Integration of Hydrothermal Carbonisation with Anaerobic Digestion; Opportunities for Valorisation of Digestate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source and Preparation of Materials
2.2. Hydrothermal Carbonisation
2.3. Feedstock and Hydrochar Analysis
2.3.1. Protein, Proximate, and Ultimate Analysis and Predictive Higher Heating Value (HHV)
2.3.2. Metal and Inorganics Analysis
2.3.3. Predictive Slagging and Fouling Indices
2.4. Process Water Analysis
2.5. Experimental Biochemical Methane Potential (BMPexp)
2.6. Assessment of Error and Statistical Analysis
3. Results
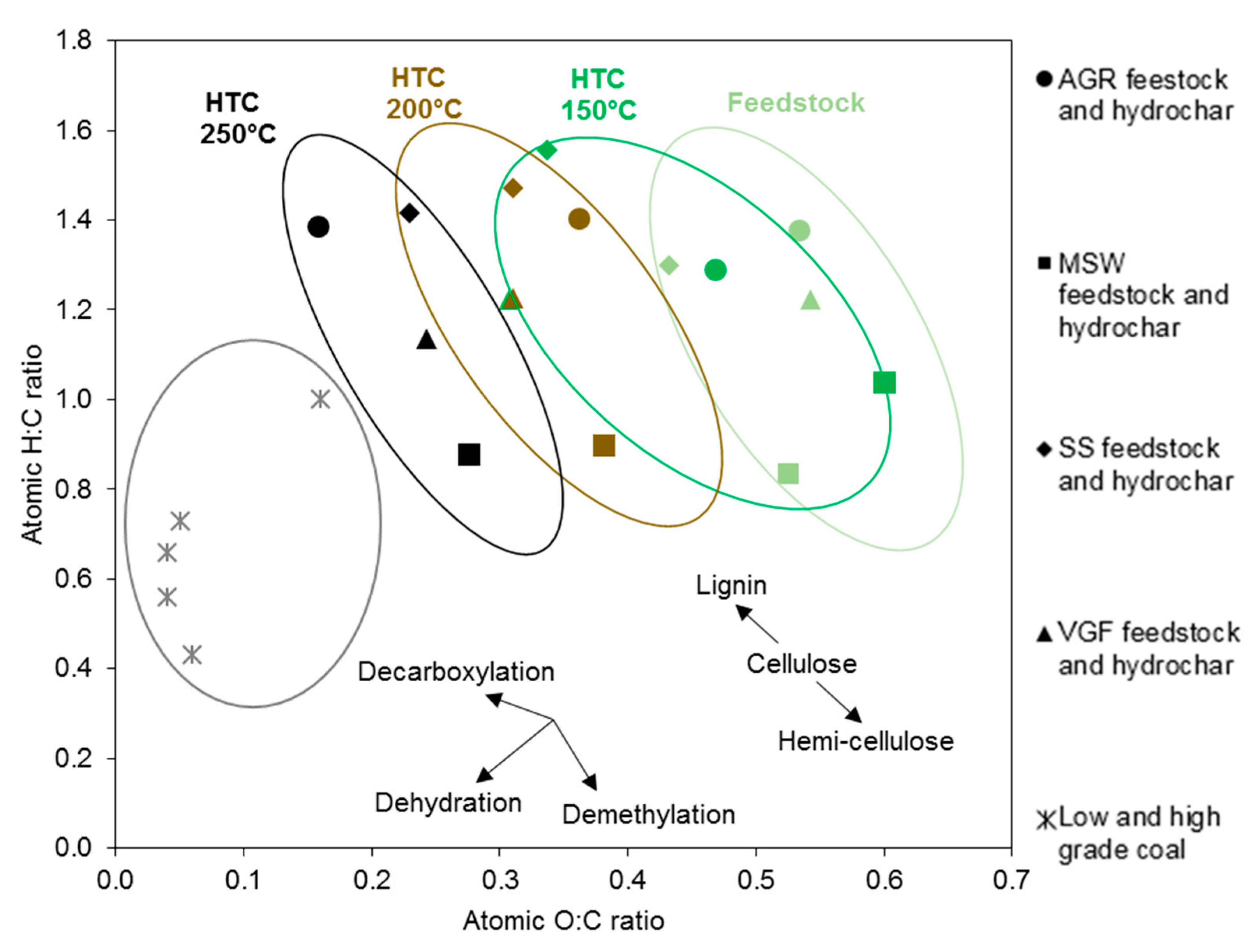
3.1. Feedstock Characterisation
3.2. Hydrothermal Carbonisation of Digestate
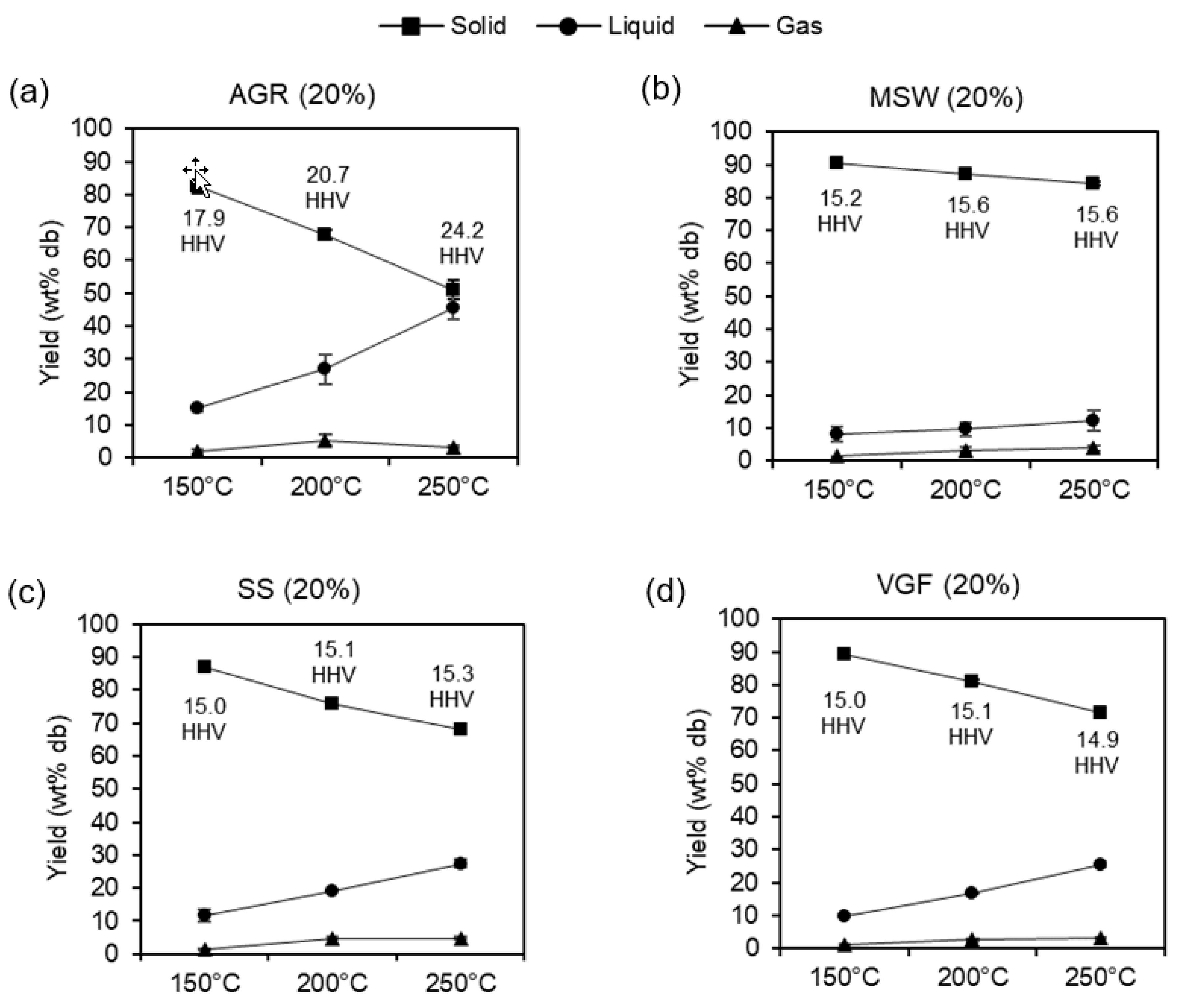
3.2.1. Effects of Temperature and Loading
3.2.2. Hydrochar Combustion Properties
3.3. Process Water Composition and Biomethane Yeild
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WRAP. Optimising the Value of Digestate and Digestion Systems; Waste & Resources Action Programme: Oxon, UK, 2015. [Google Scholar]
- WRAP. PAS 110: Specification for Whole Digestate, Separated Liquor and Separated Fibre Derived from the Anaerobic Digestion of Source-Segregated Biodegradable Materials; British Standards Institution: London, UK, 2014; p. 46. [Google Scholar]
- Monlau, F.; Francavilla, M.; Sambusiti, C.; Antoniou, N.; Solhy, A.; Libutti, A.; Zabaniotou, A.; Barakat, A.; Monteleone, M. Toward a functional integration of anaerobic digestion and pyrolysis for a sustainable resource management. Comparison between solid-digestate and its derived pyrochar as soil amendment. Appl. Energy 2016, 169, 652–662. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Strezov, V.; Kan, T. Product based evaluation of pyrolysis of food waste and its digestate. Energy 2015, 92, 349–354. [Google Scholar] [CrossRef]
- Wiśniewski, D.; Gołaszewski, J.; Białowiec, A. The pyrolysis and gasification of digestate from agricultural biogas plant. Arch. Environ. Prot. 2015, 41, 70–75. [Google Scholar] [CrossRef]
- Hung, C.Y.; Tsai, W.T.; Chen, J.W.; Lin, Y.Q.; Chang, Y.M. Characterization of biochar prepared from biogas digestate. Waste Manag. 2017, 66, 53–60. [Google Scholar] [CrossRef]
- Monlau, F.; Sambusiti, C.; Antoniou, N.; Barakat, A.; Zabaniotou, A. A new concept for enhancing energy recovery from agricultural residues by coupling anaerobic digestion and pyrolysis process. Appl. Energy 2015, 148, 32–38. [Google Scholar] [CrossRef]
- Opatokun, S.A.; Yousef, L.F.; Strezov, V. Agronomic assessment of pyrolysed food waste digestate for sandy soil management. J. Environ. Manag. 2017, 187, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Parker, W. A technical and economic evaluation of the pyrolysis of sewage sludge for the production of bio-oil. Bioresour. Technol. 2008, 99, 1409–1416. [Google Scholar] [CrossRef]
- Schouten, S.; van Groenigen, J.W.; Oenema, O.; Cayuela, M.L. Bioenergy from cattle manure? Implications of anaerobic digestion and subsequent pyrolysis for carbon and nitrogen dynamics in soil. GCB Bioenergy 2012, 4, 751–760. [Google Scholar]
- Monlau, F.; Sambusiti, C.; Ficara, E.; Aboulkas, A.; Barakat, A.; Carrère, H. New opportunities for agricultural digestate valorization: Current situation and perspectives. Energy Environ. Sci. 2015, 8, 2600–2621. [Google Scholar] [CrossRef]
- Rodriguez Correa, C.; Bernardo, M.; Ribeiro, R.P.P.L.; Esteves, I.A.A.C.; Kruse, A. Evaluation of hydrothermal carbonization as a preliminary step for the production of functional materials from biogas digestate. J. Anal. Appl. Pyrolysis 2017, 124, 461–474. [Google Scholar] [CrossRef]
- Wiedner, K.; Rumpel, C.; Steiner, C.; Pozzi, A.; Maas, R.; Glaser, B. Chemical evaluation of chars produced by thermochemical conversion (gasification, pyrolysis and hydrothermal carbonization) of agro-industrial biomass on a commercial scale. Biomass Bioenergy 2013, 59, 264–278. [Google Scholar] [CrossRef]
- Bridgwater, A.V.; Meier, D.; Radlein, D. An overview of fast pyrolysis of biomass. Org. Geochem. 1999, 30, 1479–1493. [Google Scholar] [CrossRef]
- Cottam, M.L.; Bridgwater, A.V. Techno-economic modelling of biomass flash pyrolysis and upgrading systems. Biomass Bioenergy 1994, 7, 267–273. [Google Scholar] [CrossRef]
- Jaroenkhasemmeesuk, C.; Tippayawong, N. Technical and Economic Analysis of A Biomass Pyrolysis Plant. Energy Procedia 2015, 79, 950–955. [Google Scholar] [CrossRef] [Green Version]
- Conesa, J.A.; Font, R.; Fullana, A.; Martín-Gullón, I.; Aracil, I.; Gálvez, A.; Moltó, J.; Gómez-Rico, M.F. Comparison between emissions from the pyrolysis and combustion of different wastes. J. Anal. Appl. Pyrolysis 2009, 84, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, D.; Westover, T.L.; Czernik, S.; Jablonski, W. Biomass feedstocks for renewable fuel production: A review of the impacts of feedstock and pretreatment on the yield and product distribution of fast pyrolysis bio-oils and vapors. Green Chem. 2014, 16, 384–406. [Google Scholar] [CrossRef]
- Gollakota, A.R.K.; Reddy, M.; Subramanyam, M.D.; Kishore, N. A review on the upgradation techniques of pyrolysis oil. Renew. Sustain. Energy Rev. 2016, 58, 1543–1568. [Google Scholar] [CrossRef]
- Williams, C.L.; Westover, T.L.; Emerson, R.M.; Tumuluru, J.S.; Li, C. Sources of Biomass Feedstock Variability and the Potential Impact on Biofuels Production. Bioenergy Res. 2016, 9, 1–14. [Google Scholar] [CrossRef]
- Neumann, J.; Binder, S.; Apfelbacher, A.; Gasson, J.R.; Ramírez García, P.; Hornung, A. Production and characterization of a new quality pyrolysis oil, char and syngas from digestate—Introducing the thermo-catalytic reforming process. J. Anal. Appl. Pyrolysis 2015, 113, 137–142. [Google Scholar] [CrossRef]
- Prado, G.H.C.; Rao, Y.; De Klerk, A. Nitrogen removal from oil: A review. Energy Fuels 2017, 31, 14–36. [Google Scholar] [CrossRef]
- Troy, S.M.; Nolan, T.; Leahy, J.J.; Lawlor, P.G.; Healy, M.G.; Kwapinski, W. Effect of sawdust addition and composting of feedstock on renewable energy and biochar production from pyrolysis of anaerobically digested pig manure. Biomass Bioenergy 2013, 49, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Pedroza, M.M.; Sousa, J.F.; Vieira, G.E.G.; Bezerra, M.B.D. Characterization of the products from the pyrolysis of sewage sludge in 1 kg/h rotating cylinder reactor. J. Anal. Appl. Pyrolysis 2014, 105, 108–115. [Google Scholar] [CrossRef]
- Sousa, A.A.T.C.; Figueiredo, C.C. Sewage sludge biochar: Effects on soil fertility and growth of radish. Biol. Agric. Hortic. 2016, 32, 127–138. [Google Scholar] [CrossRef]
- Weidemann, E.; Buss, W.; Edo, M.; Mašek, O.; Jansson, S. Influence of pyrolysis temperature and production unit on formation of selected PAHs, oxy-PAHs, N-PACs, PCDDs, and PCDFs in biochar—A screening study. Environ. Sci. Pollut. Res. 2018, 25, 3933–3940. [Google Scholar] [CrossRef]
- Busch, D.; Stark, A.; Kammann, C.I.; Glaser, B. Genotoxic and phytotoxic risk assessment of fresh and treated hydrochar from hydrothermal carbonization compared to biochar from pyrolysis. Ecotoxicol. Environ. Saf. 2013, 97, 59–66. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A comparative review of biochar and hydrochar in terms of production, physico-chemical properties and applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Judex, J.W.; Gaiffi, M.; Burgbacher, H.C. Gasification of dried sewage sludge: Status of the demonstration and the pilot plant. Waste Manag. 2012, 32, 719–723. [Google Scholar] [CrossRef]
- Pecchi, M.; Baratieri, M. Coupling anaerobic digestion with gasification, pyrolysis or hydrothermal carbonization: A review. Renew. Sustain. Energy Rev. 2019, 105, 462–475. [Google Scholar] [CrossRef]
- Antoniou, N.; Monlau, F.; Sambusiti, C.; Ficara, E.; Barakat, A.; Zabaniotou, A. Contribution to Circular Economy options of mixed agricultural wastes management: Coupling anaerobic digestion with gasification for enhanced energy and material recovery. J. Clean. Prod. 2019, 209, 505–514. [Google Scholar] [CrossRef]
- Rollinson, A.N. Gasification reactor engineering approach to understanding the formation of biochar properties. Proc. R. Soc. A Math. Phys. Eng. Sci. 2016, 472, 20150841. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Han, Z.; Liu, X.; Li, J.; Liu, F.; Feng, S. Study on the effect and mechanism of hydrothermal pretreatment of dewatered sewage sludge cake for dewaterability. J. Air Waste Manag. Assoc. 2013, 63, 997–1002. [Google Scholar] [CrossRef] [Green Version]
- Escala, M.; Zumbühl, T.; Koller, C.; Junge, R.; Krebs, R. Hydrothermal carbonization as an energy-efficient alternative to established drying technologies for sewage sludge: A feasibility study on a laboratory scale. Energy Fuels 2013, 27, 454–460. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Zhang, G.; Li, J.; Li, Z.; Yu, G.; Wang, Y. A process combining hydrothermal pretreatment, anaerobic digestion and pyrolysis for sewage sludge dewatering and co-production of biogas and biochar: Pilot-scale verification. Bioresour. Technol. 2018, 254, 187–193. [Google Scholar] [CrossRef]
- Reza, M.T.; Andert, J.; Wirth, B.; Busch, D.; Pielert, J.; Lynam, J.G.; Mumme, J. Hydrothermal Carbonization of Biomass for Energy and Crop Production. Appl. Bioenergy 2014, 1, 11–29. [Google Scholar] [CrossRef]
- Lynam, J.G.; Reza, M.T.; Yan, W.; Vásquez, V.R.; Coronella, C.J. Hydrothermal carbonization of various lignocellulosic biomass. Biomass Convers. Biorefinery 2015, 5, 173–181. [Google Scholar] [CrossRef]
- Reza, M.T.; Lynam, J.G.; Uddin, M.H.; Coronella, C.J. Hydrothermal carbonization: Fate of inorganics. Biomass Bioenergy 2013, 49, 86–94. [Google Scholar] [CrossRef]
- Berge, N.D.; Ro, K.S.; Mao, J.; Flora, J.R.V.; Chappell, M.A.; Bae, S. Hydrothermal carbonization of municipal waste streams. Environ. Sci. Technol. 2011, 45, 5696–5703. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Balasubramanian, R. Production of solid biochar fuel from waste biomass by hydrothermal carbonization. Fuel 2013, 103, 943–949. [Google Scholar] [CrossRef]
- Smith, A.M.; Ross, A.B. The Influence of Residence Time during Hydrothermal Carbonisation of Miscanthus on Bio-Coal Combustion Chemistry. Energies 2019, 12, 523. [Google Scholar] [CrossRef]
- Smith, A.M.; Singh, S.; Ross, A.B. Fate of inorganic material during hydrothermal carbonisation of biomass: Influence of feedstock on combustion behaviour of hydrochar. Fuel 2016, 169, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Biller, P.; Ross, A.B. Production of biofuels via hydrothermal conversion. In Handbook of Biofuels Production: Processes and Technologies, 2nd ed.; Woodhead Publishing: Duxford, UK, 2016; ISBN 9780081004562. [Google Scholar]
- Gai, C.; Chen, M.; Liu, T.; Peng, N.; Liu, Z. Gasification characteristics of hydrochar and pyrochar derived from sewage sludge. Energy 2016, 113, 957–965. [Google Scholar] [CrossRef] [Green Version]
- Wilk, M.; Magdziarz, A.; Jayaraman, K.; Szymańska-Chargot, M.; Gökalp, I. Hydrothermal carbonization characteristics of sewage sludge and lignocellulosic biomass. A comparative study. Biomass Bioenergy 2019, 120, 166–175. [Google Scholar] [CrossRef]
- Libra, J.A.; Ro, K.S.; Kammann, C.; Funke, A.; Berge, N.D.; Neubauer, Y.; Titirici, M.-M.; Fühner, C.; Bens, O.; Kern, J.; et al. Hydrothermal carbonization of biomass residuals: A comparative review of the chemistry, processes and applications of wet and dry pyrolysis. Biofuels 2011, 2, 71–106. [Google Scholar] [CrossRef]
- Yu, Y.; Lou, X.; Wu, H. Some recent advances in hydrolysis of biomass in hot-compressed water and its comparisons with other hydrolysis methods. Energy Fuels 2008, 22, 46–60. [Google Scholar] [CrossRef]
- Razavi, A.S.; Hosseini Koupaie, E.; Azizi, A.; Hafez, H.; Elbeshbishy, E. Hydrothermal pretreatment of source separated organics for enhanced solubilization and biomethane recovery. Bioresour. Technol. 2019, 274, 502–511. [Google Scholar] [CrossRef]
- Posmanik, R.; Labatut, R.A.; Kim, A.H.; Usack, J.G.; Tester, J.W.; Angenent, L.T. Coupling hydrothermal liquefaction and anaerobic digestion for energy valorization from model biomass feedstocks. Bioresour. Technol. 2017, 233, 134–143. [Google Scholar] [CrossRef]
- Kim, D.; Lee, K.; Park, K.Y. Enhancement of biogas production from anaerobic digestion of waste activated sludge by hydrothermal pre-treatment. Int. Biodeterior. Biodegrad. 2015, 101, 42–46. [Google Scholar] [CrossRef]
- Wirth, B.; Reza, T.; Mumme, J. Influence of digestion temperature and organic loading rate on the continuous anaerobic treatment of process liquor from hydrothermal carbonization of sewage sludge. Bioresour. Technol. 2015, 198, 215–222. [Google Scholar] [CrossRef]
- Aragón-Briceño, C.; Ross, A.B.; Camargo-Valero, M.A. Evaluation and comparison of product yields and bio-methane potential in sewage digestate following hydrothermal treatment. Appl. Energy 2017, 208, 1357–1369. [Google Scholar] [CrossRef]
- Fakkaew, K.; Koottatep, T.; Polprasert, C. Faecal sludge treatment and utilization by hydrothermal carbonization. J. Environ. Manag. 2018, 216, 421–426. [Google Scholar] [CrossRef]
- Merzari, F.; Langone, M.; Andreottola, G.; Fiori, L. Methane production from process water of sewage sludge hydrothermal carbonization. A review. Valorising sludge through hydrothermal carbonization. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1–42. [Google Scholar] [CrossRef]
- De la Rubia, M.A.; Villamil, J.A.; Rodriguez, J.J.; Borja, R.; Mohedano, A.F. Mesophilic anaerobic co-digestion of the organic fraction of municipal solid waste with the liquid fraction from hydrothermal carbonization of sewage sludge. Waste Manag. 2018, 76, 315–322. [Google Scholar] [CrossRef]
- Villamil, J.A.; Mohedano, A.F.; Rodriguez, J.J.; De la Rubia, M.A. Anaerobic co-digestion of the aqueous phase from hydrothermally treated waste activated sludge with primary sewage sludge. A kinetic study. J. Environ. Manag. 2019, 231, 726–733. [Google Scholar] [CrossRef]
- De la Rubia, M.A.; Villamil, J.A.; Rodriguez, J.J.; Mohedano, A.F. Effect of inoculum source and initial concentration on the anaerobic digestion of the liquid fraction from hydrothermal carbonisation of sewage sludge. Renew. Energy 2018, 127, 697–704. [Google Scholar] [CrossRef]
- Reza, M.T.; Werner, M.; Pohl, M.; Mumme, J. Evaluation of Integrated Anaerobic Digestion and Hydrothermal Carbonization for Bioenergy Production. J. Vis. Exp. 2014, 88, e51734. [Google Scholar] [CrossRef]
- Zhao, K.; Li, Y.; Zhou, Y.; Guo, W.; Jiang, H.; Xu, Q. Characterization of hydrothermal carbonization products (hydrochars and spent liquor) and their biomethane production performance. Bioresour. Technol. 2018, 267, 9–16. [Google Scholar] [CrossRef]
- Qiao, W.; Yan, X.; Ye, J.; Sun, Y.; Wang, W.; Zhang, Z. Evaluation of biogas production from different biomass wastes with/without hydrothermal pretreatment. Renew. Energy 2011, 36, 3313–3318. [Google Scholar] [CrossRef]
- Friedl, A.; Padouvas, E.; Rotter, H.; Varmuza, K. Prediction of heating values of biomass fuel from elemental composition. Anal. Chim. Acta 2005, 544, 191–198. [Google Scholar] [CrossRef]
- Xing, P.; Mason, P.E.; Chilton, S.; Lloyd, S.; Jones, J.M.; Williams, A.; Nimmo, W.; Pourkashanian, M. A comparative assessment of biomass ash preparation methods using X-ray fluorescence and wet chemical analysis. Fuel 2016, 182, 161–165. [Google Scholar] [CrossRef] [Green Version]
- Holliger, C.; Alves, M.; Andrade, D.; Angelidaki, I.; Astals, S.; Baier, U.; Bougrier, C.; Buffière, P.; Carballa, M.; De Wilde, V.; et al. Towards a standardization of biomethane potential tests. Water Sci. Technol. 2016, 74, 2515–2522. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.P.; Zhang, C.; Li, X.; Yu, S.H.; Tan, P.; Fang, Q.Y.; Chen, G. A two-step process for sewage sludge treatment: Hydrothermal treatment of sludge and catalytic hydrothermal gasification of its derived liquid. Fuel Process. Technol. 2018, 180, 67–74. [Google Scholar] [CrossRef]
- Danso-Boateng, E.; Holdich, R.G.; Wheatley, A.D.; Martin, S.J.; Shama, G. Hydrothermal carbonization of primary sewage sludge and synthetic faeces: Effect of reaction temperature and time on filterability. Environ. Prog. Sustain. Energy 2015, 34, 1279–1290. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Gupta, R.; Lee, Y.Y.; Gupta, R.B. Cellulose pretreatment in subcritical water: Effect of temperature on molecular structure and enzymatic reactivity. Bioresour. Technol. 2010, 101, 1337–1347. [Google Scholar] [CrossRef]
- Ravber, M. Hydrothermal Degradation of Fats, Carbohydrates and Proteins in Sunflower Seeds after Treatment with Subcritical Water. Chem. Biochem. Eng. Q. 2016, 29, 351–355. [Google Scholar] [CrossRef]
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefin. 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Kang, S.; Li, X.; Fan, J.; Chang, J. Characterization of hydrochars produced by hydrothermal carbonization of lignin, cellulose, d-xylose, and wood meal. Ind. Eng. Chem. Res. 2012, 51, 9023–9031. [Google Scholar] [CrossRef]
- Hills, D.J. Effects of carbon: Nitrogen ratio on anaerobic digestion of dairy manure. Agric. Wastes 1979, 1, 267–278. [Google Scholar] [CrossRef]
- Stanton, W.R. Anaerobic digestion: Principles and practice for biogas systems. Biol. Wastes 2003, 1, 8–16. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Wang, J.; Meng, L. Effects of volatile fatty acid concentrations on methane yield and methanogenic bacteria. Biomass Bioenergy 2009, 33, 848–853. [Google Scholar] [CrossRef]
- Ghanim, B.M.; Kwapinski, W.; Leahy, J.J. Hydrothermal carbonisation of poultry litter: Effects of initial pH on yields and chemical properties of hydrochars. Bioresour. Technol. 2017, 238, 78–85. [Google Scholar] [CrossRef]


| Slagging and Fouling Indices | Equation | Interpretation |
|---|---|---|
| Alkali index | AI < 0.17 safe combustion | |
| AI > 0.17 < 0.34 likely slagging and fouling | ||
| AI > 0.34 almost certain slagging and fouling | ||
| Bed agglomeration index | BAI < 0.15 bed agglomeration likely | |
| Acid base ratio | < 0.5 low slagging risk | |
| Slagging index | SI < 0.6 low slagging inclination | |
| SI > 0.6 < 2.0 medium slagging inclination | ||
| SI > 2.0 high slagging inclination | ||
| Fouling index | FI < 0.6 low fouling | |
| FI > 0.6 < 40.0 medium fouling | ||
| FI > 40.0 indicate high fouling | ||
| Slag viscosity index | SVI > 72 low slagging inclination | |
| SVI > 63 < 72 medium slagging inclination | ||
| SVI < 65 high slagging inclination |
| AGR | MSW | SS | VGF | |
|---|---|---|---|---|
| % C (db) | 44.1 ± 0.1 | 24.1 ± 0.0 | 28.6 ± 0.3 | 29.5 ± 0.1 |
| % H (db) | 5.1 ± 0.0 | 1.7 ± 0.0 | 3.1 ± 0.1 | 3.0 ± 0.1 |
| % N (db) | 3.2 ± 0.0 | 1.5 ± 0.0 | 3.4 ± 0.0 | 2.0 ± 0.0 |
| % S (db) | 0.3 ± 0.0 | 0.2 ± 0.0 | 1.5 ± 0.0 | 0.3 ± 0.0 |
| % O a (db) | 31.3 ± 0.3 | 16.9 ± 0.0 | 16.4 ± 0.3 | 21.3 ± 0.6 |
| H/C (daf) | 1.38 | 0.83 | 1.3 | 1.22 |
| O/C (daf) | 0.53 | 0.53 | 0.43 | 0.54 |
| % Protein (db) | 17.7 | 6.8 | 24.3 | 9.8 |
| % VM (db) | 70.2 ± 0.3 | 36.2 ± 0.1 | 51.0 ± 0.1 | 47.2 ± 1.1 |
| % FC (db) | 13.8 ± 0.0 | 8.3 ± 0.0 | 2.1 ± 0.1 | 9.0 ± 0.2 |
| % Ash a (db) | 16.0 ± 0.3 | 55.5 ± 0.1 | 46.9 ± 0.0 | 43.8 ± 0.8 |
| HHV (MJ/kg db) b | 17.8 | 15.6 | 14.9 | 14.9 |
| % Na (db) | 0.3 | 0.9 | 0.5 | 0.5 |
| % Mg (db) | 0.7 | 1.4 | 0.9 | 0.8 |
| % P (db) | 1.2 | 0.7 | 2.7 | 2.6 |
| % K (db) | 1.6 | 1.6 | 0.7 | 0.7 |
| % Ca (db) | 1.3 | 10.4 | 4.6 | 4.3 |
| % Fe (db) | 2.3 | 3.2 | 3.3 | 3.0 |
| % Si (db) | 1.8 | 10.2 | 7.6 | 7.1 |
| Material | Ultimate Analysis (wt% db) | Proximate Analysis (wt% db) | HHV (MJ/kg, db) | H/C (daf) | O/C (daf) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | O a | VM | FC | Ash a | ||||
| AGR digestate | 44.1 ± 0.1 | 5.1 ± 0.0 | 3.2 ± 0.0 | 0.3 ± 0.0 | 31.3 ± 0.3 | 70.2 ± 0.3 | 13.8 ± 0.0 | 16.0 ± 0.3 | 17.8 | 1.38 | 0.53 |
| 150 °C at 20% | 44.2 ± 1.9 | 4.8 ± 0.3 | 3.1 ± 0.1 | 0.0 ± 0.0 | 27.5 ± 0.5 | 62.2 ± 3.2 | 17.5 ± 0.6 | 20.4 ± 2.7 | 17.9 | 1.29 | 0.47 |
| 200 °C at 20% | 50.8 ± 0.8 | 6.0 ± 0.7 | 3.3 ± 0.5 | 0.1 ± 0.1 | 24.4 ± 3.3 | 67.4 ± 0.5 | 17.3 ± 1.9 | 15.3 ± 1.4 | 20.7 | 1.40 | 0.36 |
| 250 °C at 20% | 57.1 ± 1.4 | 6.6 ± 1.5 | 3.9 ± 0.3 | 0.2 ± 0.0 | 12.0 ± 3.1 | 58.2 ± 0.0 | 21.6 ± 0.1 | 20.2 ± 0.1 | 24.2 | 1.39 | 0.16 |
| MSW digestate | 24.1 ± 0.0 | 1.7 ± 0.0 | 1.5 ± 0.0 | 0.2 ± 0.0 | 16.9 ± 0.0 | 36.2 ± 0.1 | 8.3 ± 0.0 | 55.5 ± 0.1 | 15.6 | 0.83 | 0.53 |
| 150 °C at 20% | 23.8 ± 1.7 | 2.1 ± 0.3 | 1.2 ± 0.1 | 0.0 ± 0.0 | 19.0 ± 4.5 | 39.4 ± 6.8 | 6.6 ± 0.5 | 54.0 ± 6.3 | 15.2 | 1.04 | 0.60 |
| 200 °C at 20% | 21.4 ± 0.2 | 1.6 ± 0.0 | 0.9 ± 0.0 | 0.1 ± 0.0 | 13.9 ± 0.1 | 32.3 ± 0.8 | 5.6 ± 0.7 | 62.1 ± 0.1 | 15.6 | 0.87 | 0.49 |
| 250 °C at 20% | 21.7 ± 1.5 | 1.6 ± 0.1 | 0.8 ± 0.0 | 0.0 ± 0.0 | 7.9 ± 0.3 | 25.7 ± 3.1 | 6.3 ± 1.2 | 68.0 ± 2.0 | 15.6 | 0.88 | 0.28 |
| SS digestate | 28.6 ± 0.3 | 3.1 ± 0.1 | 3.4 ± 0.0 | 1.5 ± 0.0 | 16.4 ± 0.3 | 51.0 ± 0.1 | 2.1 ± 0.1 | 46.9 ± 0.0 | 14.9 | 1.30 | 0.43 |
| 150 °C at 20% | 33.4 ± 1.8 | 4.4 ± 0.6 | 3.2 ± 0.2 | 0.3 ± 0.1 | 15.0 ± 2.3 | 50.5 ± 4.7 | 5.7 ± 0.2 | 43.8 ± 4.9 | 15.0 | 1.56 | 0.34 |
| 200 °C at 20% | 34.0 ± 1.1 | 4.2 ± 0.2 | 2.4 ± 0.1 | 0.9 ± 0.1 | 14.0 ± 0.6 | 49.1 ± 0.9 | 6.5 ± 0.2 | 44.4 ± 0.7 | 15.1 | 1.47 | 0.31 |
| 250 °C at 20% | 34.7 ± 0.4 | 4.1 ± 0.0 | 2.4 ± 0.1 | 0.7 ± 0.3 | 10.6 ± 0.6 | 45.9 ± 0.5 | 6.6 ± 0.2 | 47.5 ± 0.3 | 15.3 | 1.42 | 0.23 |
| VGF digestate | 29.5 ± 0.1 | 3.0 ± 0.1 | 2.0 ± 0.0 | 0.3 ± 0.0 | 21.3 ± 0.6 | 47.2 ± 1.1 | 9.0 ± 0.2 | 43.8 ± 0.8 | 14.9 | 1.22 | 0.54 |
| 150 °C at 20% | 29.7 ± 1.8 | 3.0 ± 0.1 | 1.9 ± 0.1 | 0.0 ± 0.0 | 12.2 ± 1.2 | 37.6 ± 0.7 | 9.3 ± 0.0 | 53.1 ± 0.7 | 15.0 | 1.22 | 0.31 |
| 200 °C at 20% | 32.2 ± 1.9 | 3.3 ± 0.2 | 1.5 ± 0.1 | 0.3 ± 0.0 | 13.3 ± 0.0 | 41.7 ± 1.5 | 9.0 ± 0.7 | 49.4 ± 2.3 | 15.1 | 1.23 | 0.31 |
| 250 °C at 20% | 27.8 ± 0.7 | 2.7 ± 0.0 | 1.4 ± 0.0 | 0.1 ± 0.1 | 9.0 ± 1.6 | 31.7 ± 1.7 | 9.2 ± 0.7 | 59.0 ± 2.4 | 14.9 | 1.14 | 0.24 |
| Material | Slagging and Fouling Indices | |||||
|---|---|---|---|---|---|---|
| AI | BAI | R b/a | SI | FI | SVI | |
| AGR digestate | 1.25 | 1.46 | 2.1 | 0.6 | 29.2 | 38 |
| 150 °C at 20% | 1.54 | 0.29 | 1.7 | 0.1 | 32.2 | 49 |
| 200 °C at 20% | 1.18 | 0.35 | 1.3 | 0.2 | 20.9 | 54 |
| 250 °C at 20% | 0.59 | 0.95 | 1.0 | 0.2 | 7.3 | 53 |
| MSW digestate | 2.05 | 1.44 | 0.9 | 0.2 | 5.3 | 50 |
| 150 °C at 20% | 1.21 | 2.80 | 1.5 | 0.0 | 5.2 | 35 |
| 200 °C at 20% | 1.31 | 2.70 | 1.0 | 0.1 | 3.4 | 45 |
| 250 °C at 20% | 1.06 | 3.88 | 0.5 | 0.0 | 1.2 | 61 |
| SS digestate | 1.04 | 3.00 | 0.6 | 1.0 | 2.1 | 56 |
| 150 °C at 20% | 0.90 | 2.62 | 0.4 | 0.1 | 1.3 | 65 |
| 200 °C at 20% | 0.75 | 3.43 | 0.4 | 0.4 | 1.1 | 64 |
| 250 °C at 20% | 0.95 | 2.81 | 0.5 | 0.3 | 1.4 | 63 |
| VGF digestate | 1.55 | 0.85 | 0.4 | 0.1 | 2.2 | 73 |
| 150 °C at 20% | 1.41 | 1.13 | 0.5 | 0.0 | 2.0 | 67 |
| 200 °C at 20% | 0.86 | 1.73 | 0.4 | 0.1 | 1.1 | 71 |
| 250 °C at 20% | 0.89 | 1.96 | 0.4 | 0.1 | 0.8 | 73 |
| Process Waters | pH | TN (g/L) | TOC (g/L) | C:N Ratio | COD (g/L) | Total VFA (g/L) | Total Phenols (g/L) | BMPexp (NmlCH4/gCOD) |
|---|---|---|---|---|---|---|---|---|
| AGR digestate | ||||||||
| 150 °C at 20% | 6.7 | 3.1 | 13.9 | 4.5 | 45.5 | 1.8 | 1.4 | 100.0 |
| 200 °C at 20% | 6.2 | 1.9 | 14.9 | 8.0 | 42.2 | 2.1 | 1.6 | 180.7 |
| 250 °C at 20% | 6.1 | 2.2 | 16.5 | 7.4 | 46.3 | 4.2 | 0.8 | 155.5 |
| MSW digestate | ||||||||
| 150 °C at 20% | 6.5 | 1.1 | 3.4 | 3.1 | 22.6 | 0.7 | 0.3 | 84.6 |
| 200 °C at 20% | 7.1 | 2.4 | 5.7 | 2.4 | 18.1 | 0.9 | 0.4 | 137.7 |
| 250 °C at 20% | 7.8 | 1.7 | 6.0 | 3.6 | 16.4 | 1.3 | 0.6 | 134.6 |
| SS digestate | ||||||||
| 150 °C at 20% | 5.6 | 2.4 | 16.6 | 6.9 | 31.0 | 1.1 | 0.2 | 100.2 |
| 200 °C at 20% | 6.2 | 4.5 | 17.1 | 3.8 | 38.9 | 1.8 | 0.9 | 181.7 |
| 250 °C at 20% | 7.6 | 4.7 | 18.4 | 3.9 | 43.6 | 5.3 | 0.8 | 151.9 |
| VGF digestate | ||||||||
| 150 °C at 20% | 6.5 | 1.0 | 5.7 | 5.9 | 11.9 | 0.9 | 0.3 | 121.4 |
| 200 °C at 20% | 5.7 | 1.4 | 8.5 | 6.3 | 22.5 | 1.1 | 0.9 | 158.6 |
| 250 °C at 20% | 6.4 | 1.5 | 9.8 | 6.5 | 27.8 | 2.2 | 1.2 | 125.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parmar, K.R.; Ross, A.B. Integration of Hydrothermal Carbonisation with Anaerobic Digestion; Opportunities for Valorisation of Digestate. Energies 2019, 12, 1586. https://doi.org/10.3390/en12091586
Parmar KR, Ross AB. Integration of Hydrothermal Carbonisation with Anaerobic Digestion; Opportunities for Valorisation of Digestate. Energies. 2019; 12(9):1586. https://doi.org/10.3390/en12091586
Chicago/Turabian StyleParmar, Kiran R., and Andrew B. Ross. 2019. "Integration of Hydrothermal Carbonisation with Anaerobic Digestion; Opportunities for Valorisation of Digestate" Energies 12, no. 9: 1586. https://doi.org/10.3390/en12091586





