SiNW/C@Pt Arrays for High-Efficiency Counter Electrodes in Dye-Sensitized Solar Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of SiNWs
2.2. Fabrication of SiNW/C@Pt
2.3. Fabrication of DSSCs
2.4. Characterization and Measurements
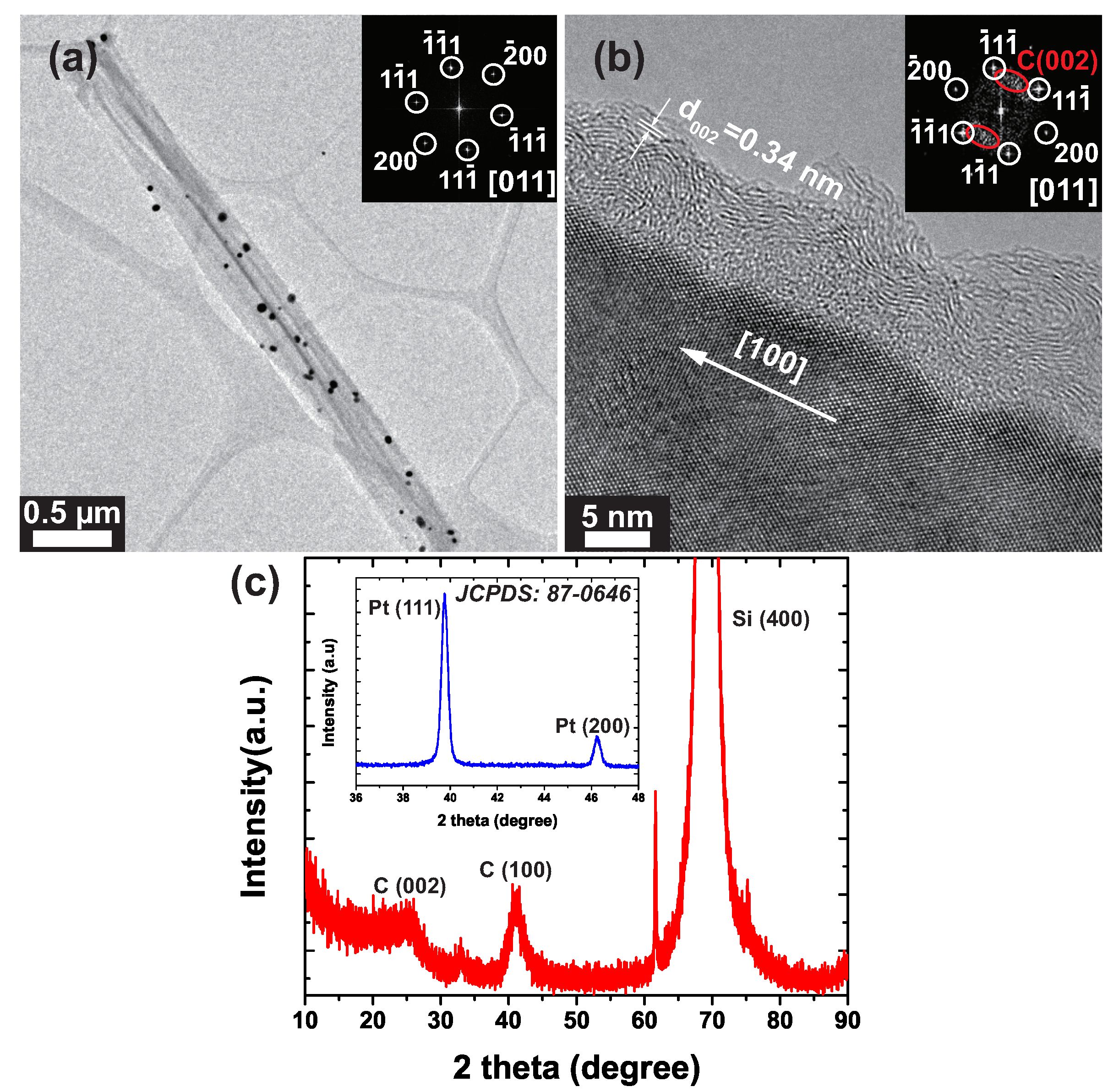
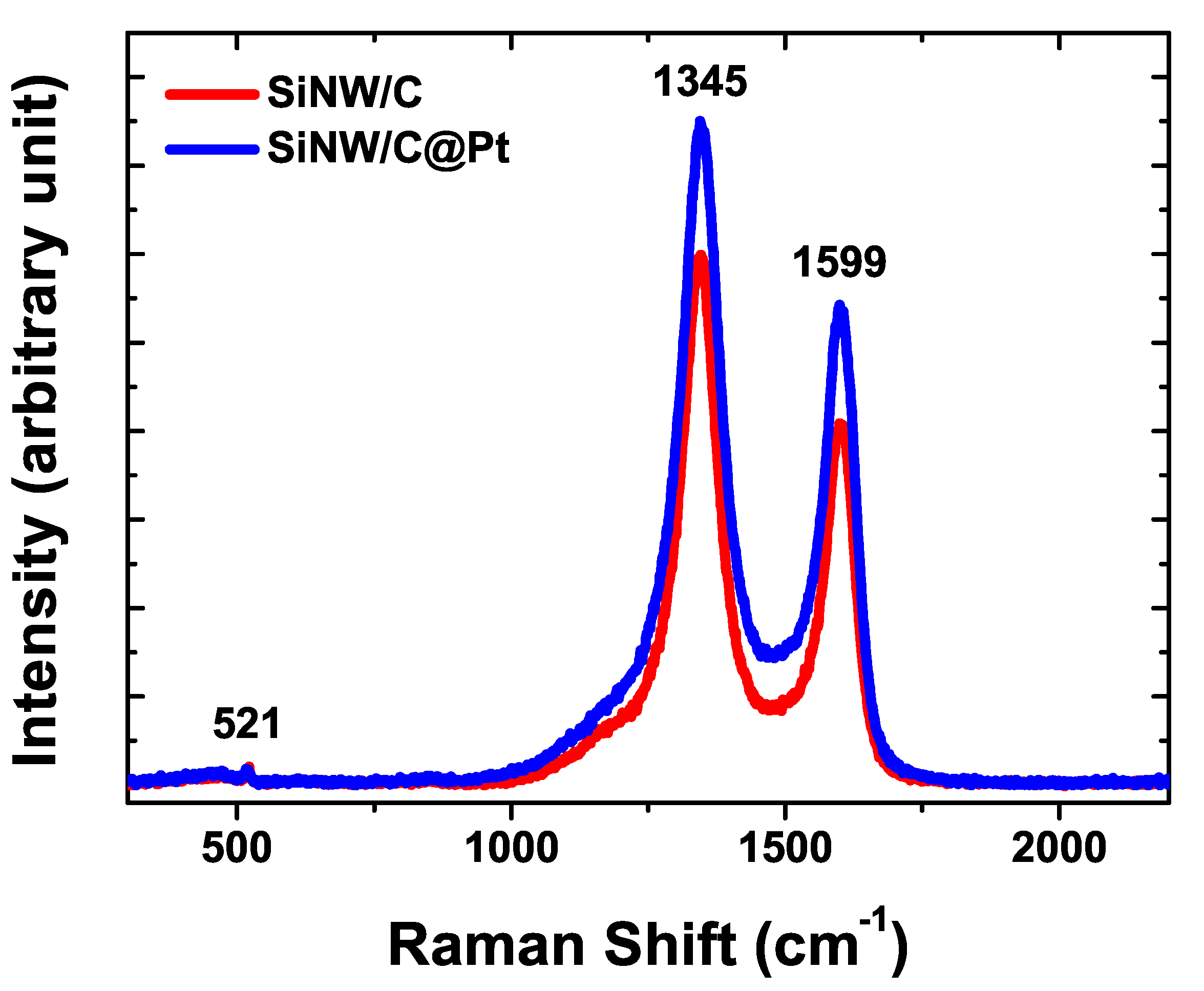
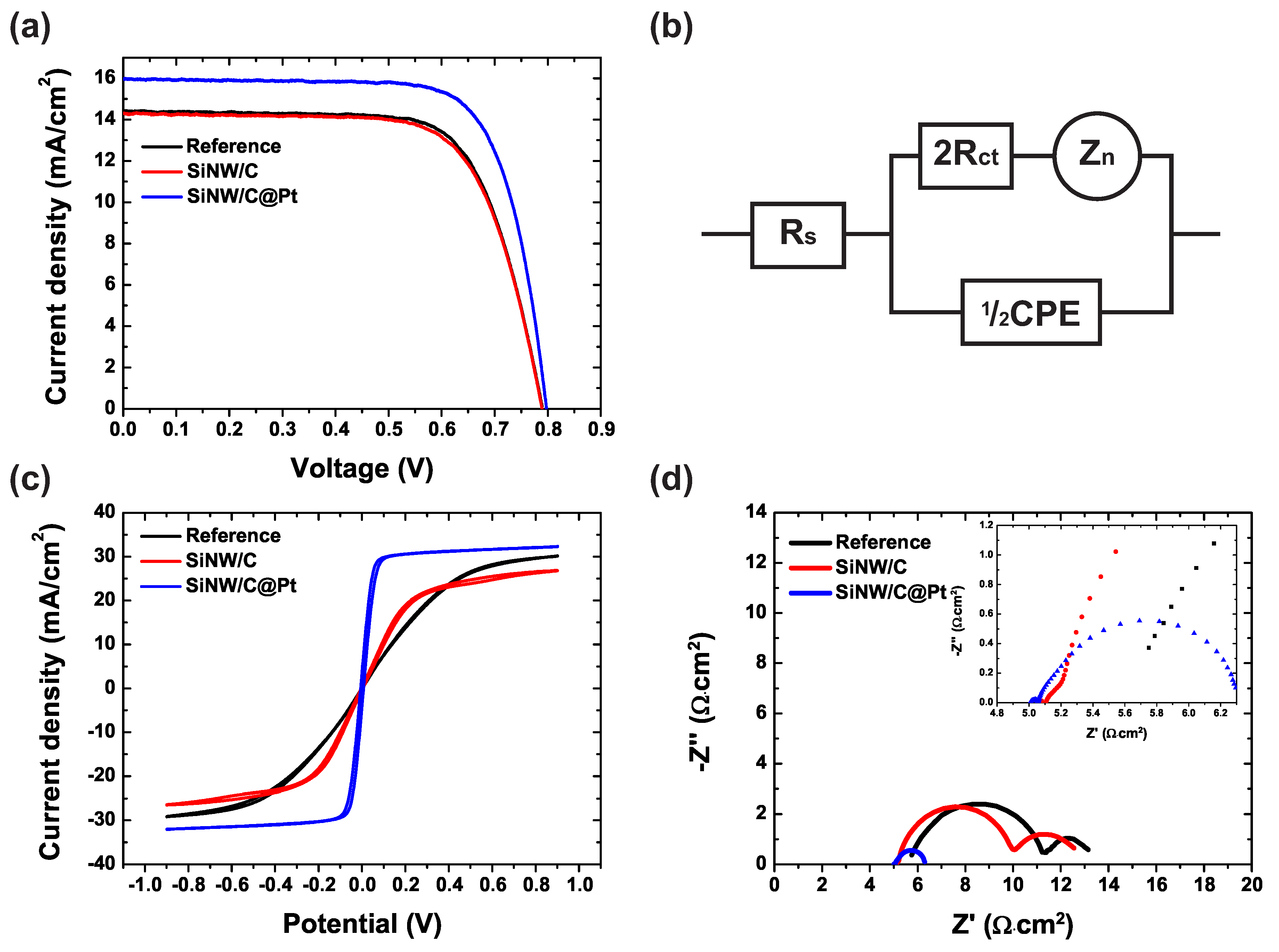
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DSSCs | Dye-sensitized solar cells |
| CEs | Counter electrodes |
| ORR | Oxygen reduction reaction |
| SiNWs | Silicon nanowire arrays |
| MaCE | Metal-assisted chemical etching |
| NPs | Nanoparticles |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| EDX | Energy-dispersive X-ray fluorescence spectroscopy |
| XPS | X-ray Photoelectron spectroscopy |
| XRD | X-ray diffraction |
| EIS | Electrochemical impedance spectroscopy |
| CV | Cyclic voltammogram |
| FTO | Fluorine-doped tin oxide |
References
- Xia, X.; Tu, J.; Zhang, Y.; Wang, X.; Gu, C.; Zhao, X.B.; Fan, H.J. High-Quality Metal Oxide Core/Shell Nanowire Arrays on Conductive Substrates for Electrochemical Energy Storage. ACS Nano 2012, 6, 5531–5538. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.F.; Chen, X.Y.; Zhou, W.; Tong, Y.X.; Li, G.R. α-Fe2O3@PANI Core–Shell Nanowire Arrays as Negative Electrodes for Asymmetric Supercapacitors. ACS Appl. Mater. Interfaces 2015, 7, 14843–14850. [Google Scholar]
- Wang, C.; Wu, L.; Wang, H.; Zuo, W.; Li, Y.; Liu, J. Fabrication and Shell Optimization of Synergistic TiO2-MoO3 Core–Shell Nanowire Array Anode for High Energy and Power Density Lithium-Ion Batteries. Adv. Func. Mater. 2015, 25, 3524–3533. [Google Scholar] [CrossRef]
- Chen, S.; Yang, G.; Jia, Y.; Zheng, H. Three-dimensional NiCo2O4@NiWO4 core–shell nanowire arrays for high performance supercapacitors. J. Mater. Chem. A 2017, 5, 1028–1034. [Google Scholar] [CrossRef]
- Chen, S.; Xie, Y.; De, A.; Berg, A.; Carlen, E.T. Electrical shielding for siliconnanowire biosensor in microchannels. Appl. Phys. Lett. 2013, 103, 173702. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Chua, J.-H.; Chee, R.E.; Agarwal, A.; Wong, S.-M. Label-free direct detection of MiRNAs with silicon nanowire biosensors. Biosens. Bioelectron. 2009, 24, 2504–2508. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y.; Rajan, N.K.; Routenberg, D.A.; Modis, Y.; Reed, M.A. Quantification of the affinities and kinetics of protein interactions using silicon nanowire biosensors. Nat. Nanotechnol. 2012, 7, 401–407. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Zhu, G.; Hu, Y.; Yu, J.-W.; Song, J.; Cheng, K.-Y.; Peng, L.-H.; Chou, L.-J.; Wang, Z.L. Gallium Nitride Nanowire BasedNanogenerators and Light-Emitting Diodes. ACS Nano 2012, 6, 5687–5692. [Google Scholar] [CrossRef]
- Zimmler, M.A.; Voss, T.; Ronning, C.; Capasso, F. Exciton-related electroluminescence from ZnO nanowire light-emitting diodes. Appl. Phys. Lett. 2009, 94, 241120. [Google Scholar] [CrossRef]
- Ahn, Y.; Jeong, Y.; Lee, D.; Lee, Y. Copper Nanowire–Graphene Core–Shell Nanostructure for Highly Stable Transparent Conducting Electrodes. ACS Nano 2015, 9, 3125–3133. [Google Scholar] [CrossRef]
- Fang, X.; Li, Y.; Wang, X.; Ding, J.; Yuan, N. Ultrathin interdigitated back-contacted silicon solar cell with light-trapping structures of Si nanowire arrays. Sol. Energy 2015, 116, 100–107. [Google Scholar] [CrossRef]
- Kim, H.T.; Lee, K.; Jin, W.; Um, H.D.; Lee, M.; Hwang, E.; Kwon, T.H.; Seo, K. Phosphorescent Energy Downshifting for Diminishing Surface Recombination in Silicon Nanowire Solar Cells. Sci. Rep. 2018, 8, 16974–16982. [Google Scholar] [CrossRef] [PubMed]
- Karvounis, A.; Gholipour, B.; MacDonald, K.F.; Zheludev, N.I. Giant Electro-Optical Effect through Electrostriction in a Nanomechanical Metalmaterial. Adv. Mater. 2019, 31, 1804801. [Google Scholar] [CrossRef] [PubMed]
- Karvounis, A.; Aspiotis, N.; Zeimpekis, I.; Ou, J.-Y.; Huang, C.-C.; Hewak, D.; Zheludev, N.I. Mechanochromic Reconfigurable Metasurfaces. Adv. Sci. 2019, 6, 1900974. [Google Scholar] [CrossRef]
- Yao, Z.; Xia, X.; Xie, D.; Wang, Y.; Zhou, C.; Liu, S.; Deng, S.; Wang, X.; Tu, J. Enhancing Ultrafast Lithium Ion Storage of Li4Ti5O12 by Tailored TiC/C Core/Shell Skeleton Plus Nitrogen Doping. Adv. Func. Mater. 2018, 28, 1802756. [Google Scholar] [CrossRef]
- Bai, L.; Cai, X.; Lu, J.; Li, L.; Zhong, S.; Wu, L.; Gong, P.; Chen, J.; Bai, S. Surface and Interface Engineering in Ag2S@MoS2 Core–Shell Nanowire Heterojunctions for Enhanced Visible Photocatalytic Hydrogen Production. ChemCatChem 2018, 10, 2107–2114. [Google Scholar] [CrossRef]
- Shen, A.; Zou, Y.; Wang, Q.; Dryfe, R.A.W.; Huang, X.; Dou, S.; Dai, L.; Wang, S. Oxygen Reduction Reaction in a Droplet on Graphite: Direct Evidence that the Edge Is More Active than the Basal Plane. Angew. Chem. Int. Ed. 2014, 53, 10804–10808. [Google Scholar] [CrossRef]
- Tao, L.; Wang, Q.; Dou, S.; Ma, Z.; Huo, J.; Wang, S.; Dai, L. Edge-rich and dopant-free graphene as a highly efficient metal-free electrocatalyst for the oxygen reduction reaction. Chem. Commun. 2016, 52, 2764–2767. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, L.; Sun, T.; Zhao, J.; Lyu, Z.; Zhuo, O.; Wang, X.; Wu, Q.; Ma, J.; Hu, Z. Significant Contribution of Intrinsic Carbon Defects to Oxygen Reduction Activity. ACS Catal. 2015, 5, 6707–6712. [Google Scholar] [CrossRef]
- Chen, Z.; Wu, R.; Liu, Y.; Guo, Y.; Sun, D.; Liu, M.; Fang, F. Ultrafine Co Nanoparticles Encapsulated in Carbon-Nanotubes-Grafted Graphene Sheets as Advanced Electrocatalysts for the Hydrogen Evolution Reaction. Adv. Mater. 2018, 30, 1802011. [Google Scholar] [CrossRef]
- Wan, T.-H.; Chiu, Y.-F.; Chen, C.-W.; Hsu, C.-C.; Cheng, I.-C.; Chen, J.-Z. Atmospheric-Pressure Plasma Jet Processed Pt-Decorated Reduced Graphene Oxides for Counter-Electrodes of Dye-Sensitized Solar Cells. Coatings 2016, 6, 44. [Google Scholar] [CrossRef]
- Wang, C.; Ciganda, R.; Yate, L.; Tuninetti, J.; Shalabaeva, V.; Salmon, L.; Moya, S.; Ruiz, J.; Astruc, D. Redox synthesis and high catalytic efficiency of transition-metal nanoparticle–graphene oxide nanocomposites. J. Mater. Chem. A 2017, 5, 21947–21954. [Google Scholar] [CrossRef]
- Govindhan, M.; Chen, A. Simultaneous synthesis of gold nanoparticle/graphene nanocomposite for enhanced oxygen reduction reaction. J. Power Sources 2015, 274, 928–936. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, Y.; Lin, Y.; Li, Y.; Liu, Y.; Liu, C. Nickel phosphide nanoparticles-nitrogen-doped graphene hybrid as an efficient catalyst for enhanced hydrogen evolution activity. J. Power Sources 2015, 297, 45–52. [Google Scholar] [CrossRef]
- Song, F.-Z.; Zhu, Q.-L.; Yang, X.; Zhan, W.-W.; Pachfule, P.; Tsumori, N.; Xu, Q. Metal-Organic Framework Templated Porous Carbon-Metal Oxide/Reduced Graphene Oxide as Superior Support of Bimetallic Nanoparticles for Efficient Hydrogen Generation from Formic Acid. Adv. Energy Mater. 2018, 8, 170146. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Wu, C.-S.; Chou, C.-J.; Yen, T.-J. Morphological Control of Single-Crystalline Silicon Nanowire Arrays near Room Temperature. Adv. Mater. 2008, 20, 3811–3815. [Google Scholar] [CrossRef]
- Zhang, M.-L.; Peng, K.-Q.; Fan, X.; Jie, J.-S.; Zhang, R.-Q.; Lee, S.-T.; Wong, N.-B. Preparation of Large-Area Uniform Silicon Nanowires Arrays through Metal-Assisted Chemical Etching. J. Phys. Chem. C 2008, 112, 4444–4450. [Google Scholar] [CrossRef]
- Togonal, A.S.; He, L.; Roca, I.C.; Rusli. Effect of Wettability on the Agglomeration of Silicon Nanowire Arrays Fabricated by Metal-Assisted Chemical Etching. Langmuir 2014, 30, 10290–10298. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095. [Google Scholar] [CrossRef]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1291. [Google Scholar] [CrossRef]
- Cho, S.H.; Yang, H.N.; Lee, D.C.; Park, S.H.; Kim, W.J. Electrochemical properties of Pt/graphene intercalated by carbon black and its application in polymer electrolyte membrane fuel cell. J. Power Sources 2013, 225, 200–206. [Google Scholar] [CrossRef]


| Counter | J | V | FF | R | R | |
|---|---|---|---|---|---|---|
| Electrode | (mA/cm) | (V) | (%) | (%) | ( · cm) | ( · cm) |
| Reference | 14.41 ± 0.2 | 0.789 ± 0.002 | 71.06 ± 0.3 | 8.08 ± 0.17 | 5.75 | 2.725 |
| SiNW/C | 14.30 ± 0.2 | 0.789 ± 0.004 | 70.19 ± 0.5 | 7.92 ± 0.21 | 5.10 | 2.448 |
| SiNW/C@Pt | 15.95 ± 0.1 | 0.797 ± 0.002 | 74.41 ± 0.4 | 9.46 ± 0.13 | 5.01 | 0.025 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Jung, S.; Kim, H.-J.; Kim, Y.; Lee, C.; Kim, S.M.; Kim, D.; Jun, Y. SiNW/C@Pt Arrays for High-Efficiency Counter Electrodes in Dye-Sensitized Solar Cells. Energies 2020, 13, 139. https://doi.org/10.3390/en13010139
Kim J, Jung S, Kim H-J, Kim Y, Lee C, Kim SM, Kim D, Jun Y. SiNW/C@Pt Arrays for High-Efficiency Counter Electrodes in Dye-Sensitized Solar Cells. Energies. 2020; 13(1):139. https://doi.org/10.3390/en13010139
Chicago/Turabian StyleKim, Junhee, Sanghoon Jung, Han-Jung Kim, Yoonkap Kim, Chanyong Lee, Soo Min Kim, Donghwan Kim, and Yongseok Jun. 2020. "SiNW/C@Pt Arrays for High-Efficiency Counter Electrodes in Dye-Sensitized Solar Cells" Energies 13, no. 1: 139. https://doi.org/10.3390/en13010139
APA StyleKim, J., Jung, S., Kim, H.-J., Kim, Y., Lee, C., Kim, S. M., Kim, D., & Jun, Y. (2020). SiNW/C@Pt Arrays for High-Efficiency Counter Electrodes in Dye-Sensitized Solar Cells. Energies, 13(1), 139. https://doi.org/10.3390/en13010139








