Potential Sites for Underground Energy and CO2 Storage in Greece: A Geological and Petrological Approach
Abstract
1. Introduction
2. Analytical Techniques
3. Geological Background and Petrological Investigation of Rock Types Considered for Storage
3.1. Evaporite Formations
3.2. Sedimentary Basins
3.3. Pleistocene Alkaline Basalt Occurrences
4. Types of Energy and CO2 Storage Considered
4.1. Underground Thermal Energy Storage (UTES)
4.1.1. Thermal Capacity Properties of Aquifers
4.1.2. UTES Heat Capacity Properties
4.2. Underground Gas Storage Potential
4.3. Hydrogen Storage Potential
4.4. Recommended CO2 Storage Sites
5. Conclusions
- Thermal energy capacity was examined in 22 sandstone aquifers throughout several regions of Northern, Central Greece and Aegean islands. Research results indicate that the aquifers of Mesohellenic Trough, Western Thessaloniki basin and those of Botsara flysch exhibit the highest thermal capacity values, which can reach up to 4175 MJ.
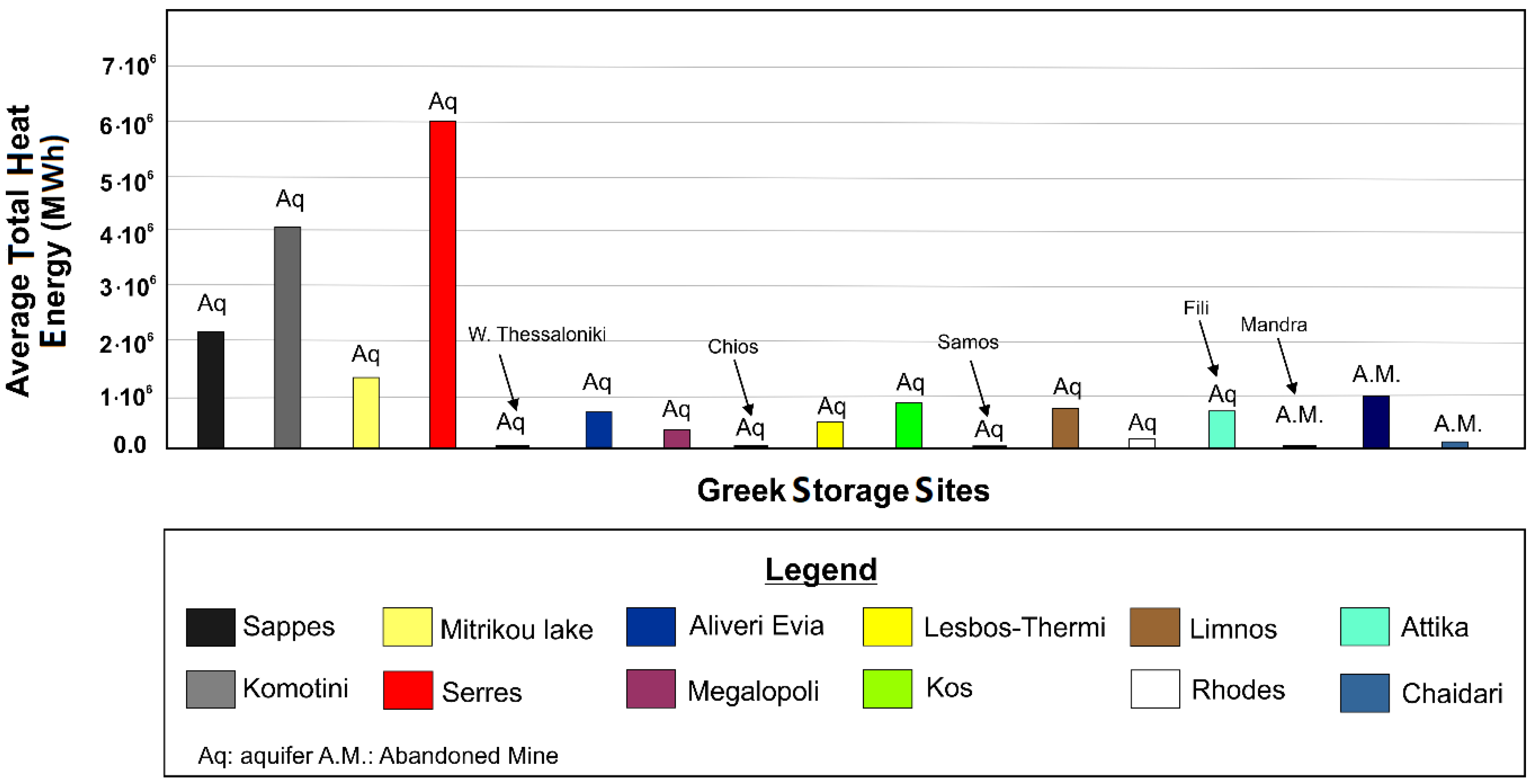
- Heat capacity was investigated in 14 aquifers throughout the Northern and Southern Greece, as well as on three abandoned mines from Attica region and Aliveri regions (Central Greece). In general, the aquifers tend to present higher average total heat energy values that reach up to ~6.05 × 106 MWh(th) compared to those of the abandoned mines. In particular, the Sappes, Serres and Komotini (Agioi Theodoroi) aquifers could cover the space heating energy consumption of East Macedonia-Thrace region.
- Underground gas storage technologies present better performance in the gas fields of South Kavala and Prinos basins compare to the studied evaporite formations and aquifers. These two gas fields could cover the electricity needs of the households in the region of East Macedonia and Thrace.
- Hydrogen storage capacity was based on a capsule-shaped cavern scenario in Trifos and Achira regions (Central-Western Greece), as well as in Kefalonia and Corfu islands. Calculations indicate storage capacity of 26,600 MWh(e) for each of the studied regions. These calculated hydrogen capacity values for Corfu and Kefalonia islands, could efficiently cover the electricity needs of 6770 households in the Ionian Sea region.
- Petrological studies of the Mesohellenic trough sandstones coupled with basaltic rocks from Volos region (Central Greece) indicate that these rocks could serve as potential sites for CO2 storage via CO2-mineralisation. This is attributed to their high porosity, low alteration grade and abundance on Fe–Mg–Ca–K silicate minerals.
- Based on CO2 capacity data provided by the literature, geographical distribution criteria, potential for implementation of CO2 storage techniques and cost-considerations, we recommend the: (a) Volos basalts, (b) Mesohellenic trough sandstones, (c) saline aquifers of Western Thessaloniki basin and (d) the oil reservoir of Prinos basin, as the most promising sites for CO2 storage in Greece.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Symbol | Description | Unit |
|---|---|---|
| Cp | heat capacity | MJ/kg·°C |
| rho | dry bulk density | kg/m3 |
| phi | porosity | % |
| T | transmissivity | m2/day |
| K | hydraulic conductivity | m/day |
| D | aquifer thickness | m |
| Kh | permeability-thickness product | m2-m |
| PFa | average formation pressure | Pa |
| T | temperature | °C |
| VISO | oil viscosity | mm2/s |
| Qg | productivity gas | m3/day |
| c | volumetric specific heat of the reservoir rock | MJ/kg·°C |
| V | volume of the reservoir | m3 |
| TR | the characteristic reservoir temperature | °C |
| TREF | the reference temperature | °C |
| H | heat in place | MJ |
| Vrock | the denoting rock volume | m3 |
| Prock | the rock density | kg/m3 |
| H | total heat energy storage capacity | MJ |
| P | effective porosity | % |
| ρm | density of the rock matrix | kg/m3 |
| ρw | density of water | kg/m3 |
| cm | specific heat capacity of the rock matrix | MJ/kg·°C |
| cw | specific heat capacity of water | MJ/kg·°C |
| Tt | mean temperature of the compartment | °C |
| A | surface area under consideration | m2 |
| Δz | aquifer thickness | m |
| Taverage | average temperature of stored hydrogen | °C |
| depth | depth | m |
| cavernHeight | height of the cavern | m |
| KV1 = 7.5 × 10−6 m3/day | ||
| KV2 = 1 × 10−5 m3/day | ||
| Density of water: 997 kg/m3 | ||
| Density of rock matrix (e.g., sandstone): 2323 kg/m3 | ||
References
- Davis, W. The Relationship between Atmospheric Carbon Dioxide Concentration and Global Temperature for the Last 425 Million Years. Climate 2017, 5, 76. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: New York, NY, USA, 2013; p. 1535. [Google Scholar]
- Koukouzas, N.; Koutsovitis, P.; Tyrologou, P.; Karkalis, C.; Arvanitis, A. Potential for Mineral Carbonation of CO2 in Pleistocene Basaltic Rocks in Volos Region (Central Greece). Minerals 2019, 9, 627. [Google Scholar] [CrossRef]
- Rosenbauer, R.J.; Thomas, B.; Bischoff, J.L.; Palandri, J. Carbon sequestration via reaction with basaltic rocks: Geochemical modeling and experimental results. Geochim. Cosmochim. Acta 2012, 89, 116–133. [Google Scholar] [CrossRef]
- Bott, C.; Dressel, I.; Bayer, P. State-of-technology review of water-based closed seasonal thermal energy storage systems. Renew. Sustain. Energy Rev. 2019, 113, 109241. [Google Scholar] [CrossRef]
- Montcoudiol, N.; Burnside, N.M.; Györe, D.; Mariita, N.; Mutia, T.; Boyce, A. Surface and groundwater hydrochemistry of the Menengai Caldera Geothermal Field and surrounding Nakuru County, Kenya. Energies 2019, 12, 3131. [Google Scholar] [CrossRef]
- Xu, X.; Wei, Z.; Ji, Q.; Wang, C.; Gao, G. Global renewable energy development: Influencing factors, trend predictions and countermeasures. Resour. Policy 2019, 63, 101470. [Google Scholar] [CrossRef]
- Nataraj Barath, J.G.; Husev, O.; Manonmani, N. Overview of Energy Storage Technologies For Renewable Energy. IJISET 2015, 2, 6. [Google Scholar]
- Mofijur, M.; Mahlia, T.M.I.; Silitonga, A.S.; Ong, H.C.; Silakhori, M.; Hasan, M.H.; Putra, N.; Rahman, S.A. Phase Change Materials (PCM) for Solar Energy Usages and Storage: An Overview. Energies 2019, 12, 3167. [Google Scholar] [CrossRef]
- Dincer, I.; Rosen, M. Thermal Energy Storage: Systems and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Paksoy, H.Ö.; Beyhan, B. Thermal energy storage (TES) systems for greenhouse technology. In Advances in Thermal Energy Storage Systems. Methods and Applications; Cabeza, L.F., Ed.; Woodhead Publishing, Elsevier: Cambridge, UK, 2015; p. 612. [Google Scholar]
- De Schepper, G.; Paulus, C.; Bolly, P.-Y.; Hermans, T.; Lesparre, N.; Robert, T. Assessment of short-term aquifer thermal energy storage for demand-side management perspectives: Experimental and numerical developments. Appl. Energy 2019, 242, 534–546. [Google Scholar] [CrossRef]
- Park, J.-W.; Rutqvist, J.; Ryu, D.; Park, E.-S.; Synn, J.-H. Coupled thermal-hydrological-mechanical behavior of rock mass surrounding a high-temperature thermal energy storage cavern at shallow depth. Int. J. Rock Mech. Min. Sci. 2016, 83, 149–161. [Google Scholar] [CrossRef]
- Arvanitis, A. Investigation of the Possibilities for the Subsurface Energy Storage in the frame of the EC-funded ESTMAP Project. In Proceedings of the 11th National Conference on Renewable Energy Sources, Thessaloniki, Greece, 14–16 March 2018. [Google Scholar]
- Breeze, P. Power System Energy Storage Technologies. In Power Generation Technologies, 3rd ed.; Breeze, P., Ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2019; pp. 219–249. [Google Scholar]
- Lemieux, A.; Sharp, K.; Shkarupin, A. Preliminary assessment of underground hydrogen storage sites in Ontario, Canada. Int. J. Hydrog. Energy 2019, 44, 15193–15204. [Google Scholar] [CrossRef]
- Caglayan, D.; Weber, N.; Heinrichs, H.; Linssen, J.; Robinius, M.; Kukla, P.; Stolten, D. Technical potential of salt caverns for hydrogen storage in Europe. Int. J. Hydrog. Energy 2020, 45, 6793–6805. [Google Scholar] [CrossRef]
- Crotogino, F.; Donadei, S.; Bünger, U.; Landinger, H. Large-Scale Hydrogen Underground Storage for Securing Future Energy Supplies. In Proceedings of the 18th World Hydrogen Energy Conference, Essen, Germany, 16–21 May 2010. [Google Scholar]
- Arvanitis, A.; Koukouzas, N.; Koutsovitis, P.; Karapanos, D.; Manoukian, E. Combined CO2 Geological Storage and Geothermal Energy Utilization in Greece. In Proceedings of the 15th International Congress of the Geological Society of Greece Harokopio University of Athens, Athens, Greece, 22–24 May 2019. [Google Scholar]
- Hasan, M.M.F.; Boukouvala, F.; First, E.L.; Floudas, C.A. Nationwide, Regional, and Statewide CO2 Capture, Utilization, and Sequestration Supply Chain Network Optimization. Ind. Eng. Chem. Res 2014, 53, 7489–7506. [Google Scholar] [CrossRef]
- Celia, M.A.; Bachu, S.; Nordbotten, J.M.; Bandilla, K.W. Status of CO2 storage in deep saline aquifers with emphasis on modeling approaches and practical simulations. Water Resour. Res. 2015, 51, 6846–6892. [Google Scholar] [CrossRef]
- Jalili, P.; Saydam, S.; Cinar, Y. CO2 Storage in Abandoned Coal Mines. In Proceedings of the Underground Coal Operators’ Conference Wollongong, Wollongong, Australia, 10–11 February 2011; p. 410. [Google Scholar]
- Xie, L.Z.; Zhou, H.; Xie, H. Research advance of CO2 storage in rock salt caverns. Yantu Lixue/Rock Soil Mech. 2009, 30, 7. [Google Scholar]
- Baran, P.; Zarębska, K.; Krzystolik, P.; Hadro, J.; Nunn, A. CO2-ECBM and CO2 sequestration in Polish coal seam—Experimental study. J. Sustain. Min. 2014, 13, 22–29. [Google Scholar] [CrossRef]
- Raza, A.; Gholami, R.; Rezaee, R.; Bing, C.H.; Nagarajan, R.; Hamid, M.A. Well selection in depleted oil and gas fields for a safe CO2 storage practice: A case study from Malaysia. Petroleum 2017, 3, 167–177. [Google Scholar] [CrossRef]
- Györe, D.; Stuart, F.M.; Gilfillan, S.M.V.; Waldron, S. Tracing injected CO2 in the Cranfield enhanced oil recovery field (MS, USA) using He, Ne and Ar isotopes. Int. J. Greenh. Gas Control 2015, 42, 554–561. [Google Scholar] [CrossRef]
- Snæbjörnsdóttir, S.Ó.; Wiese, F.; Fridriksson, T.; Ármansson, H.; Einarsson, G.M.; Gislason, S.R. CO2 storage potential of basaltic rocks in Iceland and the oceanic ridges. Energy Procedia 2014, 63, 4585–4600. [Google Scholar] [CrossRef]
- Ragnheidardottir, E.; Sigurdardottir, H.; Kristjansdottir, H.; Harvey, W. Opportunities and challenges for CarbFix: An evaluation of capacities and costs for the pilot scale mineralization sequestration project at Hellisheidi, Iceland and beyond. Int. J. Greenh. Gas Control 2011, 5, 1065–1072. [Google Scholar] [CrossRef]
- Koukouzas, N.; Kypritidou, Z.; Purser, G.; Rochelle, C.A.; Vasilatos, C.; Tsoukalas, N. Assessment of the impact of CO2 storage in sandstone formations by experimental studies and geochemical modeling: The case of the Mesohellenic Trough, NW Greece. Int. J. Greenh. Gas Control 2018, 71, 116–132. [Google Scholar] [CrossRef]
- Garcia-Rios, M.; Luquot, L.; Soler, J.; Cama, J. Laboratory-Scale Interaction between CO2-Rich Brine and Reservoir Rocks (Limestone and Sandstone). Procedia Earth Planet. Sci. 2013, 7, 109–112. [Google Scholar] [CrossRef]
- Boschi, C.; Dini, A.; Dallai, L.; Ruggieri, G.; Gianelli, G. Enhanced CO2-mineral sequestration by cyclic hydraulic fracturing and Si-rich fluid infiltration into serpentinites at Malentrata (Tuscany, Italy). Chem. Geol. 2009, 265, 209–226. [Google Scholar] [CrossRef]
- Dichicco, M.C.; Laurita, S.; Paternoster, M.; Rizzo, G.; Sinisi, R.; Mongelli, G. Serpentinite Carbonation for CO2 Sequestration in the Southern Apennines: Preliminary Study. Energy Procedia 2015, 76, 477–486. [Google Scholar] [CrossRef]
- Bennaceur, K. CO2 Capture and Sequestration. In Future Energy, 2nd ed.; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 583–611. [Google Scholar]
- Piessens, K.; Dusar, M. CO2-sequestration in abandoned coal mines. In Proceedings of the International Coalbed Methane Symposium, Tuscaloosa, AL, USA, 5–8 May 2003; p. 11. [Google Scholar]
- Kelektsoglou, K. Carbon Capture and Storage: A Review of Mineral Storage of CO2 in Greece. Sustainability 2018, 10, 4400. [Google Scholar] [CrossRef]
- Petrounias, P.; Giannakopoulou, P.; Rogkala, A.; Kalpogiannaki, M.; Koutsovitis, P.; Damoulianou, M.-E.; Koukouzas, N. Petrographic Characteristics of Sandstones as a Basis to Evaluate Their Suitability in Construction and Energy Storage Applications. A Case Study from Klepa Nafpaktias (Central Western Greece). Energies 2020, 13, 1119. [Google Scholar] [CrossRef]
- Tasianas, A.; Koukouzas, N. CO2 storage capacity estimate in the lithology of the Mesohellenic Trough, Greece. Energy Procedia 2016, 86, 334–341. [Google Scholar] [CrossRef]
- Koukouzas, N.; Gemeni, V.; Ziock, H.J. Sequestration of CO2 in magnesium silicates, in Western Macedonia, Greece. Int. J. Miner. Process. 2009, 93, 179–186. [Google Scholar] [CrossRef]
- ESTMAP, Energy Storage Mapping and Planning Home Page. Available online: http://estmap.eu/deliverables.html (accessed on 5 May 2020).
- Karakitsios, V. Western Greece and Ionian Sea petroleum systems. AAPG Bull. 2013, 97, 1567–1595. [Google Scholar] [CrossRef]
- Karakitsios, V.; Rigakis, N. Evolution and petroleum potential of Western Greece. J. Pet. Geol. 2007, 30, 197–218. [Google Scholar] [CrossRef]
- Tserolas, P.; Mpotziolis, C.; Maravelis, A.; Zelilidis, A. Preliminary Geochemical and Sedimentological Analysis in NW Corfu: The Miocene Sediments in Agios Georgios Pagon. In Proceedings of the 14th International Congress of Geological Society of Greece, Thessaloniki, Greece, 25–27 May 2016; pp. 402–411. [Google Scholar]
- Karakitsios, V.; Roveri, M.; Lugli, S.; Manzi, V.; Gennari, R.; Antonarakou, A.; Triantaphyllou, M.; Agiadi, K.; Kontakiotis, G.; Kafousia, N.; et al. A Record of the Messinian Salinity Crisis in the Eastern Ionian Tectonically Active Domain (Greece, Eastern Mediterrenean). Basin Res. 2017, 29, 203–233. [Google Scholar] [CrossRef]
- Kokinou, E.; Kamberis, E.; Kotsi, F.; Lioni, K.; Velaj, T. The Impact of Evaporites in the Greek and Albanian Oil Systems. In Proceedings of the 79th EAGE Conference and Exhibition 2017, Paris, France, 12–15 June 2017. [Google Scholar]
- Velaj, T. The structural style and hydrocarbon exploration of the subthrust in the Berati Anticlinal Belt, Albania. J. Pet. Explor. Prod. Technol. 2015, 5, 123–145. [Google Scholar] [CrossRef]
- Velaj, T. Tectonic style in Western Albania Thrustbelt and its implication on hydrocarbon exploration. AAPG Search Discov. Artic. 2011, 123–148. [Google Scholar]
- Kontopoulos, N.; Zelilidis, A.; Piper, D.J.W.; Mudie, P.J. Messinian evaporites in Zakynthos, Greece. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1997, 129, 361–367. [Google Scholar] [CrossRef]
- Bourli, N.; Kokkaliari, M.; Iliopoulos, I.; Pe-Piper, G.; Piper, D.J.W.; Maravelis, A.G.; Zelilidis, A. Mineralogy of siliceous concretions, cretaceous of ionian zone, western Greece: Implication for diagenesis and porosity. Mar. Pet. Geol. 2019, 105, 45–63. [Google Scholar] [CrossRef]
- Tsipoura-Vlachou, M. Diagenesis of the Marly-Gypsum Formations, Igoumenitsa Area, N.W. Greece. Bull. Geol. Soc. Greece 2007, 40, 1009–1021. [Google Scholar] [CrossRef]
- Getsos, K.; Papaioannou, F.; Zelilidis, A. Triassic Carbonate and Evaporite Sedimentation in the Ionian Zone (Western Greece): Palaeogeographic and Palaeoclimatic Implication. Bull. Geol. Soc. Greece 2004, 36, 699–707. [Google Scholar] [CrossRef]
- Flugel, E. Mikrofazielle Untersuchungen in der Alpinen Trias: Methoden and Probleme. Mitt. Ges. Geol. Bergbaustud. 1972, 21, 9–64. [Google Scholar]
- Vamvaka, A.; Spiegel, C.; Frisch, W.; Danisik, M. Fission track data from the Mesohellenic Trough and the Pelagonian zone in NW Greece: Cenozoic tectonics and exhumation of source areas. Int. Geol. Rev. 2010, 52, 223–248. [Google Scholar] [CrossRef]
- Rassios, A.; Moores, E. Heterogeneous mantle complex, crustal processes, and obduction kinematics in a unified Pindos-Vourinos ophiolitic slab (northern Greece). Geol. Soc. Lond. Spec. Publ. 2006, 260, 237–266. [Google Scholar] [CrossRef]
- Kilias, A.; Vamvaka, A.; Falalakis, G.; Sfeikos, A.; Papadimitriou, E.; Gkarlaouuni, C.; Karakostas, B. The Mesohellenic Trough and the Paleogene Thrace Basin on the Rhodope Massif, their Structural Evolution and Geotectonic Significance in the Hellenides. J. Geol. Geosci. 2015, 4, 2. [Google Scholar] [CrossRef]
- Brunn, J.H. Contribution à l’étude géologique du Pinde septentrional et d’une partie de la Macédoine occidentale. Ann. Géol. Pays Hell. 1956, 8, 346–358. [Google Scholar]
- Papanikolaou, D.; Lekkas, E.; Mariolakos, H.; Mirkou, R. Contribution on the geodynamic evolution of the Mesohellenic trough. Bull. Geol. Soc. Greece 1988, 20, 17–36. [Google Scholar]
- Aubouin, J. Contribution à l’ étude géologique de la Grèce septentrionale: Le confins de l’ Epire et de la Thessalie. Ann. Géol. Pays Hell. 1959, 10, 525. [Google Scholar]
- Koukouzas, C.; Koukouzas, N. Coals of Greece: Distribution, quality and reserves. Geol. Soc. Spec. Publ. 1995, 82, 171–180. [Google Scholar] [CrossRef]
- Wilson, M.; Monea, M. IEA GHG Weyburn CO2 monitoring and storage project: Summary report 2000–2001. In Proceedings of the 7th International Conference Greenhouse Gas Control Technology (GHGT-7), Vancouver, BC, Canada, 5–9 September 2005. [Google Scholar]
- Koukouzas, N.; Ziogou, F.; Gemeni, V. Preliminary assessment of CO2 geological storage opportunities in Greece. Int. J. Greenh. Gas Control 2009, 3, 502–513. [Google Scholar] [CrossRef]
- Tasianas, A.; Koukouzas, N. Assessing the Potential of the Mesohellenic Trough and Other Sites, in Greece, for CO2 Storage. Procedia Earth Planet. Sci. 2015, 15, 607–612. [Google Scholar] [CrossRef][Green Version]
- Saccani, E.; Photiades, A. Mid-ocean ridge and supra-subduction affinities in the Pindos ophiolites (Greece): Implications for magma genesis in a forearc setting. Lithos 2004, 73, 229–253. [Google Scholar] [CrossRef]
- Pomonis, P.; Tsikouras, B.; Hatzipanagiotou, K. Geological evolution of the Koziakas ophiolitic complex (W. Thessaly, Greece). Ofioliti 2005, 30, 77–86. [Google Scholar]
- Koutsovitis, P. Gabbroic rocks in ophiolitic occurrences from East Othris, Greece: Petrogenetic processes and geotectonic environment implications. Mineral. Petrol. 2011, 104, 249–265. [Google Scholar] [CrossRef]
- Saccani, E.; Beccaluva, L.; Photiades, A.; Zeda, O. Petrogenesis and tectono-magmatic significance of basalts and mantle peridotites from the Albanian–Greek ophiolites and sub-ophiolitic mélanges. New constraints for the Triassic–Jurassic evolution of the Neo-Tethys in the Dinaride sector. Lithos 2011, 124, 227–242. [Google Scholar] [CrossRef]
- Stouraiti, C.; Pantziris, I.; Vasilatos, C.; Kanellopoulos, C.; Mitropoulos, P.; Pomonis, P.; Moritz, R.; Chiaradia, M. Ophiolitic Remnants from the Upper and Intermediate Structural Unit of the Attic-Cycladic Crystalline Belt (Aegean, Greece): Fingerprinting Geochemical Affinities of Magmatic Precursors. Geosciences 2017, 7, 14. [Google Scholar] [CrossRef]
- Mortazavi, M.; Sparks, R. Origin of rhyolite and rhyodacite lavas and associated mafic inclusions of Cape Akrotiri, Santorini: The role of wet basalt in generating calcalkaline silicic magmas. Contrib. Mineral. Petrol. 2004, 146, 397–413. [Google Scholar] [CrossRef]
- Bachmann, O.; Deering, C.; Ruprecht, J.; Huber, C.; Skopelitis, A.; Schnyder, C. Evolution of silicic magmas in the Kos-Nisyros volcanic center, Greece: A petrological cycle associated with caldera collapse. Contrib. Mineral. Petrol. 2012, 163, 151–166. [Google Scholar] [CrossRef]
- Pe-Piper, G.; Panagos, A.G. Geochemical characteristics of the Triassic volcanic rocks of Evia: Petrogenetic and tectonic implications. Ofioliti 1989, 14, 33–50. [Google Scholar]
- Pe-Piper, G.; Piper, D. Neogene backarc volcanism of the Aegean: New insights into the relationship between magmatism and tectonics. Geol. Soc. Am. 2007, 418, 17–31. [Google Scholar] [CrossRef]
- Fytikas, M.; Innocenti, F.; Manetti, P.; Peccerillo, A.; Mazzuoli, R.; Villari, L. Tertiary to Quaternary evolution of volcanism in the Aegean region: The Geological Evolution of the Eastern Mediterranean. In Geological Society Special Publication; Dixon, J.E., Robertson, A.H.F., Eds.; The Geological Society Publishing House: Bath, UK, 1984; Volume 17, pp. 687–699. [Google Scholar]
- Innocenti, F.; Agostini, S.; Doglioni, C.; Piero, M.; Tonarini, S. Geodynamic evolution of the Aegean: Constraints from the Plio-Pleistocene volcanism of the Volos-Evia area. J. Geol. Soc. Lond. 2010, 167, 475–489. [Google Scholar] [CrossRef]
- Nicholls, I.A. Petrology of Santorini Volcano, Cyclades, Greece. J. Petrol. 1971, 12, 67–119. [Google Scholar] [CrossRef]
- Nicholls, I.A. Santorini volcano, greece—Tectonic and petrochemical relationships with volcanics of the Aegean region. Tectonophysics 1971, 11, 377–385. [Google Scholar] [CrossRef]
- Bridger, D.W.; Allen, D.M. Designing aquifer thermal energy storage systems. ASHRAE J. 2005, 47, S32. [Google Scholar]
- Vance, D. Heat in Groundwater Systems Part I—The Fundamentals. Natl. Environ. J. 1996, 6, 30–31. [Google Scholar]
- Brandt, M.J.; Johnson, K.M.; Elphinston, A.J.; Ratnayaka, D.D. Twort’s Water Supply, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Maidment, D.R. Handbook of Hydrology; McGraw-Hill: New York, NY, USA, 1992. [Google Scholar]
- Price, M. Introducing Groundwater, 2nd ed.; Chapman and Hall: London, UK, 1996. [Google Scholar]
- Crain, E.R. Crain’s Petrophysical Handbook; Spectrum 2000 Mindware: Rocky Mountain House, AB, Canada, 1987. [Google Scholar]
- Nathenson, M. Physical Factors Determining the Fraction of Stored Energy Recoverable from Hydrothermal Convection Systems and Conduction-Dominated Areas; U.S. Geological Survey Open-File Report: Menlo Park, CA, USA, 1975; p. 50. [Google Scholar]
- White, D.E.; Williams, D.L. Assessment of Geothermal Resources of the United Sates; U.S. Geological Survey Circular: Arlington, VA, USA, 1975; p. 155. [Google Scholar]
- Muffler, L.J.P. Assessment of Geothermal Resources of the United States; U.S. Geological Survey Circular: Roosevelt, UT, USA, 1979; p. 163. [Google Scholar]
- Franco, A.; Donatini, F. Methods for the estimation of the energy stored in geothermal reservoirs. J. Phys. Conf. Ser. 2017, 796, 012025. [Google Scholar] [CrossRef]
- Beardsmore, G.; Rybach, L.; Blackwell, D.; Baron, C. A Protocol for Estimating and Mapping Global EGS Potential. GRC Trans. 2010, 34, 301–312. [Google Scholar]
- Gao, L.; Zhao, J.; An, Q.; Liu, X.; Du, Y. Thermal performance of medium-to-high-temperature aquifer thermal energy storage systems. Appl. Therm. Eng. 2019, 146, 898–909. [Google Scholar] [CrossRef]
- Van Lopik, J.H.; Hartog, N.; Jan Zaadnoordijk, W. The use of salinity contrast for density difference compensation to improve the thermal recovery efficiency in high-temperature aquifer thermal energy storage systems. Hydrogeol. J. 2016, 24, 1255–1271. [Google Scholar] [CrossRef]
- Berger, B.; Anderson, K. Modern Petroleum: A Basic Primer of the Industry, 3rd ed.; Pennwell Books: Houston, TX, USA, 1992; p. 517. [Google Scholar]
- North, F.K. Petroleum Geology; Allen & Unwin: Boston, MA, USA, 1985. [Google Scholar]
- Franz, P.; Neville-Lamb, M.; Azwar, L.; Quinao, J. Calculation of Geothermal Stored Heat from a Numerical Model for Reserve Estimation. In Proceedings of the World Geothermal Congress, Melbourne, Australia, 19–25 April 2015. [Google Scholar]
- Muffler, P.; Cataldi, R. Methods for regional assessment of geothermal resources. Geothermics 1978, 7, 53–89. [Google Scholar] [CrossRef]
- Schellschmidt, R.; Hurter, S. Atlas of Geothermal Resources in Europe. Geothermics 2003, 32, 779–787. [Google Scholar] [CrossRef]
- Pellegrini, M.; Bloemendal, M.; Hoekstra, N.; Spaak, G.; Andreu Gallego, A.; Rodriguez Comins, J.; Grotenhuis, T.; Picone, S.; Murrell, A.J.; Steeman, H.J. Low carbon heating and cooling by combining various technologies with Aquifer Thermal Energy Storage. Sci. Total Environ. 2019, 665, 1–10. [Google Scholar] [CrossRef]
- Todorov, O.; Alanne, K.; Virtanen, M.; Kosonen, R. A method and analysis of aquifer thermal energy storage (ATES) system for district heating and cooling: A case study in Finland. Sustain. Cities Soc. 2020, 53, 101977. [Google Scholar] [CrossRef]
- Hooimeijer, F.; Maring, L. The significance of the subsurface in urban renewal. IRPUS 2018, 11, 303–328. [Google Scholar] [CrossRef][Green Version]
- Kostakis, I. Socio-demographic determinants of household electricity consumption: Evidence from Greece using quantile regression analysis. CRSUST 2020, in press. [Google Scholar] [CrossRef]
- Azeiteiro, U.; Davim, J. Higher Education and Sustainability, 1st ed.; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Tichler, R.; Bauer, S. Power to gas. In Storing Energy with Special Reference to Renewable Energy Sources; Letcher, T.M., Ed.; Elsevier: Oxford, UK, 2016; p. 590. [Google Scholar]
- Renpu, W. Basis of Well Completion Engineering. In Advanced Well Completion Engineering, 3rd ed.; Renpu, W., Ed.; Gulf Professional Publishing: Oxford, UK, 2011; pp. 1–74. [Google Scholar]
- Speight, J.G. Recovery, storage, and transportation. In Natural Gas, 2nd ed.; Speight, J.G., Ed.; Gulf Professional Publishing: Boston, MA, USA, 2019; pp. 149–186. [Google Scholar]
- Speight, J.G. (Ed.) Reservoirs and Reservoir Fluids. In Handbook of Hydraulic Fracturing; Wiley: Hoboken, NJ, USA, 2016; pp. 27–54. [Google Scholar]
- Papadopoulou, D.; Tourkolias, C.N.; Mirasgedis, S. Assessing the macroeconomic effect of gas pipeline projects: The case of Trans-Adriatic Pipeline on Greece. SPOUDAI 2015, 65, 100–118. [Google Scholar]
- Khalova, G.O.; Illeritskiy, N.I.; Smirnova, V.A. Prospects for the Construction of the Poseidon Gas Pipeline as a Factor in Supplying the Needs of the Southern Europe Countries with Natural Gas. Int. J. Energy Econom. Policy 2019, 9, 143–148. [Google Scholar]
- Kotek, P.; Granado, P.C.d.; Egging, R.; Toth, B.T. European Natural Gas Infrastructure in the Energy Transition. In Proceedings of the 16th International Conference on the European Energy Market (EEM), Ljubljana, Slovenia, 18–20 September 2019; pp. 1–6. [Google Scholar]
- Kitsilis, M.-C. Issues for Underground Gas Storage (UGS) in ‘South Kavala’ offshore gas field. In Proceedings of the 5th South East Europe Energy Dialogue, Thessaloniki, Greece, 2–3 June 2011. [Google Scholar]
- Ozarslan, A. Large-scale hydrogen energy storage in salt caverns. Int. J. Hydrog. Energy 2012, 37, 14265–14277. [Google Scholar] [CrossRef]
- Winter, C.-J.; Nitch, J. Hydrogen as an Energy Carrier: Technologies, Systems, Economy; Spiringer: Stuttgart, Germany, 1988. [Google Scholar]
- Eiken, O.; Ringrose, P.; Hermanrud, C.; Nazarian, B.; Torp, T.A.; Høier, L. Lessons learned from 14 years of CCS operations: Sleipner, In Salah and Snøhvit. Energy Procedia 2011, 4, 5541–5548. [Google Scholar] [CrossRef]
- Beaubien, S.E.; Jones, D.G.; Gal, F.; Barkwith, A.K.A.P.; Braibant, G.; Baubron, J.-C.; Ciotoli, G.; Graziani, S.; Lister, T.R.; Lombardi, S.; et al. Monitoring of near-surface gas geochemistry at the Weyburn, Canada, CO2-EOR site, 2001–2011. Int. J. Greenh. Gas Control 2013, 16, S236–S262. [Google Scholar] [CrossRef]
- Andritsos, N.; Arvanitis, A.; Papachristou, M.; Fytikas, M.; Dalabakis, P. Geothermal Activities in Greece During 2005-2009. In Proceedings of the World Geothermal Congress Bali, Bali, Indonesia, 25–29 April 2010; pp. 25–29. [Google Scholar]
- Jin, C.; Liu, L.; Yiman, l.; Zeng, R. Capacity assessment of CO2 storage in deep saline aquifers by mineral trapping and the implications for Songliao Basin, Northeast China. Energy Sci. Eng. 2017, 5, 81–89. [Google Scholar] [CrossRef]
- Hannis, S.; Lu, J.; Chadwick, A.; Hovorka, S.; Kirk, K.; Romanak, K.; Pearce, J. CO2 storage in depleted or depleting oil and gas fields: What can we learn from existing projects? Energy Procedia 2017, 114, 5680–5690. [Google Scholar] [CrossRef]







| Aquifers Considered | Thermal Capacity (MJ) | Productivity (m3/day) | Heat in Place (MJ) |
|---|---|---|---|
| Sappes | 3.370 | 353 | 3 × 10−3 |
| Agioi Theodoroi-Komotini | 2.930 | 1193 | 3 × 10−3 |
| Mitrikou lake | 3.009 | 788 | 9 × 10−4 |
| Serres | 2.435 | 11,169 | 1 × 10−3 |
| W. Thessaloniki Shallow Thermal | 2.382 | 162 | 4 × 10−4 |
| W. Thessaloniki-Alexandria | 3.577 | 1516 | 1 × 10−3 |
| Mesohellenic Trough_ South Grevena | 3.700 | 57,709 | 25 × 10−3 |
| Flysch Botsara syncline | 3.822 | 64,304 | 18 × 10−3 |
| Aliveri Evia | 2.703 | 405 | 1 × 10−3 |
| Megalopoli | 2.291 | 109 | 4 × 10−4 |
| Thimiana Chios | 3.021 | 80 | 5 × 10−4 |
| Lesbos-Thermi | 2.885 | 44.5 | 2 × 10−3 |
| Kos | 2.394 | 404 | 1 × 10−3 |
| Samos | 2.461 | 37.5 | 7 × 10−4 |
| Limnos | 2.692 | 38.5 | 1 × 10−3 |
| Rhodes | 2.249 | 832 | 9 × 10−4 |
| Mesohellenic Trough_Felio | 4.175 | 92,747 | 18 × 10−3 |
| W. Thessaloniki_ SG | 3.93 | 11,082 | 7 × 10−3 |
| W. Thessaloniki_ DG | 4.651 | 3253 | 1 × 10−3 |
| Fili landfill_Attica | 2.589 | 4352 | 1 × 10−3 |
| North Mesohellenic basin_ SG | 3.700 | 43,282 | 22 × 10−3 |
| North Mesohellenic basin_ DG | 4.006 | 93,778 | 3 × 10−3 |
| Aquifers Considered | Transmissivity (m2) |
|---|---|
| Aquifer Sappes | 6.9 × 10−14 |
| Aquifer Agioi Theodoroi-Komotinis | 2.21 × 10−13 |
| Aquifer Mitrikou lake | 1.77 × 10−13 |
| Aquifer Serres | 4.14 × 10−13 |
| Aquifer Western Thessaloniki_Shallow Thermal | 5.92 × 10−14 |
| Aquifer_Western Thessaloniki_Alexandria | 1.18 × 10−13 |
| Aquifer_Mesohellenic Trough_South Grevena | 1.97 × 10−12 |
| Aquifer Flysch Botsara syncline | 2.96 × 10−13 |
| Aquifer Aliveri Evia | 3.45 × 10−13 |
| Aquifer Megalopoli | 7.89 × 10−14 |
| Aquifer Thimiana Chios | 7.4 × 10−14 |
| Aquifer Lesbos-Thermi island | 5.92 × 10−15 |
| Aquifer Kos island | 6.9 × 10−13 |
| Aquifer Samos island | 2.96 × 10−14 |
| Aquifer Limnos island | 2.96 × 10−14 |
| Aquifer Rhodes | 1.18 × 10−13 |
| Aquifer_Mesohellenic Trough_Felio | 1.48 × 10−12 |
| Aquifer_Western Thessaloniki_SG | 2.96 × 10−13 |
| Aquifer_Western Thessaloniki_DG | 8.29 × 10−14 |
| Aquifer_Fili landfill_Attica | 2.24 × 10−13 |
| North Mesohellenic basin_SG | 1.97 × 10−12 |
| North Mesohellenic basin_DG | 1.48 × 10−12 |
| Sites Considered (* Aquifer, ** Abandoned Mine) | Avgerage Total Heat Energy Storage Capacity (MWh[th]) | Average Area Heat Energy Storage Capacity (MWh[th]) |
|---|---|---|
| Sappes * | 2,172,617 | 1,284,404 |
| Agioi Theodoroi Komotinis * | 4,076,831 | 1,365,479 |
| Mitrikou lake * | 1,335,808 | 448,048 |
| Serres * | 6,052,932 | 793,685 |
| W. Thessaloniki Shallow Thermal * | 13,232 | 249,200 |
| Aliveri Evia * | 723,255 | 691,274 |
| Megalopoli * | 384,557 | 227,864 |
| Thimiana Chios * | 9599 | 256,027 |
| Lesbos-Thermi * | 509,167 | 1,024,109 |
| Kos * | 847,815 | 836,356 |
| Samos * | 21,801 | 384,041 |
| Limnos * | 767,700 | 473,650 |
| Rhodes * | 162,278 | 477,918 |
| Fili landfill_Attica * | 673,119 | 621,720 |
| Mandra Attica ** | 34,543 | 2,997,653 |
| Aliveri ** | 936,938 | 5,440,280 |
| Chaidari ** | 65,198 | 1,181,993 |
| Sites Considered (* Gas Field, ** Aquifer, *** Salt Structure) | Total Gas Volume (Mm3) | Working Gas Volume (Mm3) | Cushion Gas Volume (Mm3) | Energy Storage Capacity (MWh[e]) |
|---|---|---|---|---|
| South Kavala * | 847 | 720 | 127 | 2,672,920 |
| Epanomi * | 500 | 250 | 250 | 928,097 |
| Katakolo * | 300 | 150 | 150 | 556,858 |
| Prinos * | 2280 | 1300 | 980 | 4,826,105 |
| Western Thessaloniki_ Alexandria ** | 3 | 1 | 2 | 3712 |
| Mesohellenic Trough_ South Grevena ** | 44 | 13 | 31 | 48,261 |
| Flysch Botsara syncline ** | 33 | 10 | 23 | 37,123 |
| Mazarakia *** | 281 | 175 | 106 | 649,668 |
| Heraklion *** | 328 | 204 | 124 | 757,327 |
| Mesohellenic Trough Filio ** | 25 | 8 | 17 | 29,699 |
| Western Thessaloniki SG ** | 136 | 41 | 95 | 152,208 |
| Western Thessaloniki DG ** | 29 | 93 | 20 | 345,252 |
| North Mesohellenic basin SG ** | 130 | 39 | 91 | 144,783 |
| North Mesohellenic basin DG ** | 214 | 64 | 150 | 237,593 |
| Delvinaki *** | 218 | 175 | 106 | 649,668 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arvanitis, A.; Koutsovitis, P.; Koukouzas, N.; Tyrologou, P.; Karapanos, D.; Karkalis, C.; Pomonis, P. Potential Sites for Underground Energy and CO2 Storage in Greece: A Geological and Petrological Approach. Energies 2020, 13, 2707. https://doi.org/10.3390/en13112707
Arvanitis A, Koutsovitis P, Koukouzas N, Tyrologou P, Karapanos D, Karkalis C, Pomonis P. Potential Sites for Underground Energy and CO2 Storage in Greece: A Geological and Petrological Approach. Energies. 2020; 13(11):2707. https://doi.org/10.3390/en13112707
Chicago/Turabian StyleArvanitis, Apostolos, Petros Koutsovitis, Nikolaos Koukouzas, Pavlos Tyrologou, Dimitris Karapanos, Christos Karkalis, and Panagiotis Pomonis. 2020. "Potential Sites for Underground Energy and CO2 Storage in Greece: A Geological and Petrological Approach" Energies 13, no. 11: 2707. https://doi.org/10.3390/en13112707
APA StyleArvanitis, A., Koutsovitis, P., Koukouzas, N., Tyrologou, P., Karapanos, D., Karkalis, C., & Pomonis, P. (2020). Potential Sites for Underground Energy and CO2 Storage in Greece: A Geological and Petrological Approach. Energies, 13(11), 2707. https://doi.org/10.3390/en13112707








