Hyperthermophilic Treatment of Grass and Leaves to Produce Hydrogen, Methane and VFA-Rich Digestate: Preliminary Results
Abstract
:1. Introduction
2. Materials and Methods
2.1. Feedstock and Inoculum
2.2. Experimental Design
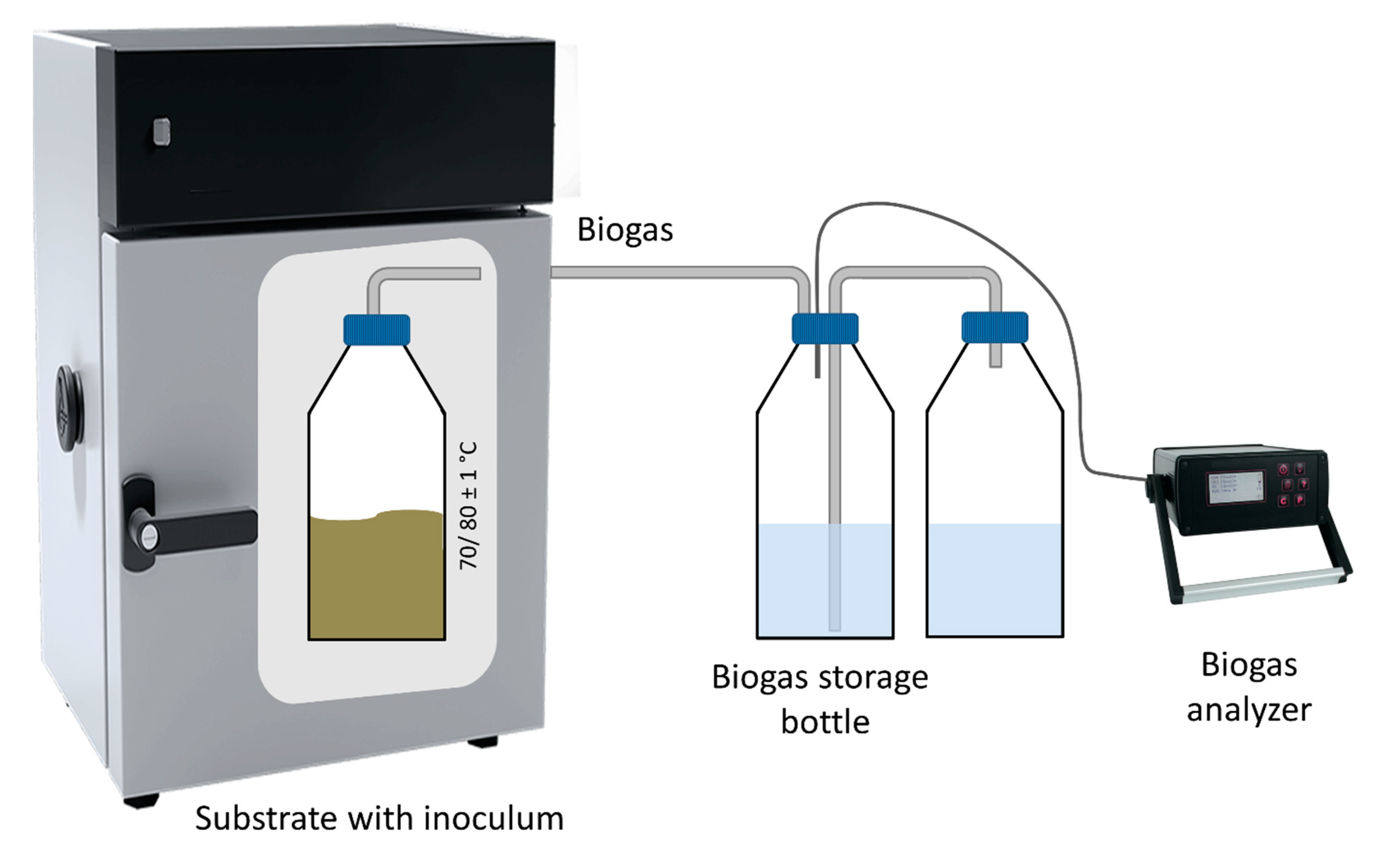
2.2.1. Batch Thermal Treatment—Experiments in Flasks
2.2.2. Anaerobic Digestion Batch Tests
2.3. Analytical Methods
3. Results and Discussion
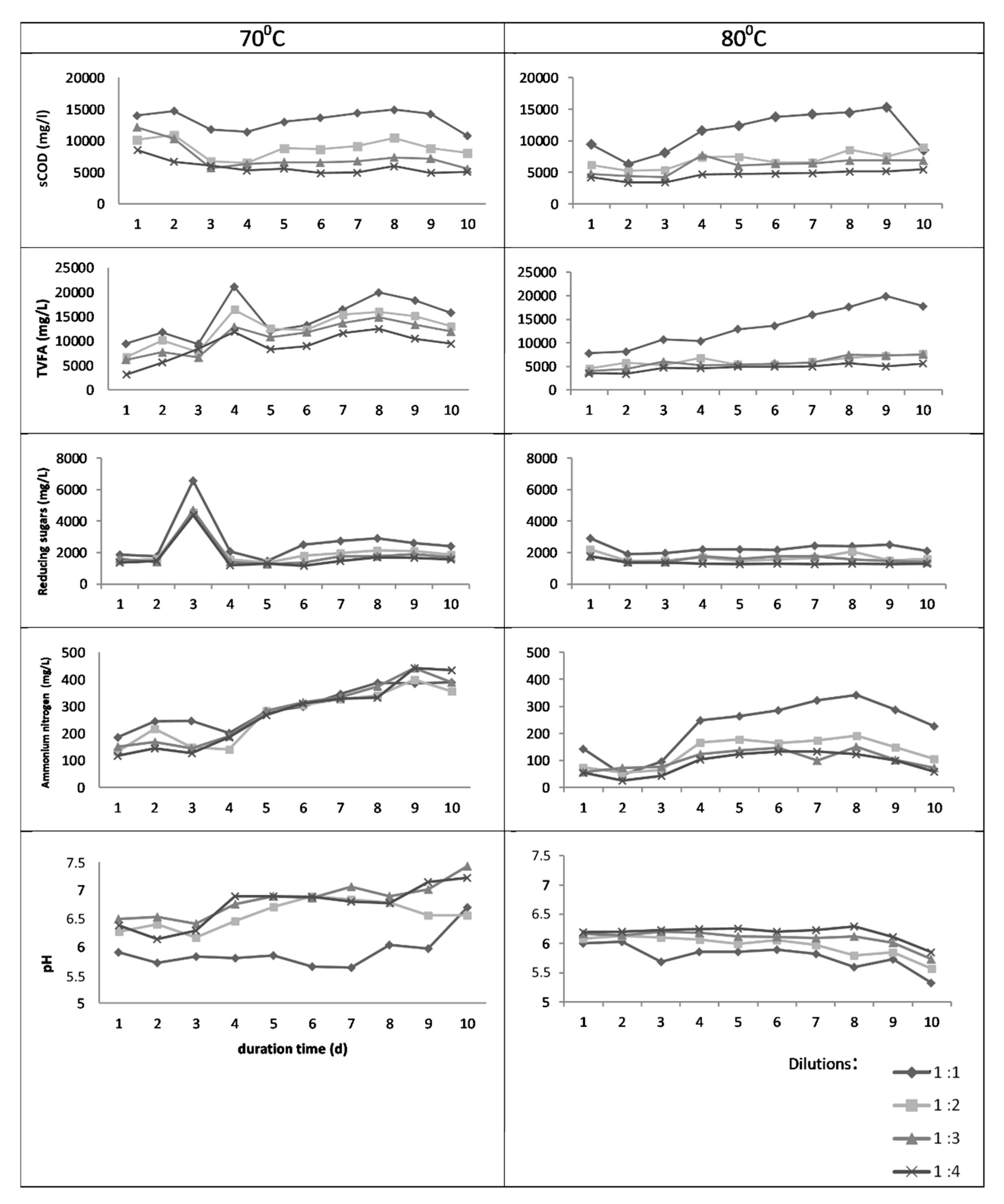
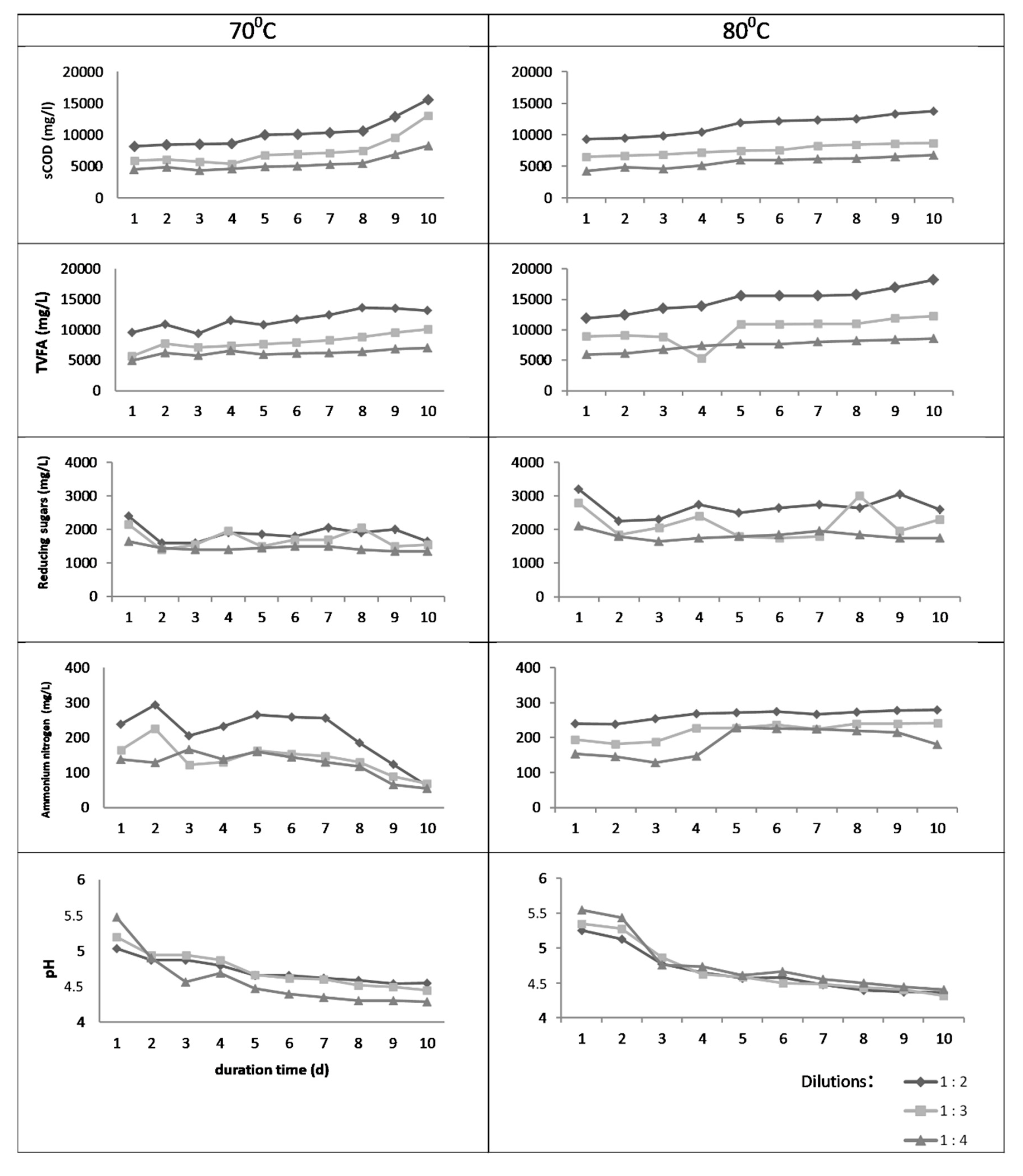
3.1. Batch Thermal Treatment—Experiments in Flasks
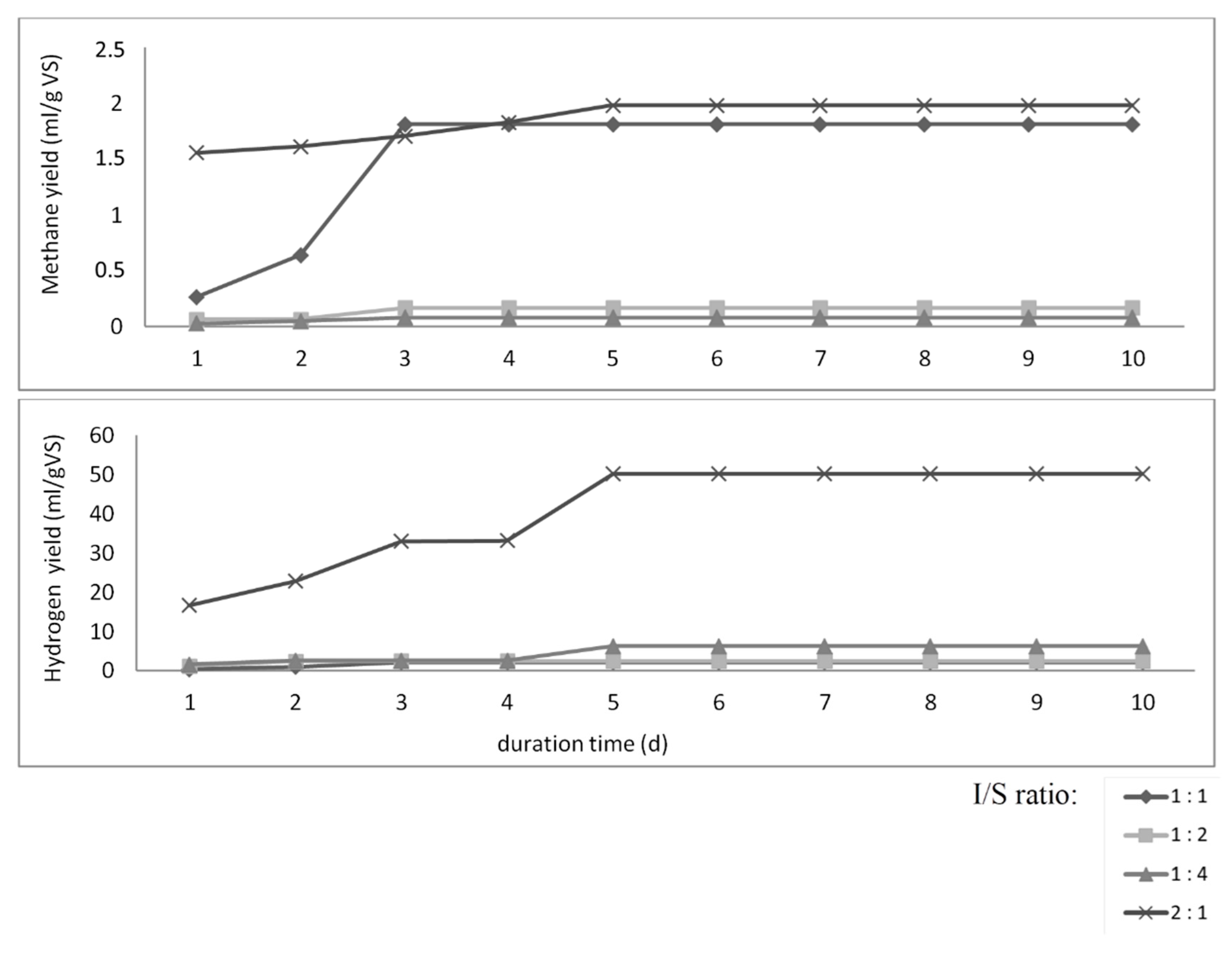
3.2. Anaerobic Digestion Batch Tests
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- GUS Environment 2019; Statistics Poland: Warsaw, Poland, 2019.
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen production from biomass using dark fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Cardona Alzate, C.A.; Sánchez Toro, O.J. Energy consumption analysis of integrated flowsheets for production of fuel ethanol from lignocellulosic biomass. Energy 2006, 31, 2447–2459. [Google Scholar] [CrossRef]
- Zhu, H.; Stadnyk, A.; Béland, M.; Seto, P. Co-production of hydrogen and methane from potato waste using a two-stage anaerobic digestion process. Bioresour. Technol. 2008, 99, 5078–5084. [Google Scholar] [CrossRef]
- Kumar, P.; Barrett, D.M.; Delwiche, M.J.; Stroeve, P. Methods for Pretreatment of Lignocellulosic Biomass for Efficient Hydrolysis and Biofuel Production. Ind. Eng. Chem. Res. 2009, 48, 3713–3729. [Google Scholar] [CrossRef]
- Sindhu, R.; Binod, P.; Pandey, A. Biological pretreatment of lignocellulosic biomass—An overview. Bioresour. Technol. 2016, 199, 76–82. [Google Scholar] [CrossRef]
- Rodriguez, C.; Alaswad, A.; Benyounis, K.Y.; Olabi, A.G. Pretreatment techniques used in biogas production from grass. Renew. Sustain. Energy Rev. 2017, 68, 1193–1204. [Google Scholar] [CrossRef] [Green Version]
- Carlsson, M.; Lagerkvist, A.; Morgan-Sagastume, F. The effects of substrate pre-treatment on anaerobic digestion systems: A review. Waste Manag. 2012, 32, 1634–1650. [Google Scholar] [CrossRef]
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.J.; Delgenès, J.P.; Steyer, J.P.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. J. Hazard. Mater. 2010, 183, 1–15. [Google Scholar] [CrossRef]
- Bolzonella, D.; Pavan, P.; Zanette, M.; Cecchi, F. Two-phase anaerobic digestion of waste activated sludge: Effect of an extreme thermophilic prefermentation. Ind. Eng. Chem. Res. 2007, 46, 6650–6655. [Google Scholar] [CrossRef]
- Qin, Y.; Higashimori, A.; Wu, L.J.; Hojo, T.; Kubota, K.; Li, Y.Y. Phase separation and microbial distribution in the hyperthermophilic-mesophilic-type temperature-phased anaerobic digestion (TPAD) of waste activated sludge (WAS). Bioresour. Technol. 2017, 245, 401–410. [Google Scholar] [CrossRef]
- Wandera, S.M.; Qiao, W.; Jiang, M.; Mahdy, A.; Yin, D.; Dong, R. Enhanced methanization of sewage sludge using an anaerobic membrane bioreactor integrated with hyperthermophilic biological hydrolysis. Energy Convers. Manag. 2019, 196, 846–855. [Google Scholar] [CrossRef]
- Lee, M.; Hidaka, T.; Tsuno, H. Effect of temperature on performance and microbial diversity in hyperthermophilic digester system fed with kitchen garbage. Bioresour. Technol. 2008, 99, 6852–6860. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Hidaka, T.; Hagiwara, W.; Tsuno, H. Comparative performance and microbial diversity of hyperthermophilic and thermophilic co-digestion of kitchen garbage and excess sludge. Bioresour. Technol. 2009, 100, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Alqaralleh, R.M.; Kennedy, K.; Delatolla, R. Improving biogas production from anaerobic co-digestion of Thickened Waste Activated Sludge (TWAS) and fat, oil and grease (FOG) using a dual-stage hyper-thermophilic/thermophilic semi-continuous reactor. J. Environ. Manag. 2018, 217, 416–428. [Google Scholar] [CrossRef] [PubMed]
- Dooms, M.; Benbelkacem, H.; Buffière, P. High solid temperature phased anaerobic digestion from agricultural wastes: Putting several reactors in sequence. Biochem. Eng. J. 2018, 130, 21–28. [Google Scholar] [CrossRef]
- Wang, F.; Hidaka, T.; Tsumori, J. Enhancement of anaerobic digestion of shredded grass by co-digestion with sewage sludge and hyperthermophilic pretreatment. Bioresour. Technol. 2014, 169, 299–306. [Google Scholar] [CrossRef]
- Urbaniec, K.; Bakker, R.R. Biomass residues as raw material for dark hydrogen fermentation—A review. Int. J. Hydrogen Energy 2015, 40, 3648–3658. [Google Scholar] [CrossRef]
- Algapani, D.E.; Qiao, W.; Su, M.; di Pumpo, F.; Wandera, S.M.; Adani, F.; Dong, R. Bio-hydrolysis and bio-hydrogen production from food waste by thermophilic and hyperthermophilic anaerobic process. Bioresour. Technol. 2016, 216, 768–777. [Google Scholar] [CrossRef]
- Dessì, P.; Lakaniemi, A.M.; Lens, P.N.L. Biohydrogen production from xylose by fresh and digested activated sludge at 37, 55 and 70 °C. Water Res. 2017, 115, 120–129. [Google Scholar] [CrossRef]
- Yilmazel, Y.D.; Johnston, D.; Duran, M. Hyperthermophilic hydrogen production from wastewater biosolids by Caldicellulosiruptor bescii. Int. J. Hydrogen Energy 2015, 40, 12177–12186. [Google Scholar] [CrossRef]
- Saidi, R.; Liebgott, P.P.; Hamdi, M.; Auria, R.; Bouallagui, H. Enhancement of fermentative hydrogen production by Thermotoga maritima through hyperthermophilic anaerobic co-digestion of fruit-vegetable and fish wastes. Int. J. Hydrogen Energy 2018, 43, 23168–23177. [Google Scholar] [CrossRef]
- American Public Health Association. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Arras, W.; Hussain, A.; Hausler, R.; Guiot, S.R. Mesophilic, thermophilic and hyperthermophilic acidogenic fermentation of food waste in batch: Effect of inoculum source. Waste Manag. 2019, 87, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Bayr, S.; Rantanen, M.; Kaparaju, P.; Rintala, J. Mesophilic and thermophilic anaerobic co-digestion of rendering plant and slaughterhouse wastes. Bioresour. Technol. 2012, 104, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Peng, L.; Su, H. The effect of a buffer function on the semi-continuous anaerobic digestion. Bioresour. Technol. 2013, 139, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Dechrugsa, S.; Kantachote, D.; Chaiprapat, S. Effects of inoculum to substrate ratio, substrate mix ratio and inoculum source on batch co-digestion of grass and pig manure. Bioresour. Technol. 2013, 146, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Xie, L.; Kinh, C.T.; Suenaga, T.; Hori, T.; Riya, S.; Terada, A.; Hosomi, M. Influence of feedstock-to-inoculum ratio on performance and microbial community succession during solid-state thermophilic anaerobic co-digestion of pig urine and rice straw. Bioresour. Technol. 2018, 252, 127–133. [Google Scholar] [CrossRef]
- Dhar, B.R.; Elbeshbishy, E.; Hafez, H.; Lee, H.S. Hydrogen production from sugar beet juice using an integrated biohydrogen process of dark fermentation and microbial electrolysis cell. Bioresour. Technol. 2015, 198, 223–230. [Google Scholar] [CrossRef]
- Wicher, E.; Seifert, K.; Zagrodnik, R.; Pietrzyk, B.; Laniecki, M. Hydrogen gas production from distillery wastewater by dark fermentation. Int. J. Hydrogen Energy 2013, 38, 7767–7773. [Google Scholar] [CrossRef]
- Guo, X.M.; Trably, E.; Latrille, E.; Carrre, H.; Steyer, J.P. Hydrogen production from agricultural waste by dark fermentation: A review. Int. J. Hydrogen Energy 2010, 35, 10660–10673. [Google Scholar] [CrossRef]
- Abreu, A.A.; Tavares, F.; Alves, M.M.; Cavaleiro, A.J.; Pereira, M.A. Garden and food waste co-fermentation for biohydrogen and biomethane production in a two-step hyperthermophilic-mesophilic process. Bioresour. Technol. 2019, 278, 180–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Hidaka, T.; Tsuno, H.; Tsubota, J. Co-digestion of polylactide and kitchen garbage in hyperthermophilic and thermophilic continuous anaerobic process. Bioresour. Technol. 2012, 112, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Material | Indicator | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total Solids (g/kg) | Volatile Solids (g/kg) | Volatile Solids (%TS) | Carbon (%TS) | Nitrogen (%TS) | Phosphorus (%TS) | Hydrogen (%TS) | Sulfur (%TS) | C/N | |
| Grass | 127.68 ± 10.51 | 104.18 ± 8.79 | 81.59 ± 3.80 | 59.8 ± 3.22 | 2.63 ± 0.12 | 0.98 ± 0.06 | 5.31 ± 0.45 | 0.89 ± 0.02 | 22.74 |
| Leaves | 928.76 ± 1.70 | 815.55 ± 3.78 | 87.81 ± 0.28 | 58.4 ± 1.95 | 2.91 ± 0.20 | 0.24 ± 0.01 | 5.33 ± 0.36 | 0.36 ± 0.01 | 20.07 |
| Inoculum | 26.29 ± 0.61 | 21.84 ± 0.20 | 83.07 ± 1.34 | 64.6 ± 2.30 | 6.43 ± 0.28 | 1.23 ± 0.06 | 5.82 ± 0.30 | 0.58 ± 0.03 | 10.05 |
| Grass | Leaves | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| I/S | 2:1 | 1:1 | 1:2 | 1:4 | 2:1 | 1:1 | 1:2 | 1:4 | |
| Mass of inoculum added (g) | 500 | 500 | 500 | 500 | 500 | 500 | 500 | 500 | |
| Initial substrate TS content (gTS) | 6.69 | 13.38 | 26.77 | 53.53 | 6.22 | 12.44 | 24.87 | 49.74 | |
| Initial substrate vs. content (gVS) | 5.46 | 10.92 | 21.84 | 43.68 | 5.46 | 10.92 | 21.84 | 43.68 | |
| Duration time (d) | 14 | 14 | 14 | 14 | 14 | 14 | 14 | 14 | |
| Cumulative hydrogen yield (mL/gVS) | 70 °C | 50.09 ± 6.33 | 2.09 ± 0.27 | 2.47 ± 2.45 | 6.23 ± 1.91 | 69.64 ± 10.50 | 42.85 ± 4.68 | 17.52 ± 3.67 | 11.66 ± 2.98 |
| 80 °C | 0.00 ± 0 | 0.00 ± 0 | 0.00 ± 0 | 0.00 ± 0 | 0.00 ± 0 | 0.00 ± 0 | 0.00 ± 0 | 0.00 ± 0 | |
| Cumulative methane yield (mL/gVS) | 70 °C | 1.98 ± 0.47 | 1.81 ± 0.07 | 0.17 ± 0.08 | 0.08 ± 0.04 | 38.63 ± 8.42 | 25.85 ± 4.84 | 1.03 ± 0.03 | 0.38 ± 0.01 |
| 80 °C | 1.53 ± 0.29 | 0.76 ± 0.28 | 0.25 ± 0.004 | 0.17 ± 0.03 | 4.09 ± 1.07 | 0.58 ± 0.06 | 0.35 ± 0.07 | 1.57 ± 0.17 | |
| Final pH | 70 °C | 6.51 ± 0.05 | 6.12 ± 0.7 | 6.05 ± 0.015 | 5.76 ± 0.025 | 7.26 ± 0.08 | 7.27 ± 0.19 | 5.07 ± 0.055 | 4.73 ± 0.07 |
| 80 °C | 6.97 ± 0.15 | 6.52 ± 0.17 | 5.79 ± 0.15 | 5.95 ± 0.03 | 7.16 ± 0.06 | 6.61 ± 0.01 | 5.775 ± 0.075 | 5.155 ± 0.055 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liczbiński, P.; Borowski, S. Hyperthermophilic Treatment of Grass and Leaves to Produce Hydrogen, Methane and VFA-Rich Digestate: Preliminary Results. Energies 2020, 13, 2814. https://doi.org/10.3390/en13112814
Liczbiński P, Borowski S. Hyperthermophilic Treatment of Grass and Leaves to Produce Hydrogen, Methane and VFA-Rich Digestate: Preliminary Results. Energies. 2020; 13(11):2814. https://doi.org/10.3390/en13112814
Chicago/Turabian StyleLiczbiński, Przemysław, and Sebastian Borowski. 2020. "Hyperthermophilic Treatment of Grass and Leaves to Produce Hydrogen, Methane and VFA-Rich Digestate: Preliminary Results" Energies 13, no. 11: 2814. https://doi.org/10.3390/en13112814
APA StyleLiczbiński, P., & Borowski, S. (2020). Hyperthermophilic Treatment of Grass and Leaves to Produce Hydrogen, Methane and VFA-Rich Digestate: Preliminary Results. Energies, 13(11), 2814. https://doi.org/10.3390/en13112814





