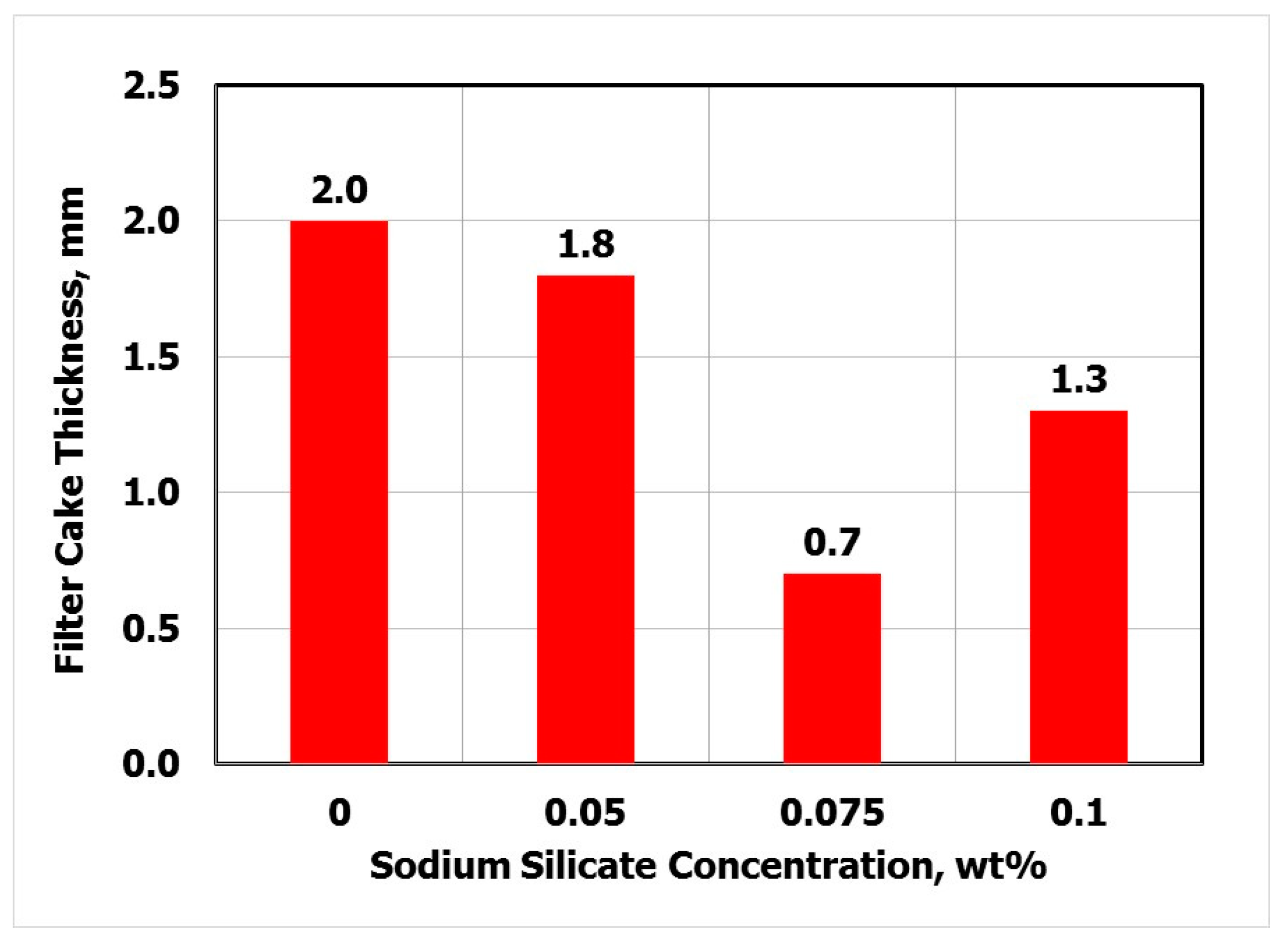
3.4. Effect of Sodium Silicate on Filtration
3.5. Retained Permeability
3.6. Mechanism of Sodium Silicate in Barite Water-Based Drilling Fluids
4. Conclusions
- Sodium silicate had no effect of fluid density and pH. In addition, there was no change in the yield point plastic viscosity ratio.
- The optimal SS concentration was 0.075 wt.% and the temperature had no effect on this value over a wide range (77–300 °F).
- Using 0.075 wt.% of SS the filtrate volume was reduced by 53% and the filter cake thickness was decreased by 65%.
- The CT scan and the return permeability calculation after the mechanical removal of the filter cake confirmed that there was no solid invasion after the filtration test at 300 °F and applied differential pressure of 300 psi.
Author Contributions
Funding
Conflicts of Interest
References
- Amanullah, M.; Allen, T. Extended Aramco Method—Its Technico-Economic Significance in Non-Damaging Drill-in Fluid Design. Presented at the SPE/IADC Middle East Drilling Technology Conference and Exhibition, Dubai, UAE, 7–9 October 2013. [Google Scholar]
- Mandal, N.G.; Jain, U.K.; Anil Kumar, B.S.; Gupta, A.K. Nondamaging Drilling Fluid Enhances Borehole Quality and Productivity in Conventional Wells of Mehsana Asset, North Cambay Basin. Presented at the SPE/IADC Indian Drilling Technology Conference and Exhibition, Mumbai, India, 16–18 October 2016. [Google Scholar]
- Elkatatny, S.M.; Mahmoud, M.A.; Nasr-El-Din, H.A. Characterization of Filter Cake Generated by Water-Based Drilling Fluids Using Ct Scan. SPE Drill. Complet. 2012, 27, 282–293. [Google Scholar] [CrossRef]
- Elkatatny, S.M.; Mahmoud, M.; Nasr-El-Din, H. Filter Cake Properties of Water-Based Drilling Fluids under Static and Dynamic Conditions Using CT Scan. J. Energy Resour. Technol. 2013, 135. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, Z.; Sun, B.; Liu, S.; Xing, X.; Wang, M. Formation damage mechanisms associated with drilling and completion fluids for deep water reservoirs. J. Pet. Sci. Eng. 2019, 173, 112–121. [Google Scholar] [CrossRef]
- Fakoya, M.F.; Ahmed, R.M. A generalized model for apparent viscosity of oil-based muds. J. Pet. Sci. Eng. 2018, 165, 777–785. [Google Scholar] [CrossRef]
- Elkatatny, S.M.; Zeeshan, T.; Mahmoud, M.A. Real Time Prediction of Drilling Fluid Rheological Properties Using Artificial Neural Networks Visible Mathematical Model (White Box). J. Pet. Sci. Eng. 2016, 146, 1202–1210. [Google Scholar] [CrossRef]
- Power, D.; Zamora, M. Drilling Fluid Yield Stress: Measurement Techniques for Improved Understanding of Critical Drilling Fluid Parameters. In Proceedings of the AADE-03 NTCE-35, AADE Technical Conference, Houston, TX, USA, 1–3 April 2003. [Google Scholar]
- Mitchell, R.F.; Miska, S.Z. Fundamentals of Drilling Engineering; Society of Petroleum Engineers: London, UK, 2011. [Google Scholar]
- Estes, J.C. Guidelines for Selecting Rotary Insert Rock Bits. Pet. Eng. 1974, 9, 30–34. [Google Scholar]
- Hussaini, S.M.; Azar, J.J. Experimental Study of Drilled Cutting Transport Using Common Drilling Muds. SPE J. 1983, 23, 11–20. [Google Scholar] [CrossRef]
- Rao, M.A. Rheology of Fluid and Semisolid Foods, 2nd ed.; Springer Science + Business Media LLC: New York, NY, USA, 2007. [Google Scholar]
- Robinson, L.; Morgan, M. Effect of hole cleaning on drilling rate performance. Presented at the AADE Drilling Fluid Conference, Houston, TX, USA, 6–7 April 2004. [Google Scholar]
- Nasiri, M.; Ashrafizadeh, S.N. Novel Equation for the Prediction of Rheological Parameters of Drilling Fluids in an Annulus. Ind. Eng. Chem. Res. 2010, 49, 3374–3385. [Google Scholar] [CrossRef]
- Zhang, F.; Miska, S.; Yu, M.; Ozbayoglu, E.M.; Takach, N. Pressure Profile in Annulus: Solids Play a Significant Role. J. Energy Resour. Technol. 2015, 137, 064502. [Google Scholar] [CrossRef]
- Taghipour, A.; Lund, B.; Ytrehus, J.D.; Skalle, P.; Saasen, A.; Reyes, A.; Abdollahi, J. Experimental Study of Hydraulics and Cuttings Transport in Circular and Noncircular Wellbores. J. Energy Resour. Technol. 2014, 136, 022904. [Google Scholar]
- Li, M.; Wu, Q.; Song, K.; De Hoop, C.; Lee, S.; Qing, Y.; Wu, Y. Cellulose Nanocrystals and Polyanionic Cellulose as Additives in Bentonite Water-Based Drilling Fluids: Rheological Modeling and Filtration Mechanisms. Ind. Eng. Chem. Res. 2016, 55, 133–143. [Google Scholar] [CrossRef]
- Halali, M.A.; Ghotbi, C.; Tahmasbi, K.; Ghazanfari, M.H. The Role of Carbon Nanotubes in Improving Thermal Stability of Polymeric Fluids: Experimental and Modeling. Ind. Eng. Chem. Res. 2016, 55, 7514–7534. [Google Scholar] [CrossRef]
- Lake, L.W.; Fanchi, J.R.; Mitchell, R.F.; Arnold, K.E.; Clegg, J.D.; Holstein, E.D.; Warner, H.R., Jr. Petroleum Engineering Handbook; Society of Petroleum Engineers: Houston, TX, USA, 2007; Volume 6. [Google Scholar]
- Van Zanten, R.; Horton, D.; Tanche-Larsen, P. Engineering Drill-in Fluids to Improve Reservoir Producibility. Presented at the SPE European Formation Damage Conference, Noordwijk, The Netherlands, 7–10 June 2011. [Google Scholar]
- Guo, J.; Yan, J.; Fan, W.; Zhang, H. Applications of strongly inhibitive silicate-based drilling fluids in troublesome shale formations in Sudan. J. Pet. Sci. Eng. 2006, 50, 195–203. [Google Scholar] [CrossRef]
- Zabala, E.; Luongo, A.; Parra, E.; Mendez, J.; Arocha, J.; Carrasquero, J. Field Experiences with Stable Mul, a Non Damaging Micro Emulsion Used as Drilling Fluid for Heavy Oil Formations in Venezuela. Presented at the SPE International Thermal Operations and Heavy Oil Symposium, Bakersfield, CA, USA, 17–19 March 1999. [Google Scholar]
- El Bialy, M.; Mohsen, M.; Ezell, R.G.; Abdulaziz, M.E.; Kompantsev, A.; Khakimov, A.; Ganizade, F.; Ashoor, A. Utilization of Non-Damaging Drilling Fluid Composed of Potassium Formate Brine and Manganese Tetra Oxide to Drill Sandstone Formation in Tight Gas Reservoir. Presented at the SPE/IADC Middle East Drilling Technology Conference and Exhibition, Muscat, Oman, 24–26 October 2011. [Google Scholar]
- Han, L.; Clinch, D.; van der Zwaag, C.; Galletti, E.; Fornasier, F.; Estevez, F.; McMillan, N.; Green, J.; Patey, I. Customizing Drill-in Fluid for Peregrino Project in Brazil: Laboratory Development and Field Experience. Presented at the SPE International Symposium and Exhibition on Formation Damage Control, Lafayette, LA, USA, 15–17 February 2012. [Google Scholar]
- Bageri, B.; Mahmoud, M.A.; Abdulraheem, A.; Al-Mutairi, S.H.; Elkatatny, S.M.; Shawabkeh, R.A. Single Stage Filter Cake Removal of Barite Weighted Water Based Drilling Fluid. J. Pet. Sci. Eng. 2017, 149, 476–484. [Google Scholar] [CrossRef]
- Volpe, D. Assessment of Iron and Manganese Sequestration. Master’s Thesis, University of Massachusetts Amherst, Amherst, MA, USA, 2012. [Google Scholar]
- Young, S.; Friedheim, J. Environmentally Friendly Drilling Fluids for Unconventional Shale. Presented at the 11th Offshore Mediterranean Conference and Exhibition, Ravenna, Italy, 20–22 March 2013. [Google Scholar]
- Elkatatny, S.M.; Nasr-El-Din, H. Removal of Water-Based Filter Cake and Stimulation of the Formation in One-Step Using an Environmentally Friendly Chelating Agent. Int. J. Oil Gas Coal Technol. 2014, 7, 169–188. [Google Scholar] [CrossRef]
- Bulatovic, S.M. Handbook of Flotation Reagents: Chemistry, Theory and Practice—Flotation of Industrial Minerals; Elsevier: Amsterdam, The Netherlands, 2007; Volume 3. [Google Scholar]
- Ives, K.J. The Scientific Basis of Flotation; Martinus Nijhoff Publishers: Hague, The Netherlands, 2012. [Google Scholar]


| Additives | Amount |
|---|---|
| Distilled Water | 241.5 cm3 |
| Soda Ash (Na2CO3) | 0.5 g |
| De-foamer | 0.01 g |
| Bentonite | 5 g |
| XC (Xanthan gum) Polymer | 1 g |
| Caustic Soda (NaOH) | 0.25 g |
| Sodium Chloride (NaCl) | 22 g |
| Starch | 4 g |
| CaCO3 (25 and ˂ 38 micron) | 3 + 3 g |
| Barite | 278 g |
| Properties | Value |
|---|---|
| Density, ppg | 14.5 |
| Plastic viscosity, cP | 27 |
| Yield point, lb/100 ft2 | 57 |
| 10 s gel strength, lb/100 ft2 | 12 |
| 10 min gel strength, lb/100 ft2 | 19 |
| pH | 10 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkatatny, S.; Jafarov, T.; Al-Majed, A.; Mahmoud, M. Formation Damage Avoidance by Reducing Invasion with Sodium Silicate-Modified Water-Based Drilling Fluid. Energies 2019, 12, 1485. https://doi.org/10.3390/en12081485
Elkatatny S, Jafarov T, Al-Majed A, Mahmoud M. Formation Damage Avoidance by Reducing Invasion with Sodium Silicate-Modified Water-Based Drilling Fluid. Energies. 2019; 12(8):1485. https://doi.org/10.3390/en12081485
Chicago/Turabian StyleElkatatny, Salaheldin, Tural Jafarov, Abdulaziz Al-Majed, and Mohamed Mahmoud. 2019. "Formation Damage Avoidance by Reducing Invasion with Sodium Silicate-Modified Water-Based Drilling Fluid" Energies 12, no. 8: 1485. https://doi.org/10.3390/en12081485
APA StyleElkatatny, S., Jafarov, T., Al-Majed, A., & Mahmoud, M. (2019). Formation Damage Avoidance by Reducing Invasion with Sodium Silicate-Modified Water-Based Drilling Fluid. Energies, 12(8), 1485. https://doi.org/10.3390/en12081485







