Explosion Characteristics of Propanol Isomer–Air Mixtures
Abstract
:1. Introduction
2. Experimental Setup and Chemicals
3. Data Evaluation
4. Results and Discussion
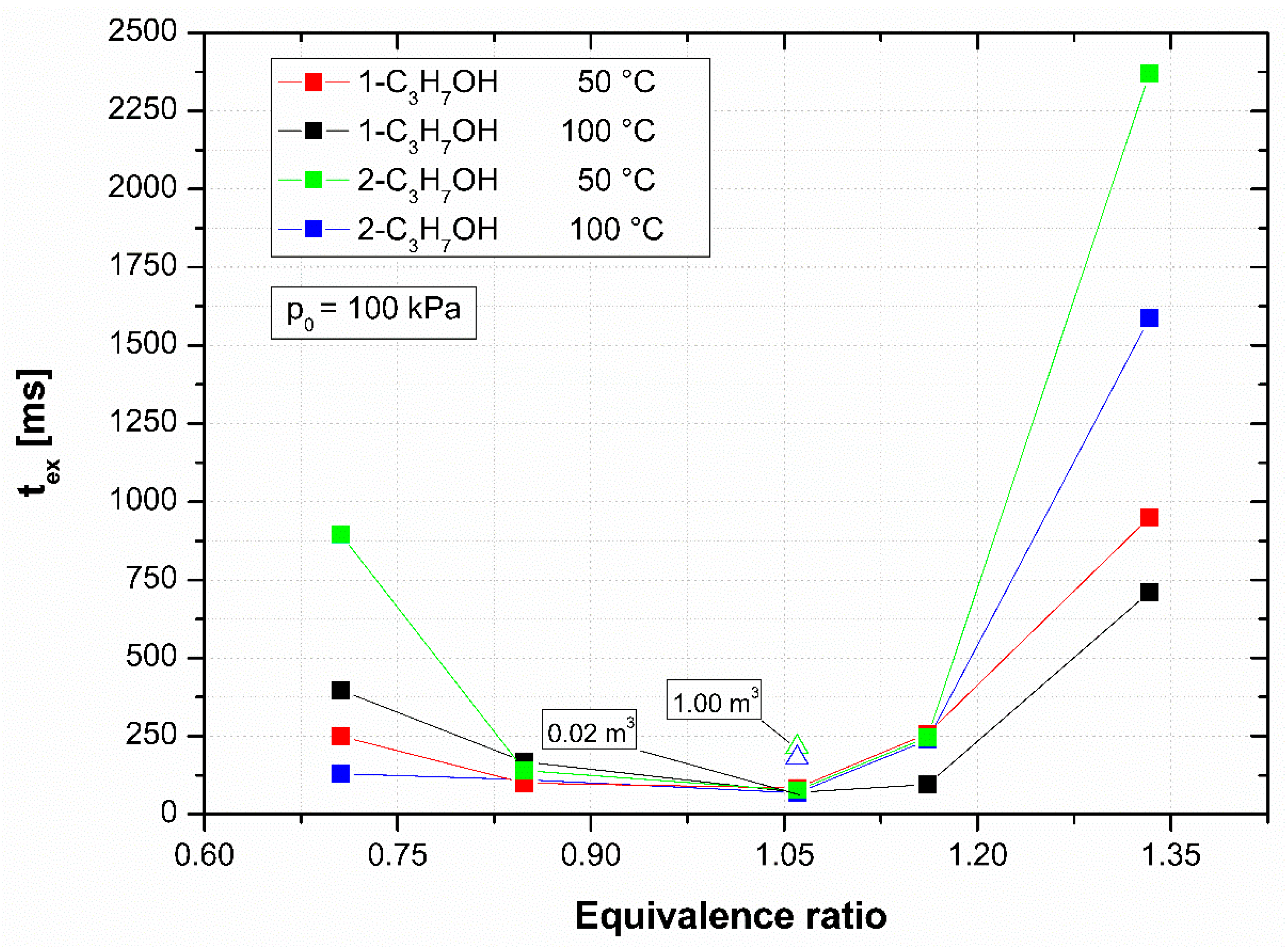
4.1. Influence of Temperature
4.2. Influence of Volume and Isomer Structure
5. Conclusions
- The propanol–air mixtures gave the maximum explosion pressures and the highest deflagration index values at the equivalence ratio of 1.06.
- Among propanol isomer–air mixtures, the maximum explosion pressure decreased in the order of 1-propanol–air, 2-propanol–air, and 1-propanol/2-propanol–air mixtures. The maximum explosion pressure decreased with increasing initial temperature and slightly decreased with increasing volume.
- The change of initial temperature only slightly influences the maximum rates of pressure rise and deflagration indexes (in both explosion vessel volumes). The maximum rate of pressure rise slightly decreased with an increasing initial temperature and slightly decreased with increasing volume.
- For 2-propanol–air mixture lower explosion limit values decreased with an increasing temperature from 3.5 ± 0.2% to 3.0 ± 0.2% for 0.02 m3 and from 2.5 ± 0.2% to 2.0 ± 0.2% for 1.00 m3. Upper explosion limit values increased with increasing temperature from 14.0 ± 0.2% to 14.5 ± 0.2% for 0.02 m3 and from 13.0 ± 0.2% to 13.5 ± 0.2% for 1.00 m3.
- For 1-propanol/2-propanol–air mixtures lower explosion limit values decreased with an increasing temperature from 3.5 ± 0.2% to 3.0 ± 0.2% for 0.02 m3 and from 3.5 ± 0.2% to 2.5 ± 0.2% for 1.00 m3. Upper explosion limit values increased with increasing temperature from 13.0 ± 0.2% to 13.5 ± 0.2% for 0.02 m3 and decreased with increasing temperature from 13.5 ± 0.2% to 13.0 ± 0.2% for 1.00 m3.
- The time to reach the maximum explosion pressure decreased with increasing initial temperature in the order of 1-propanol, 1-propanol + 2-propanol mixture, and 2-propanol. Moreover, it increased greatly with an increasing explosion vessel volume.
Author Contributions
Funding
Conflicts of Interest
References
- Chang, W.R.; Hwang, J.J.; Wu, W. Environmental impact and sustainability study on biofuels for transportation applications. Renew. Sustain. Energy Rev. 2017, 67, 277–288. [Google Scholar] [CrossRef]
- Kumar, B.R.; Muthukkumar, T.; Krishnamoorthy, V.; Saravanan, S. A comparative evaluation and optimization of performance and emission characteristics of a DI diesel engine fueled with n-propanol/diesel, n-butanol/diesel and n-pentanol/diesel blends using response surface methodology. RSC Adv. 2016, 6, 61869–61890. [Google Scholar] [CrossRef]
- Balogh, P.; Bai, A. Internet-orientated Hungarian car drivers’ knowledge and attitudes towards biofuels. Renew. Sustain. Energy Rev. 2015, 48, 17–26. [Google Scholar] [CrossRef] [Green Version]
- Saladini, F.; Patrizi, N.; Pulselli, F.N. Guidelines for emergy evaluation of first, second and third generation biofuels. Renew. Sustain. Energy Rev. 2016, 66, 221–227. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Oßwald, P.; Hansen, N.; Kohse-Höinghaus, K. Alcohol combustion chemistry. Prog. Energy Combust. Sci. 2014, 44, 40–102. [Google Scholar] [CrossRef]
- Skřínský, J.; Dolníček, P.; Skřínská, M.; Marek, J.; Lukešová, P. Flashpoint prediction for binary mixtures of alcohols with water in order to improve their safety. Chem. Eng. Technol. 2015, 38, 727–733. [Google Scholar] [CrossRef]
- Skřínský, J.; Vereš, J.; Borovec, K. Experimental Modelling of Autoignition Temperature for Alkyl/Alkenyl Products from Fischer-Tropsch Synthesis. MATEC Web Conf. 2018, 168, 07014. [Google Scholar] [CrossRef]
- Skřínský, J. Influence of Temperature and Vessel Volume on Explosion Characteristics of Propanol/Air Mixtures in Closed Spherical Vessels. Chem. Eng. Technol. 2018, 70, 1351–1355. [Google Scholar] [CrossRef]
- Skřínská, M.; Skřínský, J.; Dolníček, P.; Lukešová, P.; Přichystalová, R.; Serafínová, C. BLEVE—Cases, Causes, Consequences and Prevention. Mater. Sci. Forum 2015, 811, 91–94. [Google Scholar] [CrossRef]
- Abbasi, T.; Abbasi, S.A. The boiling liquid expanding vapour explosion (BLEVE): Mechanism, consequence assessment, management. J. Hazard. Mater. 2007, 141, 489–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, Z.; Wang, X.; Zheng, J.; Miao, H.; Wang, X. Combustion characteristics of methanol—Air and methanol–air–diluent premixed mixtures at elevated temperatures and pressures. Appl. Therm. Eng. 2009, 29, 2680–2688. [Google Scholar] [CrossRef]
- Mitu, M.; Brandes, E. Explosion parameters of methanol-air mixtures. Fuel 2015, 158, 217–223. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Y.; Huang, Z. Comparative assessment of the explosion characteristics of alcohol-air mixtures. J. Loss Prev. Proc. 2015, 37, 91–100. [Google Scholar] [CrossRef]
- Li, Q.; Cheng, Y.; Jin, W.; Huang, Z. Comparative study on the explosion characteristics of pentanol isomer-air mixtures. Fuel 2015, 161, 78–86. [Google Scholar] [CrossRef]
- Mitu, M.; Brandes, E. Influence of pressure, temperature and vessel volume on explosion characteristics of ethanol/air mixtures in closed spherical vessels. Fuel 2017, 203, 460–468. [Google Scholar] [CrossRef]
- Mitu, M.; Brandes, E.; Hirsch, W. Mitigation effects on the explosion safety characteristic data of ethanol/air mixtures in closed vessel. Process Saf. Environ. Prot. 2018, 117, 190–199. [Google Scholar] [CrossRef]
- EN 15967: Determination of Maximum Explosion Pressure and the Maximum Rate of Pressure Rise of Gases and Vapours; European Committee for Standardization: Brussels, Belgium, 2011.
- EN 1839: Determination of the Explosion Limits and the Limiting Oxygen Concentration (LOC) for Flammable Gases and Vapours; European Committee for Standardization: Brussels, Belgium, 2017.
- Chen, H.X.; Liu, N.A.; Shu, L.F.; Zong, R.W. Smoothing and differentiation of thermogravimetric data of biomass materials. J. Therm. Anal. Calorim. 2004, 78, 1025–1041. [Google Scholar] [CrossRef]
- Seifert, E. OriginPro 9.1: Scientific Data Analysis and Graphing Software—Software Review. J. Chem. Inf. Model. 2014, 54, 1552. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Tian, H.; Zhang, X.; Li, X.; Huang, Z. Explosion characteristics of n-butanol/iso-octane-air mixtures. Fuel 2017, 188, 90–97. [Google Scholar] [CrossRef]
- Glassman, I.; Yetter, R.A. Combustion, 4th ed.; Academic Press: Cambridge, MA, USA, 2008. [Google Scholar]
- NFPA 68. Guide for Venting of Deflagrations, 1st ed.; National Fire Protection Association: Quincy, MA, USA, 2000. [Google Scholar]
- IFA. GESTIS Substance Database—Information System on Hazardous Substances of the German Social Accident Insurances; Institut für Arbeitsschutz der Deutschen Gesetzlichen Unfallversicherung: Sankt Augustin, Germany, 2018. [Google Scholar]
- ILO. International Safety Chemical Cards Database. Available online: http://www.ilo.org/dyn/icsc/showcard.home (accessed on 15 February 2019).
- Merck Index, 14th ed.; Merck: Whitehouse Station, NJ, USA, 2007.
- PENTA s.r.o. Material Safety Data Sheets; Penta: Prague, Czech Republic, 2017; Available online: https://www.pentachemicals.eu/en/ (accessed on 15 February 2019).
- SFPE. Handbook of Fire Protection Engineering, 2nd ed.; Society of Fire Protection Engineers: Boston, MA, USA, 1995. [Google Scholar]
- AIChE. DIPPR Project 801—Full Version; Design Institute for Physical Property Research/AIChE: Fort Washington, PA, USA, 2015. [Google Scholar]
- Yaws, C.L. Chemical Properties Handbook: Physical, Thermodynamics, Environmental, Transport, Safety & Health Related Properties for Organic & Inorganic Chemicals, 1st ed.; McGraw-Hill: New York, NY, USA, 1999. [Google Scholar]










| Chemical | pmax (bar) | KG (bar·m/s) | Volume (m3) | Temperature (°C) | Equivalence Ratio | Reference |
|---|---|---|---|---|---|---|
| CH3OH | 7.1–8.2 | 105–107 | 0.005–0.020 | 50–100 | 1.00–1.26 | [11,12] |
| C2H5OH | 7.9–8.7 | 95–135 | 0.005–0.020 | 100–120 | 1.00–1.10 | [15,16] |
| 1-C3H7OH | 7.7–8.7 | 86–97 | 0.020–1.000 | 50–150 | 1.06 | [8] |
| 1-C4H9OH | 5.5–5.7 | 95–100 | 0.0053 1 | 120 | 1.00 | [13] |
| 1-C5H11OH | 5.6–5.7 | 90–95 | 0.0053 1 | 120–160 | 1.00 | [13,14] |
| Chemical | Formula | Purity (Mass %) | Company |
|---|---|---|---|
| 1-propanol | C3H7OH | >99.7 | Merck KGaA (Darmstadt, Germany) |
| 2-propanol | C3H7OH | >99.8 | Penta (Katowice, Poland) |
| 0.02 m3 | 1 m3 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2-Propanol | 1-Propanol/2-Propanol | 2-Propanol | 1-Propanol/2-Propanol | |||||||||
| T0 (°C) | pmax | KG | tex | pmax | KG | tex | pmax | KG | tex | pmax | KG | tex |
| 50 | 1.6% | 6.9% | 4.2% | 1.7% | 7.1% | 4.5% | 1.9% | 7.9% | 4.9% | 1.8% | 7.2% | 4.6% |
| 100 | 2.0% | 7.0% | 5.0% | 2.1% | 8.0% | 5.1% | 2.2% | 7.2% | 5.2% | 2.3% | 8.2% | 5.3% |
| 150 | 2.2% | 8.4% | 5.1% | 2.1% | 8.5% | 5.2% | 2.2% | 8.5% | 5.3% | 2.4% | 8.5% | 5.6% |
| Characteristic | Unit | 0.02 m3 | 1.00 m3 | ||||
|---|---|---|---|---|---|---|---|
| 50 °C | 100 °C | 150 °C | 50 °C | 100 °C | 150 °C | ||
| pmax/p0 | 8.7 ± 0.1 | 8.3 ± 0.2 | 7.4 ± 0.2 | 8.6 ± 0.2 | 8.1 ± 0.2 | 7.3 ± 0.2 | |
| (dp/dt)max | bar/s | 349 ± 24 | 338 ± 24 | 329 ± 28 | 361 ± 29 | 354 ± 26 | 336 ± 29 |
| KG | bar·m/s | 95 ± 7 | 92 ± 6 | 89 ± 7 | 98 ± 7 | 96 ± 7 | 91 ± 8 |
| LEL 1 | % | 3.5 ± 0.2 | 3.5 ± 0.2 | 3.0 ± 0.2 | 2.5 ± 0.2 | 2.0 ± 0.2 | 2.0 ± 0.2 |
| UEL 2 | % | 14.0 ± 0.2 | 14.0 ± 0.2 | 14.5 ± 0.2 | 13.0 ± 0.2 | 13.0 ± 0.2 | 13.5 ± 0.2 |
| tex | ms | 89 ± 4 | 87 ± 4 | 85 ± 4 | 215 ± 11 | 181 ± 9 | 176 ± 9 |
| Characteristic | Unit | 0.02 m3 | 1.00 m3 | ||||
|---|---|---|---|---|---|---|---|
| 50 °C | 100 °C | 150 °C | 50 °C | 100 °C | 150 °C | ||
| pmax/p0 | 8.2 ± 0.1 | 7.9 ± 0.2 | 7.6 ± 0.2 | 8.2 ± 0.2 | 7.9 ± 0.2 | 7.1 ± 0.2 | |
| (dp/dt)max | bar/s | 399 ± 28 | 393 ± 31 | 387 ± 33 | 402 ± 29 | 396 ± 33 | 382 ± 33 |
| KG | bar·m/s | 108 ± 8 | 107 ± 9 | 105 ± 9 | 109 ± 9 | 107 ± 9 | 104 ± 9 |
| LEL 1 | % | 3.5 ± 0.2 | 3.5 ± 0.2 | 3.0 ± 0.2 | 3.5 ± 0.2 | 3.0 ± 0.2 | 2.5 ± 0.2 |
| UEL 2 | % | 13.0 ± 0.2 | 13.0 ± 0.2 | 13.5 ± 0.2 | 13.5 ± 0.2 | 13.5 ± 0.2 | 13.0 ± 0.2 |
| tex | ms | 77 ± 4 | 75 ± 4 | 72 ± 4 | 166 ± 8 | 156 ± 8 | 142 ± 8 |
| Chemical | pmax (bar) | KG (bar·m/s) | Volume (m3) | Temperature (°C) | Equivalence Ratio | Reference |
|---|---|---|---|---|---|---|
| CH3OH | 7.1–8.2 | 105–107 | 0.005–0.020 | 50–100 | 1.00–1.26 | [11,12] |
| C2H5OH | 7.9–8.7 | 95–135 | 0.005–0.020 | 100–120 | 1.00–1.10 | [15,16] |
| 1-C3H7OH | 7.7–8.7 | 86–97 | 0.020–1.000 | 50–150 | 1.06 | [8] |
| 2-C3H7OH | 7.3–8.7 | 89–98 | 0.020–1.000 | 50–150 | 1.06 | Present |
| 1P + 2P 1 | 7.6–8.2 | 105–108 | 0.020–1.000 | 50–150 | 1.06 | Present |
| 1-C4H9OH | 5.5–5.7 | 95–100 | 0.0053 2 | 120 | 1.00 | [13] |
| 1-C5H11OH | 5.6–5.7 | 90–95 | 0.0053 2 | 120–160 | 1.00 | [13,14] |
| Chemical | IFA 1 [24] | ILO 2 [25] | Merck [26] | Penta [27] | SFPE 3 [28] | DIPPR 4 [29] | Yaws, C.L. [30] | Present |
|---|---|---|---|---|---|---|---|---|
| CH3OH | 6.0–50.0 | 5.5–44.0 | 5.5–44.0 | 6.0–36.0 | 6.7–36.5 | 7.2–36.5 | 6.0–36.0 | |
| C2H5OH | 9.0–44.0 | 3.3–19.0 | 3.1–27.7 | 3.5–15.0 | 3.3–19.0 | 3.3–19.0 | 4.3–19.0 | |
| 1-C3H7OH | 2.1–19.9 | 2.1–13.5 | 2.1–19.2 | 2.1–13.7 | 2.2–13.5 | 2.1–14.0 | 2.0–12.0 | |
| 2-C3H7OH | 2.0–13.4 | 2.0–12.7 | 2.0–13.4 | 2.0–12.7 | 2.0–11.8 | 2.0–12.7 | 2.0–12.7 | 2.0–14.0 |
| 1P + 2P 5 | 2.5–13.5 | |||||||
| 1-C4H9OH | 1.4–11.3 | 1.4–11.3 | 1.4–11.3 | 1.4–11.3 | 1.4–11.3 | 1.7–11.3 | 1.4–11.2 | |
| 1-C5H11OH | 1.3–10.5 | 1.2–10.5 | 1.6–8.0 | 1.2–10.0 | 1.2–10.0 | 1.4–10.0 | 1.2–10.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skřínský, J.; Ochodek, T. Explosion Characteristics of Propanol Isomer–Air Mixtures. Energies 2019, 12, 1574. https://doi.org/10.3390/en12081574
Skřínský J, Ochodek T. Explosion Characteristics of Propanol Isomer–Air Mixtures. Energies. 2019; 12(8):1574. https://doi.org/10.3390/en12081574
Chicago/Turabian StyleSkřínský, Jan, and Tadeáš Ochodek. 2019. "Explosion Characteristics of Propanol Isomer–Air Mixtures" Energies 12, no. 8: 1574. https://doi.org/10.3390/en12081574
APA StyleSkřínský, J., & Ochodek, T. (2019). Explosion Characteristics of Propanol Isomer–Air Mixtures. Energies, 12(8), 1574. https://doi.org/10.3390/en12081574





