Corrosion Performance of Engineered Barrier System in Deep Horizontal Drillholes
Abstract
:1. Introduction
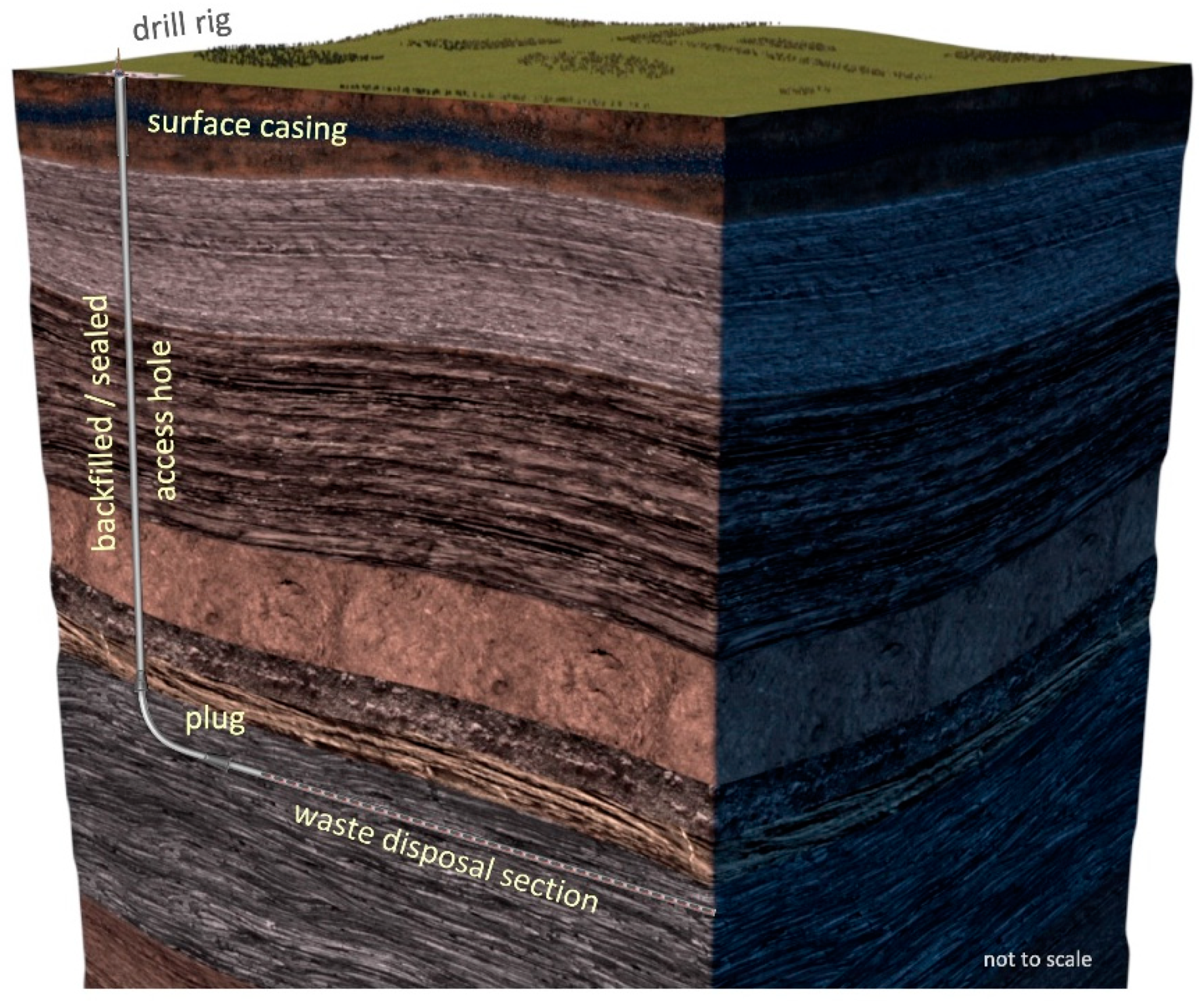
2. Representative Disposal System
3. Methods
- Description of the representative EBS design for disposal of Cs/Sr waste capsules
- Determination of the evolution of the temperature path from thermal simulation and the evolution of the aqueous environment for a deep horizontal drillhole
- Designation of a series of zones relevant to corrosion performance
- Assignment of corrosion rates for Alloy 625 and L80 steel for each zone
- Calculation of EBS corrosion performance.
4. Evolution of the Environment
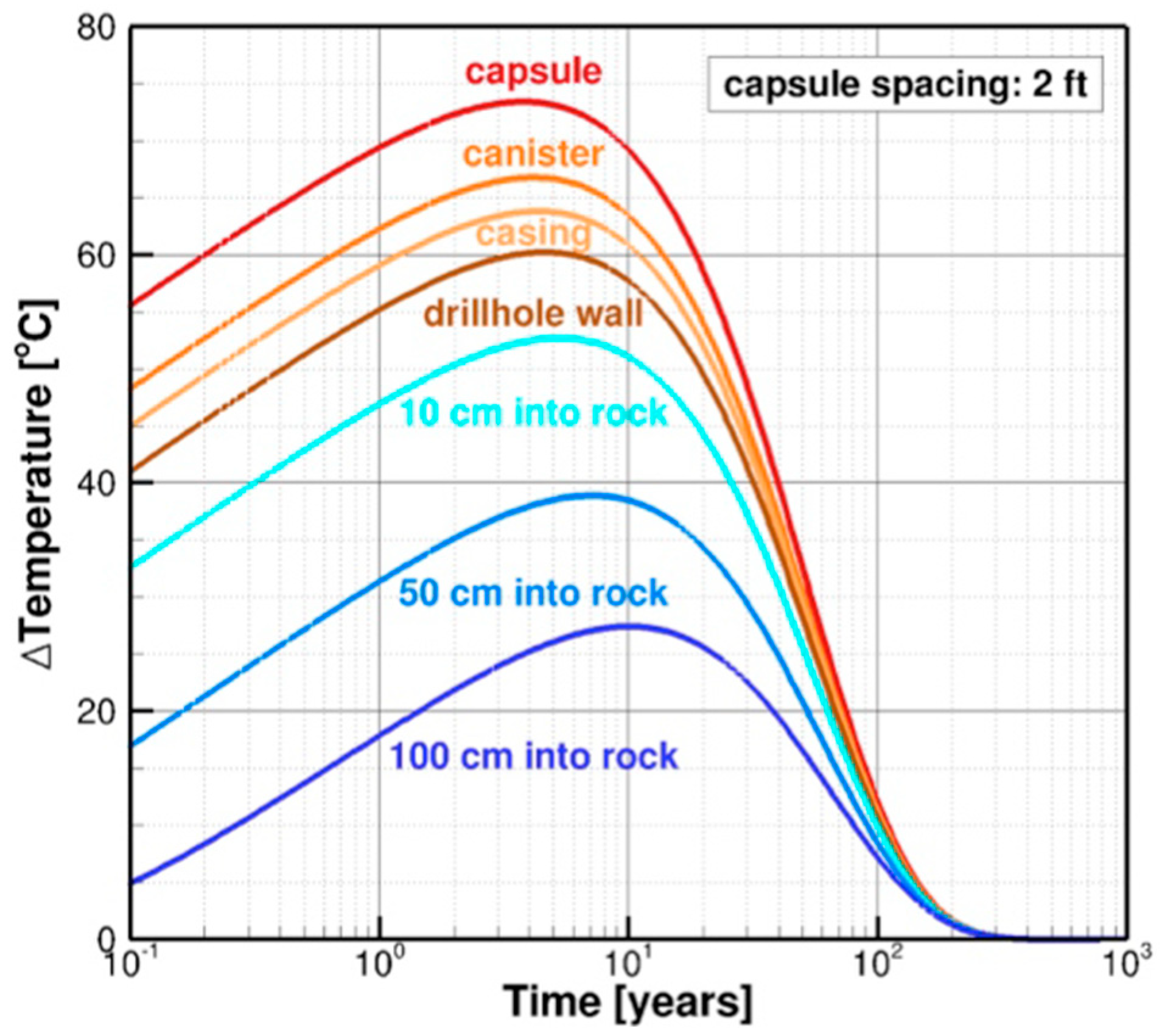
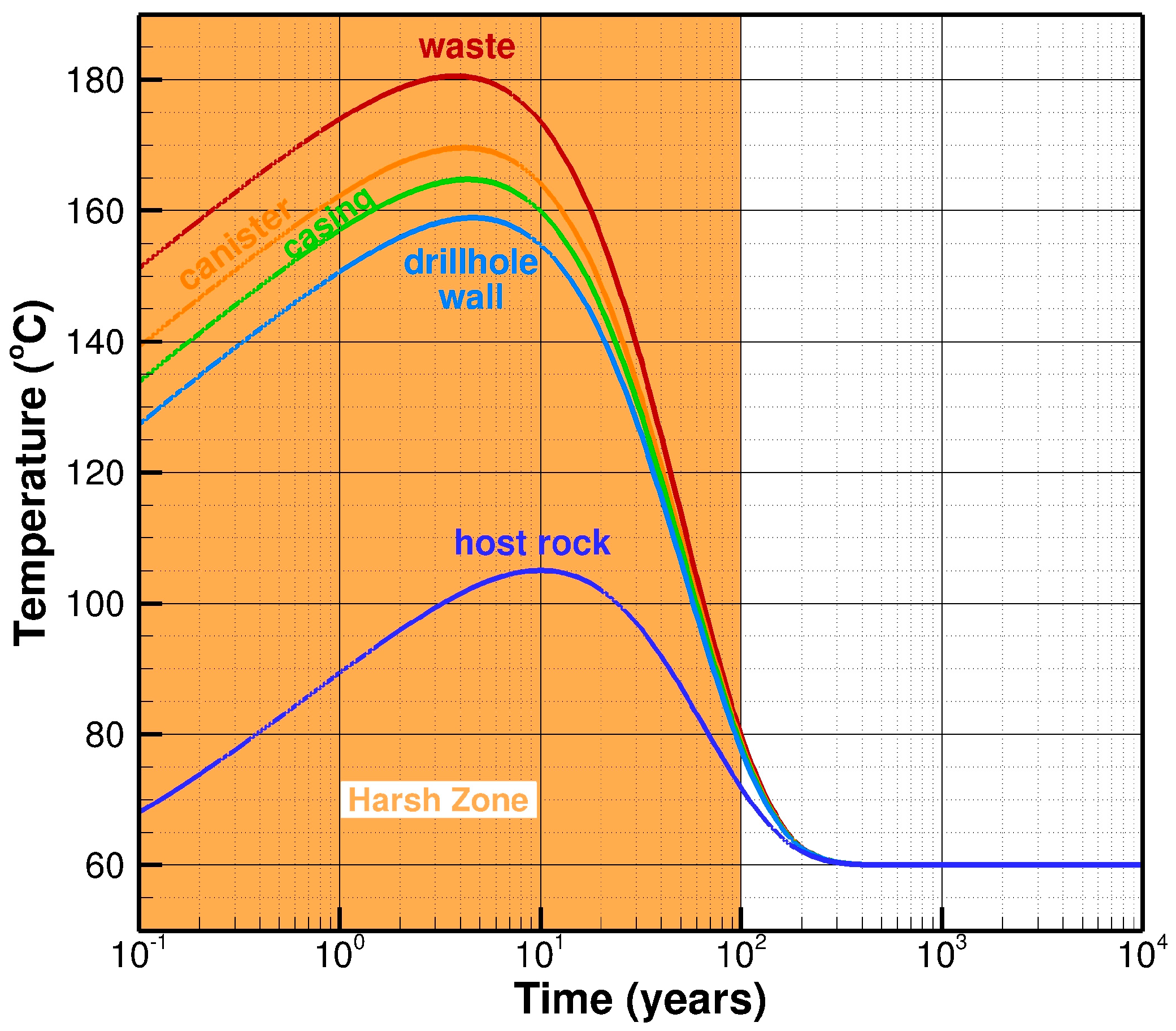
4.1. Evolution of Temperature
4.2. Evolution of the Aqueous Environment
5. Corrosion, Hydrogen Generation and Oxide Formation
5.1. Effect of Corrosion Rate on Metal Loss, Hydrogen Generation and Oxide Formation
5.2. Metal Loss Over 10,000 Years
- Zone I, Years 0–2: During this early transition period, the corrosion resistance of the metals changes and the environment evolves toward reducing conditions. Exposure conditions are moderately oxidizing from oxygen introduced during the drilling, casing installation, and canister emplacement. Initial heat-up has begun, and there are fresh metal surfaces on the canisters and casing. While oxygen is present, the corrosion reaction produces ferric (Fe3+) containing compounds such as Fe(OH)3, and no hydrogen is produced. As corrosion proceeds, oxygen is consumed, the ferric species are reduced, and hydrogen production commences. On conversion to Fe3O4, more hydrogen is produced.
- Zone II, Years 2–20: During the second period, the highest EBS temperatures are reached, cool-down begins, oxygen has been consumed, and conditions transition to anoxic and reducing.
- Zone III, Years 20–100: For the third period, the temperature has cooled from 120 °C to 80 °C. The environment throughout is anoxic and reducing.
- Zone IV, Years 100–1000: For the fourth period, the temperature has cooled further to 60 °C. The environment throughout is anoxic and reducing.
- Zone V, Years 1000–10,000: The temperature is still 60 °C (the ambient rock temperature) and remains steady for 10,000 years and beyond. The environment is anoxic and reducing.
5.3. Hydrogen Generation and Oxide Formation
5.3.1. Canister Corrosion Products
5.3.2. Casing Corrosion Products
6. Discussion
6.1. Evolution of the Environment and the Corrosion Evolutionary Path
6.2. Design and Strategies of EBS for Deep Horizontal Drillholes
6.2.1. Location and Conditions at Drillhole Depth
6.2.2. EBS Design
6.2.3. Technical Basis and Safety Case
7. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Muller, R.A.; Finsterle, S.; Grimsich, J.; Baltzer, R.; Muller, E.A.; Rector, J.W.; Payer, J.; Apps, J. Disposal of High-Level Nuclear Waste in Deep Horizontal Drillholes. Preprints 2019, 2019040153. [Google Scholar] [CrossRef]
- Deep Isolation Technology. Available online: https://www.deepisolation.com/technology/ (accessed on 13 February 2019).
- King, F. Container materials for the storage and disposal of nuclear waste. Corrosion 2013, 69, 986–1011. [Google Scholar] [CrossRef]
- Payer, J.H.; Carroll, S.A.; Gdowski, G.E.; Rebak, R.B. A Framework for the Analysis of Localized Corrosion at the Proposed Yucca Mountain Repository. In Proceedings of the International High-Level Radioactive Waste Management Conference Proceedings, Las Vegas, NV, USA, 30 April–4 May 2006. [Google Scholar]
- Payer, J.H. Corrosion Resistance of Alloy 22. In Proceedings of the USA Nuclear Waste Technical Review Board Mtg, Washington, DC, USA, 18 May 2004; Available online: www.nwtrb.gov/meetings (accessed on 24 May 2004).
- Steefel, C. The In-Drift Chemical Environment During the Above Boiling Period. In Proceedings of the USA Nuclear Waste Technical Review Board Meeting, Washington, DC, USA, 18 May 2004; Available online: www.nwtrb.gov/meetings (accessed on 19 May 2004).
- Johnson, L.; King, F. The effect of the evolution of environmental conditions on the corrosion evolutionary path in a repository for spent fuel and high-level waste in Opalinus Clay. J. Nucl. Mater. 2008, 379, 9–15. [Google Scholar] [CrossRef]
- Payer, J.H.; Finsterle, S.; Apps, J.A.; Muller, R.A. Corrosion Resistant Alloy Canisters for Nuclear Waste Disposal in Horizontal Drillholes. In Proceedings of the International High-Level Radioactive Waste Management Conference, Knoxville, TN, USA, 14–18 April 2019. [Google Scholar]
- Department of Energy. Yucca Mountain Repository License Application; DOE/RW-0573, Rev. 0; US Department of Energy: Washington, DC, USA, 2008.
- Rebak, R.B.; Mccright, R.D. Corrosion of Containment Materials for Radioactive-Waste Isolation. In ASM Handbook; ASM International: Materials Park, OH, USA, 2006. [Google Scholar] [CrossRef]
- Rebak, R.B.; Payer, J.H. Passive corrosion behavior of alloy 22. In Proceedings of the 11th International High-Level Radioactive Waste Management Conference, Las Vegas, NV, USA, 30 April–4May 2006. [Google Scholar]
- Rebak, R.B. Corrosion testing of nickel and titanium alloys for nuclear waste disposition. Corrosion 2009, 65, 252–271. [Google Scholar] [CrossRef]
- Enos, D.G.; Bryan, C.R. The long-term corrosion performance of Alloy 22 in heated brine solutions. Corrosion 2015, 71, 758–770. [Google Scholar] [CrossRef]
- Dunn, D.S.; Brossia, C.S. Assessment of Passive and Localized Corrosion Processes for Alloy 22 High Level Nuclear Waste Container Materials. In CORROSION 2002; NACE International: Houston, TX, USA, 2002. [Google Scholar]
- Lloyd, A.C.; Shoesmith, D.W.; McIntyre, N.S.; Noel, J.J. Effects of temperature and potential on the passive corrosion properties of alloys C22 and C276. J. Electrochem. Soc. 2003, 150, B120–B130. [Google Scholar] [CrossRef]
- Rebak, R.B.; Crook, P. Influence of the environment on the general corrosion rate of alloy 22 (N06022). In Proceedings of the ASME/JSME 2004 Pressure Vessels and Piping Conference, San Diego, CA, USA, 25–29 July 2004; pp. 131–136. [Google Scholar]
- Smart, N.R. Corrosion behavior of carbon steel radioactive waste packages: A summary review of Swedish and U.K. Research. Corrosion 2009, 65, 195–212. [Google Scholar] [CrossRef]
- Smart, N.R.; Rance, A.P.; Nixon, D.J.; Fennell, P.A.H.; Reddy, B.; Kursten, B. Summary of studies on the anaerobic corrosion of carbon steel in alkaline media in support of the Belgian supercontainer concept. Corros. Eng. Sci. Technol. 2017. [Google Scholar] [CrossRef]
- Reardon, E.J. Anaerobic Corrosion of Granular Iron: Measurement and Interpretation of Hydrogen Evolution Rates. Environ. Sci. Technol. 1995. [Google Scholar] [CrossRef]
- Dinh, H.T.; Kuever, J.; Mußmann, M.; Hassel, A.W.; Stratmann, M.; Widdel, F. Iron corrosion under the enrichment culture of anaerobic microorganisms utilizing metallic iron as an electron donor. Corros. Eng. 2010. [Google Scholar] [CrossRef]
- Martin, F.A.; Bataillon, C.; Schlegel, M.L. Corrosion of iron and low alloyed steel within a water saturated brick of clay under anaerobic deep geological disposal conditions: An integrated experiment. J. Nucl. Mater. 2008. [Google Scholar] [CrossRef]
- Carlson, L.; Karnland, O.; Oversby, V.M.; Rance, A.P.; Smart, N.R.; Snellman, M.; Vähänen, M.; Werme, L.O. Experimental studies of the interactions between anaerobically corroding iron and bentonite. Phys. Chem. Earth 2007. [Google Scholar] [CrossRef]
- Rebak, R.B. Selection of corrosion resistant materials for nuclear waste repositories. Mater. Sci. Technol. 2006, 6, 639. [Google Scholar]
- Shoesmith, D.W. Assessing the corrosion performance of high-level nuclear waste containers. Corrosion 2006, 62, 703–722. [Google Scholar] [CrossRef]
- King, F.; Padovani, C. Review of the corrosion performance of selected canister materials for disposal of UK HLW and/or spent fuel. Corros. Eng. Sci. Technol. 2011, 46, 82–90. [Google Scholar] [CrossRef]
- King, F.; Kolar, M. Lifetime Predictions for Nuclear Waste Disposal Containers. Corrosion 2018, 75, 309–323. [Google Scholar] [CrossRef]
- Finsterle, S.; Muller, R.A.; Baltzer, R.; Payer, J.; Rector, J.W. Thermal Evolution near Heat-Generating Nuclear Waste Canisters Disposed in Horizontal Drillholes. Energies 2019, 12, 596. [Google Scholar] [CrossRef]
- Inconel Alloy 625. Available online: www.specialmetals.com (accessed on 10 December 2018).
- Smart, N.R.; Blackwood, D.J.; Werme, L. Anaerobic corrosion of carbon steel and cast iron in artificial groundwaters: Part 2-Gas generation. Corrosion 2002. [Google Scholar] [CrossRef]
- Frankel, G.S. Pitting Corrosion of Metals A Review of critical Factors. J. Electrochem. Soc. 1998. [Google Scholar] [CrossRef]
- Hornus, E.C.; Giordano, C.M.; Rodŕiguez, M.A.; Carranza, R.M. Effect of temperature on the crevice corrosion resistance of Ni-Cr-Mo alloys as engineered barriers in nuclear waste repositories. In Materials Research Society Symposium Proceedings; Materials Research Society: Cambridge, UK, 2012. [Google Scholar] [CrossRef]
- Carranza, R.M.; Rodríguez, M.A. Crevice corrosion of nickel-based alloys considered as engineering barriers of geological repositories. NPJ Mater. Degrad. 2017. [Google Scholar] [CrossRef]
- Finsterle, S.; Muller, R.A.; Baltzer, R.; Payer, J.; Rector, J.W. Numerical Evaluation of Thermal Effects From Nuclear Waste Disposed in Horizontal Drillholes. In Proceedings of the International High-Level Radioactive Waste Management, Knoxville, TN, USA, 14–18 April 2019. [Google Scholar]
- Orme, C.A. The Passive Film on Alloy 22. In Technical Report UCRL-TR-215277; Lawrence Livermore National Laboratory: Livermore, CA, USA, 2005. [Google Scholar] [Green Version]
- Priyantha, N.; Jayaweera, P.; Macdonald, D.D.; Sun, A. An electrochemical impedance study of Alloy 22 in NaCl brine at elevated temperature. I. Corrosion behavior. J. Electroanal. Chem. 2004, 572, 409–419. [Google Scholar] [CrossRef]
- Macdonald, D.D.; Sun, A.; Priyantha, N.; Jayaweera, P. An electrochemical impedance study of Alloy-22 in NaCl brine at elevated temperature: II. Reaction mechanism analysis. J. Electroanal. Chem. 2004, 572, 421–431. [Google Scholar] [CrossRef]
- Payer, J.; Pharkya, P. Robustness of Passive Films in High Temperature Brines. In Proceedings of the 2008 MRS Fall Meetin, Boston, MA, USA, 1–5 December 2008. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Jakupi, P.; Noel, J.J.; Shoesmith, D.W.; Noël, J.J.; Shoesmith, D.W. The Role of Alloying Elements on the Crevice Corrosion Behavior of Ni-Cr-Mo Alloys. Corrosion 2015, 71, 1441–1451. [Google Scholar] [CrossRef]
- Pharkya, P.; Shan, X.; Payer, J.H. Durability Of Passive Films In High Temperature Brines. In CORROSION 2008; NACE International: Houston, TX, USA, 2008. [Google Scholar]
- Rebak, R.B. Stifling of crevice corrosion in alloy 22 during constant potential tests. J. Press. Vessel Technol. 2009, 131, 14501. [Google Scholar] [CrossRef]

| Ni | Cr | Mo | Nb + Ta | Nb | Ta | Fe | |
|---|---|---|---|---|---|---|---|
| wt% | 58 | 23 | 10 | 0 | 2 | 2 | 5 |
| at.% | 59 | 27 | 6 | 0 | 1.3 | 0.7 | 5.4 |
| Corrosion Rate Alloy 625 µm/y | Weight Loss g/m2-Year | Moles Loss mol/m2-Year | Hydrogen Formed mol/m2-Year | Oxides Formed mol/m2-Year | Volume Oxides Formed cm3/m2-Year | Solids Expansion cm3/m2-Year | Thickness of Oxide After 100 Years mm |
|---|---|---|---|---|---|---|---|
| 0.01 | 0.084 | 0.001 | 0 | 0 | 0.04 | 0.03 | 0 |
| 0.1 | 0.84 | 0.01 | 0.02 | 0.03 | 0.4 | 0.3 | 0 |
| 1 | 8.4 | 0.1 | 0.2 | 0.25 | 4.3 | 3.3 | 0.4 |
| 10 | 84 | 1.4 | 1.7 | 2.5 | 43 | 33 | 4.3 |
| Corrosion Rate L80 Steel µm/y | Weight Loss g/m2-Year | Moles Loss mol/m2-Year | Hydrogen Formed mol/m2-Year | Oxides Formed mol/m2-Year | Volume Oxides Formed cm3/m2-Year | Solids Expansion cm3/m2-Year | 100 Years Expansion mm |
|---|---|---|---|---|---|---|---|
| 0.01 | 0.08 | 0.001 | 0 | 0 | 0.01 | 0.1 | 0 |
| 0.1 | 0.8 | 0.01 | 0.02 | 0.03 | 1.2 | 1.1 | 0.1 |
| 1 | 7.7 | 0.14 | 0.2 | 0.29 | 12 | 11 | 1.1 |
| 10 | 77 | 1.4 | 2 | 3 | 120 | 110 | 11 |
| 100 | 770 | 14 | 20 | 30 | 1200 | 1100 | 110 |
| Years After Emplacement | Environment | Corrosion Rate µm/Year | Metal Loss per Zone µm | Wall Thickness mm |
|---|---|---|---|---|
| 2 | Early transition | 2 | 4 | 9.5 |
| 20 | T > 120 °C | 2 | 36 | 9.5 |
| 100 | 80 < T < 120 °C | 1 | 80 | 9.3 |
| 1000 | T < 80 °C | 0.1 | 90 | 9.1 |
| 10,000 | T = 60 °C | 0.1 | 900 | 8 |
| Years After Emplacement | Environment | Corrosion Rate µm/y | Metal Loss per Side per Zone µm | Wall Thickness mm |
|---|---|---|---|---|
| 2 | Early transition | 20 | 40 | 12.4 |
| 20 | T > 120 °C | 4 | 72 | 12.3 |
| 100 | 80 < T < 120 °C | 2 | 160 | 12 |
| 1000 | T < 80 °C | 1 | 900 | 10 |
| 6000 | T = 60 °C | 1 | 5000 | 0.00 |
| Years After Emplacement | Environment | Hydrogen Formed per m2 mols/m2 | Hydrogen Formed per Canister mols | Hydrogen Formed per Year per Canister mols/year |
|---|---|---|---|---|
| 2 | Early transition | 0.7 | 0.16 | 0.08 |
| 20 | T > 120 °C | 6 | 1.4 | 0.08 |
| 100 | 80 < T < 120 °C | 13 | 3 | 0.04 |
| 1000 | T < 80 °C | 15 | 4 | 0.004 |
| 10,000 | T = 60 °C | 150 | 35 | 0.004 |
| Years After Emplacement | Environment | Moles Oxide Formed mols/m2 | Volume Oxide Formed cm3/m2 | Volume Oxide Expansion cm3/m2 | Volume Oxide Formed per Year cm3//m2-year |
|---|---|---|---|---|---|
| 2 | Early transition | 1 | 17 | 13 | 8.5 |
| 20 | T > 120 °C | 9 | 150 | 120 | 8.5 |
| 100 | 80 < T < 120 °C | 20 | 340 | 260 | 4.3 |
| 1000 | T < 80 °C | 22 | 385 | 300 | 0.4 |
| 10,000 | T = 60 °C | 220 | 3850 | 3000 | 0.4 |
| Years After Emplacement | Environment | Hydrogen Formed mols/m2 | Hydrogen Formed on Casing ID mols/m | Total Hydrogen Formed on Casing mols/m |
|---|---|---|---|---|
| 2 | Early Transition | 8 | 3 | 7 |
| 20 | T > 120 °C | 14 | 6 | 13 |
| 100 | 80 < T < 120 °C | 30 | 13 | 30 |
| 1000 | T < 80 °C | 170 | 75 | 165 |
| 6000 | T = 60 °C | 950 | 420 | 900 |
| Years After Emplacement | Environment | Moles of Oxide Formed mols/m | Volume of Oxide Formed cm3/m | Oxide Formed per Year cm3/m-year | Solids Expansion cm3/m |
|---|---|---|---|---|---|
| 2 | Early transition | 5 | 220 | 110 | 200 |
| 20 | T > 120 °C | 20 | 900 | 50 | 820 |
| 100 | 80 < T < 120 °C | 50 | 2000 | 25 | 1800 |
| 1000 | T < 80 °C | 260 | 11,000 | 12 | 10,000 |
| 6000 | T = 60 °C | 1500 | 62,000 | 12 | 56,000 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Payer, J.H.; Finsterle, S.; Apps, J.A.; Muller, R.A. Corrosion Performance of Engineered Barrier System in Deep Horizontal Drillholes. Energies 2019, 12, 1491. https://doi.org/10.3390/en12081491
Payer JH, Finsterle S, Apps JA, Muller RA. Corrosion Performance of Engineered Barrier System in Deep Horizontal Drillholes. Energies. 2019; 12(8):1491. https://doi.org/10.3390/en12081491
Chicago/Turabian StylePayer, Joe H., Stefan Finsterle, John A. Apps, and Richard A. Muller. 2019. "Corrosion Performance of Engineered Barrier System in Deep Horizontal Drillholes" Energies 12, no. 8: 1491. https://doi.org/10.3390/en12081491
APA StylePayer, J. H., Finsterle, S., Apps, J. A., & Muller, R. A. (2019). Corrosion Performance of Engineered Barrier System in Deep Horizontal Drillholes. Energies, 12(8), 1491. https://doi.org/10.3390/en12081491






