Bio-Based Phase Change Materials Incorporated in Lignocellulose Matrix for Energy Storage in Buildings—A Review
Abstract
:1. Introduction
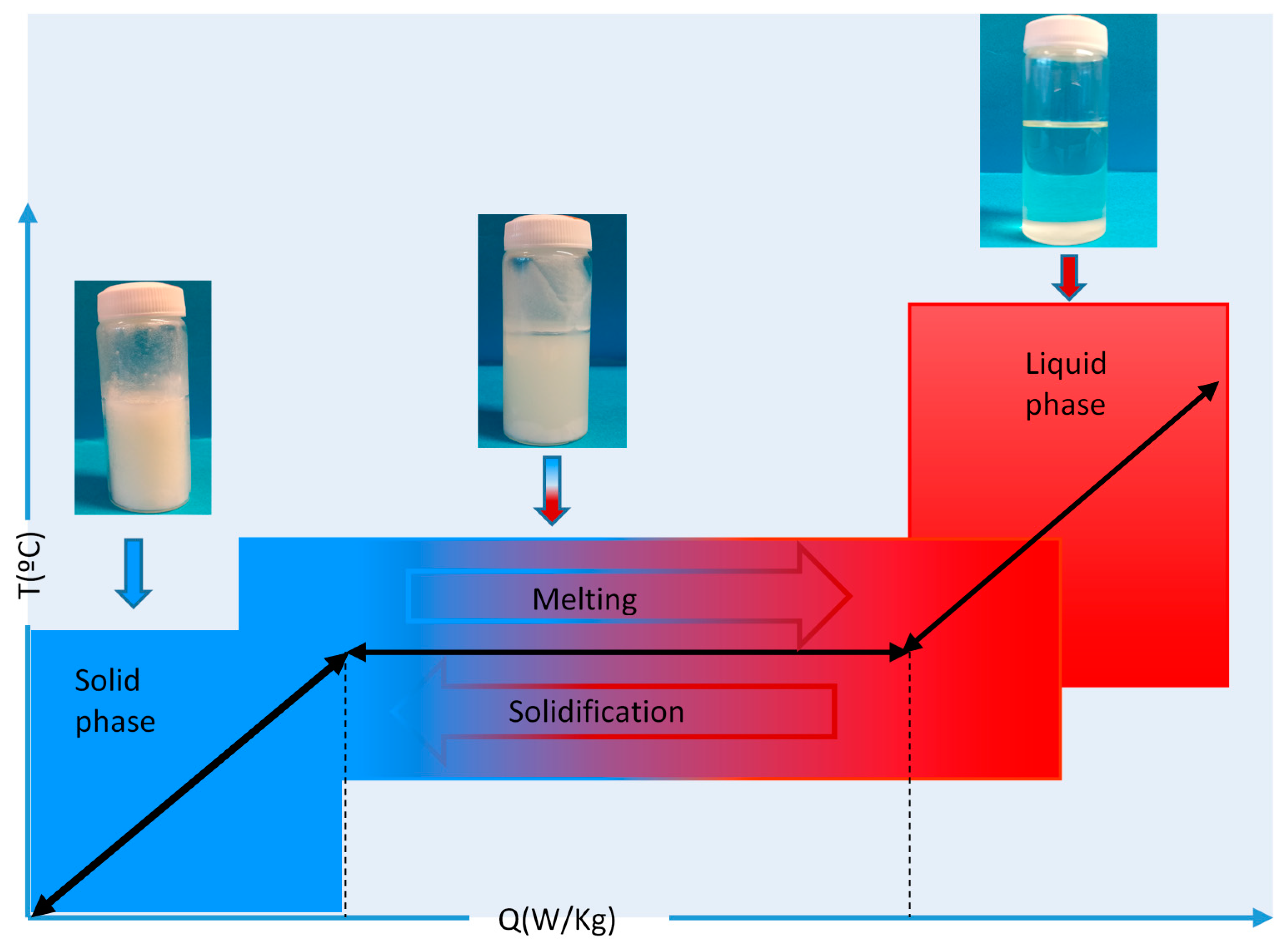
2. Basics in the Phase Change Mechanism, Systems, and Analysis
2.1. Energy Conversion Mechanism
2.2. Energy Storage System Mechanism
2.3. Available Analytical Methods of Energy and Chemical Behavior of PCM
3. Organic PCM (OPCM) for Building Applications
3.1. Paraffins
3.2. Polyethylene Glycols
3.3. Polyols
3.4. Fatty Acids
3.5. Organic-Organic Eutectics and Esters
3.6. Selection Criteria
4. BPCM Containment
4.1. Microencapsulation
4.2. Nano-encapsulation
4.3. Shape Stabilization Using Organic Polymer Matrices
4.4. Shape Stabilization Using Inorganic Porous Materials
4.5. Incorporation of PCM in Lignocellulose Materials
5. Practical Challenges for Application of BPCM in Buildings
6. Life Cycle Assessments
7. Conclusions
- Among the investigated OPCMs, fatty acids, their derivatives, and their eutectic mixtures are the most promising bio-based materials that can be used as PCMs. They have been extensively investigated for solar energy applications but have not been explored for controlling indoor temperature fluctuations.
- BPCMs benefit from some properties such as non-corrosivity, high latent heat of fusion, suitable melting temperature, non-toxicity, reasonable thermal and chemical stability, and lack of any environmental impact.
- In cold temperate regions (e.g., Canada, North-European countries) with few sunny days in the winter, using PCMs for controlling the indoor temperature intermittency is rather interesting than for storing solar energy. However, only a few pure fatty acids have phase change temperatures in the range of human comfort (i.e., 18–25 °C). A further functionalization of the fatty acids is a prerequisite for the application in a building sector.
- Most of the PCMs are incorporated in wallboard and ceiling board gypsum while only a few studies investigated the possibility of incorporating BPCMs into wood and wood-based materials. Using wood as a matrix appears as a significant improvement of the thermal mass of a “green” building.
- Encapsulation and shape stabilization are the most used approaches for incorporating PCMs. Recently, special attention has been paid to shape stabilization using porous materials and fibers. This method is cheaper and have no side effect compared to encapsulation that reduces thermal performance of the PCM.
- The main limiting factor of using BPCMs is their low thermal conductivity, which can be improved by inclusion of metallic, carbon-based nanoparticles as well as by using carbon and a graphite-based scaffold for encapsulation.
- Wood and wood-based materials, e.g., delignified wood and carbonized wood, wood-flour, and wood fibers are interesting biomaterials for cost-effective and shape-stabilized BPCMs. Utilization of these materials will result in lightweight construction materials and enhancement in the thermal mass of buildings.
- Use of BPCM can introduce a negative impact on the environment and, thus, LCA appears as a compulsory tool for selecting BPCM with a low impact for building applications.
Author Contributions
Funding
Conflicts of Interest
References
- Pasupathy, A.; Velraj, R.; Seeniraj, R. Phase change material-based building architecture for thermal management in residential and commercial establishments. Renew. Sustain. Energy Rev. 2008, 12, 39–64. [Google Scholar] [CrossRef]
- Tyagi, V.; Kaushik, S.; Tyagi, S.; Akiyama, T. Development of phase change materials based microencapsulated technology for buildings: A review. Renew. Sustain. Energy Rev. 2011, 15, 1373–1391. [Google Scholar] [CrossRef]
- Bland, A.; Khzouz, M.; Statheros, T.; Gkanas, E.I. PCMs for Residential Building Applications: A Short Review Focused on Disadvantages and Proposals for Future Development. Buildings 2017, 7, 78. [Google Scholar] [CrossRef] [Green Version]
- Souayfane, F.; Fardoun, F.; Biwole, P.H. Phase change materials (PCM) for cooling applications in buildings: A review. Energy Build. 2016, 129, 396–431. [Google Scholar] [CrossRef]
- Kenisarin, M.M.; Mahkamov, K. Passive thermal control in residential buildings using phase change materials. Renew. Sustain. Energy Rev. 2016, 55, 371–398. [Google Scholar] [CrossRef]
- Kuznik, F.; David, D.; Johannes, K.; Roux, J.-J. A review on phase change materials integrated in building walls. Renew. Sustain. Energy Rev. 2011, 15, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Mathis, D.; Blanchet, P.; Landry, V.; Lagiere, P. Thermal characterization of bio-based phase changing materials in decorative wood-based panels for thermal energy storage. Green Energy Environ. 2019, 4, 56–65. [Google Scholar] [CrossRef]
- Mathis, D.; Blanchet, P.; Landry, V.; Lagière, P. Impregnation of Wood with Microencapsulated Bio-Based Phase Change Materials for High Thermal Mass Engineered Wood Flooring. Appl. Sci. 2018, 8, 2696. [Google Scholar] [CrossRef] [Green Version]
- Asdrubali, F.; Ferracuti, B.; Lombardi, L.; Guattari, C.; Evangelisti, L.; Grazieschi, G. A review of structural, thermo-physical, acoustical, and environmental properties of wooden materials for building applications. Build. Environ. 2017, 114, 307–332. [Google Scholar] [CrossRef]
- Chen, F.; Kessel, A.; Wolcott, M. A novel energy saving wood product with phase change materials. In Proceedings of the 55th International Convention of Society of Wood Science and Technology, Beijing, China, 27–31 August 2012. [Google Scholar]
- Kalnæs, S.E.; Jelle, B. Phase change materials and products for building applications: A state-of-the-art review and future research opportunities. Energy Build. 2015, 94, 150–176. [Google Scholar] [CrossRef] [Green Version]
- Agyenim, F.; Hewitt, N.J.; Eames, P.; Smyth, M. A review of materials, heat transfer and phase change problem formulation for latent heat thermal energy storage systems (LHTESS). Renew. Sustain. Energy Rev. 2010, 14, 615–628. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Osorio, F.J.B.; Isaza-Ruiz, M.; Xu, X.; Vignarooban, K.; Phelan, P.; Inamuddin; Kannan, A.N.M. Recent developments in phase change materials for energy storage applications: A review. Int. J. Heat Mass Transf. 2019, 129, 491–523. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Barreneche, C.; Martorell, I.; Miró, L.; Sari-Bey, S.; Fois, M.; Paksoy, H.; Sahan, N.; Weber, R.R.; Constantinescu, M.; et al. Unconventional experimental technologies available for phase change materials (PCM) characterization. Part 1. Thermophysical properties. Renew. Sustain. Energy Rev. 2015, 43, 1399–1414. [Google Scholar] [CrossRef] [Green Version]
- Konuklu, Y.; Ostry, M.; Paksoy, H.; Charvát, P. Review on using microencapsulated phase change materials (PCM) in building applications. Energy Build. 2015, 106, 134–155. [Google Scholar] [CrossRef]
- Umair, M.M.; Zhang, Y.; Iqbal, K.; Zhang, S.; Tang, B. Novel strategies and supporting materials applied to shape-stabilize organic phase change materials for thermal energy storage—A review. Appl. Energy 2019, 235, 846–873. [Google Scholar] [CrossRef]
- Wei, G.; Wang, G.; Xu, C.; Ju, X.; Ju, X.; Du, X.; Yang, Y. Selection principles and thermophysical properties of high temperature phase change materials for thermal energy storage: A review. Renew. Sustain. Energy Rev. 2018, 81, 1771–1786. [Google Scholar] [CrossRef]
- Khadiran, T.; Hussein, M.Z.; Zainal, Z.; Rusli, R. Encapsulation techniques for organic phase change materials as thermal energy storage medium: A review. Sol. Energy Mater. Sol. Cells 2015, 143, 78–98. [Google Scholar] [CrossRef]
- Baetens, R.; Jelle, B.; Gustavsen, A. Phase change materials for building applications: A state-of-the-art review. Energy Build. 2010, 42, 1361–1368. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Tyagi, V.; Chen, C.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Sarier, N.; Onder, E. Organic phase change materials and their textile applications: An overview. Thermochim. Acta 2012, 540, 7–60. [Google Scholar] [CrossRef]
- Sharma, R.; Ganesan, P.B.; Tyagi, V.; Metselaar, H.S.C.; Sandaran, S. Developments in organic solid–liquid phase change materials and their applications in thermal energy storage. Energy Convers. Manag. 2015, 95, 193–228. [Google Scholar] [CrossRef] [Green Version]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater. Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Rathod, M.K.; Banerjee, J. Thermal stability of phase change materials used in latent heat energy storage systems: A review. Renew. Sustain. Energy Rev. 2013, 18, 246–258. [Google Scholar] [CrossRef]
- Amaral, C.; Vicente, R.; Marques, P.A.; Barros-Timmons, A. Phase change materials and carbon nanostructures for thermal energy storage: A literature review. Renew. Sustain. Energy Rev. 2017, 79, 1212–1228. [Google Scholar] [CrossRef]
- Turkyilmazoglu, M. Stefan problems for moving phase change materials and multiple solutions. Int. J. Therm. Sci. 2018, 126, 67–73. [Google Scholar] [CrossRef]
- Kośny, J.; Kossecka, E.; Brzeziński, A.; Tleoubaev, A.; Yarbrough, D. Dynamic thermal performance analysis of fiber insulations containing bio-based phase change materials (PCMs). Energy Build. 2012, 52, 122–131. [Google Scholar] [CrossRef]
- Ye, R.; Lin, W.; Fang, X.; Zhang, Z. A numerical study of building integrated with CaCl2· 6H2O/expanded graphite composite phase change material. App. Therm. Eng. 2017, 126, 480–488. [Google Scholar] [CrossRef]
- Ogoh, W.; Groulx, D. October. Stefan’s problem: Validation of a one–dimensional solid–liquid phase change heat transfer process. In Procceedings of the Excerpt from the Proceedings of the COMSOL Conference; ResearchGate: Boston, MA, USA, 2010. [Google Scholar]
- Gulfam, R.; Zhang, P.; Meng, Z. Advanced thermal systems driven by paraffin-based phase change materials—A review. Appl. Energy 2019, 238, 582–611. [Google Scholar] [CrossRef]
- Jeong, S.-G.; Chung, O.; Yu, S.; Kim, S.; Kim, S. Improvement of the thermal properties of Bio-based PCM using exfoliated graphite nanoplatelets. Sol. Energy Mater. Sol. Cells 2013, 117, 87–92. [Google Scholar] [CrossRef]
- Yu, S.; Jeong, S.-G.; Chung, O.; Kim, S. Bio-based PCM/carbon nanomaterials composites with enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2014, 120, 549–554. [Google Scholar] [CrossRef]
- Jeong, S.-G.; Lee, J.-H.; Seo, J.; Kim, S. Thermal performance evaluation of Bio-based shape stabilized PCM with boron nitride for energy saving. Int. J. Heat Mass Transf. 2014, 71, 245–250. [Google Scholar] [CrossRef]
- Kaizawa, A.; Maruoka, N.; Kawai, A.; Kamano, H.; Jozuka, T.; Senda, T.; Akiyama, T. Thermophysical and heat transfer properties of phase change material candidate for waste heat transportation system. Heat Mass Transf. 2007, 44, 763–769. [Google Scholar] [CrossRef]
- Jiang, Y.; Hussain, H.; Kressler, J. Poly(vinyl alcohol) Cryogel Formation Using Biocompatible Ice Nucleating Agents. Macromol. Mater. Eng. 2014, 300, 181–190. [Google Scholar] [CrossRef]
- Elefsiniotis, A.; Becker, T.; Schmid, U. Thermoelectric Energy Harvesting Using Phase Change Materials (PCMs) in High Temperature Environments in Aircraft. J. Electron. Mater. 2013, 43, 1809–1814. [Google Scholar] [CrossRef]
- Solé, A.; Neumann, H.; Niedermaier, S.; Martorell, I.; Schossig, P.; Cabeza, L.F. Stability of sugar alcohols as PCM for thermal energy storage. Sol. Energy Mater. Sol. Cells 2014, 126, 125–134. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, N.; Tao, W.; Cao, X.; He, Y. Fatty acids as phase change materials: A review. Renew. Sustain. Energy Rev. 2014, 29, 482–498. [Google Scholar] [CrossRef]
- Rozanna, D.; Chuah, T.G.; Salmiah, A.; Choong, T.S.Y.; Sa’Ari, M. Fatty Acids as Phase Change Materials (PCMs) for Thermal Energy Storage: A Review. Int. J. Green Energy 2005, 1, 495–513. [Google Scholar] [CrossRef]
- Feldman, D.; Shapiro, M.; Banu, D. Organic phase change materials for thermal energy storage. Sol. Energy Mater. 1986, 13, 1–10. [Google Scholar] [CrossRef]
- Feldman, D.; Banu, D.; Hawes, D. Development and application of organic phase change mixtures in thermal storage gypsum wallboard. Sol. Energy Mater. Sol. Cells 1995, 36, 147–157. [Google Scholar] [CrossRef]
- Hasan, A. Thermal energy storage system with stearic acid as phase change material. Energy Convers. Manag. 1994, 35, 843–856. [Google Scholar] [CrossRef]
- Hasan, A. Phase change material energy storage system employing palmitic acid. Sol. Energy 1994, 52, 143–154. [Google Scholar] [CrossRef]
- Sarı, A.; Sarı, A.; Kaygusuz, K. Thermal energy storage system using stearic acid as a phase change material. Sol. Energy 2001, 71, 365–376. [Google Scholar] [CrossRef]
- Sarı, A.; Kaygusuz, K. Thermal performance of myristic acid as a phase change material for energy storage application. Renew. Energy 2001, 24, 303–317. [Google Scholar] [CrossRef]
- Cedeño, F.O.; Prieto, M.M.; Espina, A.; García, J.R. Measurements of temperature and melting heat of some pure fatty acids and their binary and ternary mixtures by differential scanning calorimetry. Thermochim. Acta 2001, 369, 39–50. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, C.; Alva, G.; Fang, G. Palmitic acid/polyvinyl butyral/expanded graphite composites as form-stable phase change materials for solar thermal energy storage. Appl. Energy 2018, 228, 1801–1809. [Google Scholar] [CrossRef]
- Sharma, R.K.; Ganesan, P.B.; Tyagi, V.V. Long-term thermal and chemical reliability study of different organic phase change materials for thermal energy storage applications. J. Therm. Anal. Calorim. 2016, 124, 1357–1366. [Google Scholar] [CrossRef]
- Nikolic, R.; Marinović-Cincović, M.; Gadzuric, S.; Zsigrai, I. New materials for solar thermal storage—Solid/liquid transitions in fatty acid esters. Sol. Energy Mater. Sol. Cells 2003, 79, 285–292. [Google Scholar] [CrossRef]
- Sarı, A. Eutectic mixtures of some fatty acids for low temperature solar heating applications: Thermal properties and thermal reliability. Appl. Therm. Eng. 2005, 25, 2100–2107. [Google Scholar] [CrossRef]
- Sarı, A.; Biçer, A.; Karaipekli, A. Synthesis, characterization, thermal properties of a series of stearic acid esters as novel solid–liquid phase change materials. Mater. Lett. 2009, 63, 1213–1216. [Google Scholar] [CrossRef]
- Sarı, A.; Sarı, A. Thermal energy storage properties of mannitol–fatty acid esters as novel organic solid–liquid phase change materials. Energy Convers. Manag. 2012, 64, 68–78. [Google Scholar] [CrossRef]
- Aydın, A.A.; Okutan, H. High-chain fatty acid esters of myristyl alcohol with odd carbon number: Novel organic phase change materials for thermal energy storage—2. Sol. Energy Mater. Sol. Cells 2011, 95, 2417–2423. [Google Scholar] [CrossRef]
- Aydın, A.A. Diesters of high-chain dicarboxylic acids with 1-tetradecanol as novel organic phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2012, 104, 102–108. [Google Scholar] [CrossRef]
- Aydın, A.A.; Aydın, A. High–chain fatty acid esters of 1–hexadecanol for low temperature thermal energy storage with phase change materials. Sol. Energy Mater. Sol. Cells 1996, 96, 93–100. [Google Scholar]
- Xu, S.; Zou, L.; Ling, X.; Wei, Y.; Zhang, S. Preparation and thermal reliability of methyl palmitate/methyl stearate mixture as a novel composite phase change material. Energy Build. 2014, 68, 372–375. [Google Scholar] [CrossRef]
- Shilei, L.; Neng, Z.; Guohui, F. Eutectic mixtures of capric acid and lauric acid applied in building wallboards for heat energy storage. Energy Build. 2006, 38, 708–711. [Google Scholar] [CrossRef]
- Dimaano, M.N.R.; Watanabe, T. The capric–lauric acid and pentadecane combination as phase change material for cooling applications. Appl. Therm. Eng. 2002, 22, 365–377. [Google Scholar] [CrossRef]
- Pielichowski, K.; Flejtuch, K.; Pielichowska, K. Differential Scanning Calorimetry Study of Blends of Poly(ethylene glycol) with Selected Fatty Acids. Macromol. Mater. Eng. 2003, 288, 259–264. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Preparation, thermal properties and thermal reliability of eutectic mixtures of fatty acids/expanded vermiculite as novel form-stable composites for energy storage. J. Ind. Eng. Chem. 2010, 16, 767–773. [Google Scholar] [CrossRef]
- Sharma, A.; Shukla, A.; Chen, C.; Dwivedi, S. Development of phase change materials for building applications. Energy Build. 2013, 64, 403–407. [Google Scholar] [CrossRef]
- Ke, H.; Li, D.; Zhang, H.; Wang, X.; Cai, Y.; Huang, F.; Wei, Q. Electrospun form-stable phase change composite nanofibers consisting of capric acid-based binary fatty acid eutectics and polyethylene terephthalate. Fibers Polym. 2013, 14, 89–99. [Google Scholar] [CrossRef]
- Hawes, D.; Feldman, D.; Banu, D. Latent heat storage in building materials. Energy Build. 1993, 20, 77–86. [Google Scholar] [CrossRef]
- Hawlader, M.; Uddin, M.; Khin, M.M. Microencapsulated PCM thermal-energy storage system. Appl. Energy 2003, 74, 195–202. [Google Scholar] [CrossRef]
- Saihi, D.; Vroman, I.; Giraud, S.; Bourbigot, S. Microencapsulation of ammonium phosphate with a polyurethane shell part I: Coacervation technique. React. Funct. Polym. 2005, 64, 127–138. [Google Scholar] [CrossRef]
- Liang, C.; Lingling, X.; Hongbo, S.; Zhibin, Z. Microencapsulation of butyl stearate as a phase change material by interfacial polycondensation in a polyurea system. Energy Convers. Manag. 2009, 50, 723–729. [Google Scholar] [CrossRef]
- Cho, J.-S.; Kwon, A.; Cho, C.G. Microencapsulation of octadecane as a phase-change material by interfacial polymerization in an emulsion system. Colloid Polym. Sci. 2002, 280, 260–266. [Google Scholar] [CrossRef]
- Kaygusuz, K.; Alkan, C.; Sarı, A.; Uzun, O. Encapsulated Fatty Acids in an Acrylic Resin as Shape-stabilized Phase Change Materials for Latent Heat Thermal Energy Storage. Energy Sources, Part. A: Recover. Util. Environ. Eff. 2008, 30, 1050–1059. [Google Scholar] [CrossRef]
- Su, J.-F.; Wang, L.-X.; Ren, L. Preparation and characterization of double-MF shell microPCMs used in building materials. J. Appl. Polym. Sci. 2005, 97, 1755–1762. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Yu, F.; Zhang, Z.; Gao, X. Preparation and properties of graphene oxide-modified poly(melamine-formaldehyde) microcapsules containing phase change material n-dodecanol for thermal energy storage. J. Mater. Chem. A 2015, 3, 11624–11630. [Google Scholar] [CrossRef]
- Su, J.; Wang, L.; Ren, L. Fabrication and thermal properties of microPCMs: Used melamine-formaldehyde resin as shell material. J. Appl. Polym. Sci. 2006, 101, 1522–1528. [Google Scholar] [CrossRef]
- Yoo, Y.; Martinez, C.; Youngblood, J.P. Synthesis and Characterization of Microencapsulated Phase Change Materials with Poly(urea−urethane) Shells Containing Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2017, 9, 31763–31776. [Google Scholar] [CrossRef]
- Park, S.; Lee, Y.; Kim, Y.S.; Lee, H.M.; Kim, J.H.; Cheong, I.W.; Koh, W.-G. Magnetic nanoparticle-embedded PCM nanocapsules based on paraffin core and polyurea shell. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 46–51. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X. Synthesis and properties of microencapsulated n-octadecane with polyurea shells containing different soft segments for heat energy storage and thermal regulation. Sol. Energy Mater. Sol. Cells 2009, 93, 1366–1376. [Google Scholar] [CrossRef]
- Sukhorukov, G.; Fery, A.; Möhwald, H. Intelligent micro- and nanocapsules. Prog. Polym. Sci. 2005, 30, 885–897. [Google Scholar] [CrossRef]
- Shchukina, E.; Graham, M.; Zheng, Z.; Shchukin, D. Nanoencapsulation of phase change materials for advanced thermal energy storage systems. Chem. Soc. Rev. 2018, 47, 4156–4175. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Rao, Z.; Zhao, J.; Huo, Y.; Li, Y. Review on nanoencapsulted phase change materials: Preparation, Characterization and Heat transfer enhancement. Nano Energy 2015, 13, 814–826. [Google Scholar] [CrossRef]
- Rodríguez-Cumplido, F.; Gelves, E.P.; Chejne-Jana, F. Recent developments in the synthesis of microencapsulated and nanoencapsulated phase change materials. J. Energy Storage 2019, 24, 100821. [Google Scholar] [CrossRef]
- Fuensanta, M.; Paiphansiri, U.; Romero-Sánchez, M.D.; Guillem, C.; Buendia, A.M.L.; Landfester, K. Thermal properties of a novel nanoencapsulated phase change material for thermal energy storage. Thermochim. Acta 2013, 565, 95–101. [Google Scholar] [CrossRef]
- Fang, G.; Chen, Z.; Li, H. Synthesis and properties of microencapsulated paraffin composites with SiO2 shell as thermal energy storage materials. Chem. Eng. J. 2010, 163, 154–159. [Google Scholar] [CrossRef]
- Cao, L.; Tang, F.; Fang, G. Preparation and characteristics of microencapsulated palmitic acid with TiO2 shell as shape-stabilized thermal energy storage materials. Sol. Energy Mater. Sol. Cells 2014, 123, 183–188. [Google Scholar] [CrossRef]
- Pan, L.; Tao, Q.; Zhang, S.; Wang, S.; Zhang, J.; Wang, S.; Wang, Z.; Zhang, Z. Preparation, characterization and thermal properties of micro-encapsulated phase change materials. Sol. Energy Mater. Sol. Cells 2012, 98, 66–70. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Wu, D. Fabrication of multifunctional microcapsules containing n -eicosane core and zinc oxide shell for low-temperature energy storage, photocatalysis, and antibiosis. Energy Convers. Manag. 2015, 106, 873–885. [Google Scholar] [CrossRef]
- Teng, T.-P.; Yu, C.-C. Characteristics of phase-change materials containing oxide nano-additives for thermal storage. Nanoscale Res. Lett. 2012, 7, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, X.; Wang, S.; Du, Z.; Cheng, X.; Wang, H. Preparation and characterization of flame–retardant nanoencapsulated phase change materials with poly(methyl methacrylate) shells for thermal energy storage. J. Mat. Chem. A 2018, 6, 17519–17529. [Google Scholar] [CrossRef]
- Yuan, H.; Bai, H.; Zhang, J.; Zhang, Z. Synthesis of Nanoencapsulated Phase Change Materials with Ag Shell for Thermal Energy Storage. In Proceedings of the 11th International Conference on Porous Metals and Metallic Foams (MetFoam 2019) Dearborn, MI, USA; Springer Science and Business Media LLC: Cham, Switzerland, 2020; pp. 687–694. [Google Scholar]
- De Matteis, V.; Cannavale, A.; Martellotta, F.; Rinaldi, R.; Calcagnile, P.; Ferrari, F.; Ayr, U.; Fiorito, F. Nano-encapsulation of phase change materials: From design to thermal performance, simulations and toxicological assessment. Energy Build. 2019, 188–189, 1–11. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, J.; Cui, Y.; Cheng, W. Synthesis of bifunctional nanoencapsulated phase change materials with nano-TiO2 modified polyacrylate shell for thermal energy storage and ultraviolet absorption. Polym. Int. 2019, 69, 140–148. [Google Scholar] [CrossRef]
- Imran Hussain, S.; Ameelia Roseline, A.; Kalaiselvam, S. Bifunctional nanoencapsulated eutectic phase change materials core with SiO2/SnO2 nanosphere shell for thermal and electrical energy storage. Mat. Design 2018, 154, 291–301. [Google Scholar]
- Zhang, Y.; Xiu, J.; Tang, B.; Lu, R.; Zhang, S.-F. Novel semi-interpenetrating network structural phase change composites with high phase change enthalpy. AIChE J. 2017, 64, 688–696. [Google Scholar] [CrossRef]
- Regin, A.F.; Solanki, S.; Saini, J. Heat transfer characteristics of thermal energy storage system using PCM capsules: A review. Renew. Sustain. Energy Rev. 2008, 12, 2438–2458. [Google Scholar] [CrossRef]
- Wang, C.; Feng, L.; Li, W.; Zheng, J.; Tian, W.; Li, X. Shape-stabilized phase change materials based on polyethylene glycol/porous carbon composite: The influence of the pore structure of the carbon materials. Sol. Energy Mater. Sol. Cells 2012, 105, 21–26. [Google Scholar] [CrossRef]
- Krupa, I.; Miková, G.; Luyt, A.S. Phase change materials based on low-density polyethylene/paraffin wax blends. Eur. Polym. J. 2007, 43, 4695–4705. [Google Scholar] [CrossRef]
- Sánchez, L.; Lacasa, E.; Carmona, M.; Rodríguez, J.; Sánchez, P.; Silva, M.L.S.; Rodríguez, J. Applying an Experimental Design to Improve the Characteristics of Microcapsules Containing Phase Change Materials for Fabric Uses. Ind. Eng. Chem. Res. 2008, 47, 9783–9790. [Google Scholar] [CrossRef]
- Alkan, C.; Sarı, A. Fatty acid/poly(methyl methacrylate) (PMMA) blends as form-stable phase change materials for latent heat thermal energy storage. Sol. Energy 2008, 82, 118–124. [Google Scholar] [CrossRef]
- Wang, L.; Meng, D. Fatty acid eutectic/polymethyl methacrylate composite as form-stable phase change material for thermal energy storage. Appl. Energy 2010, 87, 2660–2665. [Google Scholar] [CrossRef]
- Wang, X.; Yu, X.; Tian, C.; Wang, J. Preparation and characterization of form-stable paraffin/polyurethane composites as phase change materials for thermal energy storage. Energy Convers. Manag. 2014, 77, 13–21. [Google Scholar] [CrossRef]
- Silakhori, M.; Metselaar, H.S.C.; Mahlia, T.M.I.; Fauzi, H.; Baradaran, S.; Naghavi, M.S. Palmitic acid/polypyrrole composites as form-stable phase change materials for thermal energy storage. Energy Convers. Manag. 2014, 80, 491–497. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Q.; Huang, F.; Lin, S.; Chen, F.; Gao, W. Thermal stability, latent heat and flame retardant properties of the thermal energy storage phase change materials based on paraffin/high density polyethylene composites. Renew. Energy 2009, 34, 2117–2123. [Google Scholar] [CrossRef]
- Li, Z.; He, W.; Xu, J.; Jiang, M. Preparation and characterization of in situ grafted/crosslinked polyethylene glycol/polyvinyl alcohol composite thermal regulating fiber. Sol. Energy Mater. Sol. Cells 2015, 140, 193–201. [Google Scholar] [CrossRef]
- Sari, A.; Kaygusuz, K. Poly (vinyl alcohol)/fatty acid blends for thermal energy storage. Energy Sources 2007, 29, 873–883. [Google Scholar] [CrossRef]
- Sari, A.; Akcay, M.; Soylak, M. Polymer–stearic acid blends as form–stable phase change material for thermal energy storage. Energy Sources 2005, 27, 1535–1546. [Google Scholar]
- Şentürk, S.B.; Kahraman, D.; Alkan, C.; Gokce, I. Biodegradable PEG/cellulose, PEG/agarose and PEG/chitosan blends as shape stabilized phase change materials for latent heat energy storage. Carbohydr. Polym. 2011, 84, 141–144. [Google Scholar] [CrossRef]
- Prajapati, D.G.; Kandasubramanian, B. Biodegradable Polymeric Solid Framework-Based Organic Phase-Change Materials for Thermal Energy Storage. Ind. Eng. Chem. Res. 2019, 58, 10652–10677. [Google Scholar] [CrossRef]
- Sarı, A.; Alkan, C.; Karaipekli, A.; Önal, A. Preparation, characterization and thermal properties of styrene maleic anhydride copolymer (SMA)/fatty acid composites as form stable phase change materials. Energy Convers. Manag. 2008, 49, 373–380. [Google Scholar]
- Pielichowska, K.; Pielichowski, K. Biodegradable PEO/cellulose-based solid-solid phase change materials. Polym. Adv. Technol. 2010, 22, 1633–1641. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Novel biodegradable form stable phase change materials: Blends of poly(ethylene oxide) and gelatinized potato starch. J. Appl. Polym. Sci. 2010, 116, 1725–1731. [Google Scholar] [CrossRef]
- Cao, L.; Tang, Y.; Fang, G. Preparation and properties of shape-stabilized phase change materials based on fatty acid eutectics and cellulose composites for thermal energy storage. Energy 2015, 80, 98–103. [Google Scholar] [CrossRef]
- Özonur, Y.; Mazman, M.; Paksoy, H.; Evliya, H. Microencapsulation of coco fatty acid mixture for thermal energy storage with phase change material. Int. J. Energy Res. 2006, 30, 741–749. [Google Scholar] [CrossRef]
- Lv, P.; Liu, C.; Rao, Z. Review on clay mineral-based form-stable phase change materials: Preparation, characterization and applications. Renew. Sustain. Energy Rev. 2017, 68, 707–726. [Google Scholar] [CrossRef]
- Liu, S.; Han, L.; Xie, S.; Jia, Y.; Sun, J.; Jing, Y.; Zhang, Q. A novel medium-temperature form-stable phase change material based on dicarboxylic acid eutectic mixture/expanded graphite composites. Sol. Energy 2017, 143, 22–30. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Gubbins, K.E. Free energy studies of freezing in slit pores: An order–parameter approach using Monte Carlo simulation. Mol. Phys. 1999, 96, 1249–1267. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Gubbins, K.E.; Watanabe, A.; Kaneko, K. Freezing of simple fluids in microporous activated carbon fibers: Comparison of simulation and experiment. J. Chem. Phys. 1999, 111, 9058–9067. [Google Scholar] [CrossRef]
- Sarı, A.; Karaipekli, A. Preparation, thermal properties and thermal reliability of palmitic acid/expanded graphite composite as form-stable PCM for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 571–576. [Google Scholar] [CrossRef]
- Wang, S.; Qin, P.; Fang, X.; Zhang, Z.; Wang, S.; Liu, X. A novel sebacic acid/expanded graphite composite phase change material for solar thermal medium-temperature applications. Sol. Energy 2014, 99, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Liu, T.; Hu, L.; Wang, Y.; Nie, S. Facile preparation and adjustable thermal property of stearic acid–graphene oxide composite as shape-stabilized phase change material. Chem. Eng. J. 2013, 215, 819–826. [Google Scholar] [CrossRef]
- Mehrali, M.; Latibari, S.T.; Mehrali, M.; Mahlia, T.M.I.; Metselaar, H.S.C.; Sanjani, M.S.N.; Sadeghinezhad, E.; Akhiani, A.R. Preparation and characterization of palmitic acid/graphene nanoplatelets composite with remarkable thermal conductivity as a novel shape-stabilized phase change material. Appl. Therm. Eng. 2013, 61, 633–640. [Google Scholar] [CrossRef]
- Ince, Ş.; Seki, Y.; Ezan, M.A.; Turgut, A.; Erek, A. Thermal properties of myristic acid/graphite nanoplates composite phase change materials. Renew. Energy 2015, 75, 243–248. [Google Scholar] [CrossRef]
- Sheng, N.; Nomura, T.; Zhu, C.; Habazaki, H.; Akiyama, T. Cotton-derived carbon sponge as support for form-stabilized composite phase change materials with enhanced thermal conductivity. Sol. Energy Mater. Sol. Cells 2019, 192, 8–15. [Google Scholar] [CrossRef]
- Liang, J.; Zhimeng, L.; Ye, Y.; Yanjun, W.; Jingxin, L.; Changlin, Z. Fabrication and characterization of fatty acid/wood-flour composites as novel form-stable phase change materials for thermal energy storage. Energy Build. 2018, 171, 88–99. [Google Scholar] [CrossRef]
- Yang, Z.; Deng, Y.; Li, J. Preparation of porous carbonized woods impregnated with lauric acid as shape-stable composite phase change materials. Appl. Therm. Eng. 2019, 150, 967–976. [Google Scholar] [CrossRef]
- Li, J.; Xue, P.; He, H.; Ding, W.; Han, J. Preparation and application effects of a novel form-stable phase change material as the thermal storage layer of an electric floor heating system. Energy Build. 2009, 41, 871–880. [Google Scholar] [CrossRef]
- Yang, H.; Chao, W.; Wang, S.; Yu, Q.; Cao, G.; Yang, T.; Liu, F.; Di, X.; Li, J.; Wang, C.; et al. Self-luminous wood composite for both thermal and light energy storage. Energy Storage Mater. 2019, 18, 15–22. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Yu, Q.; Cao, G.; Yang, R.; Ke, J.; Di, X.; Liu, F.; Zhang, W.; Wang, C. Composite phase change materials with good reversible thermochromic ability in delignified wood substrate for thermal energy storage. Appl. Energy 2018, 212, 455–464. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Q.; Li, L. Delignified wood/capric acid-palmitic acid mixture stable-form phase change material for thermal storage. Sol. Energy Mater. Sol. Cells 2019, 194, 215–221. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.; Barzin, R.; Farid, M. Composite of wood-plastic and micro-encapsulated phase change material (MEPCM) used for thermal energy storage. Appl. Therm. Eng. 2017, 112, 82–88. [Google Scholar] [CrossRef]
- Barreneche, C.; Vecstaudza, J.; Bajare, D.; Fernandez, A. PCM/wood composite to store thermal energy in passive building envelopes. IOP Conf. Ser. Mater. Sci. Eng. 2017, 251, 12111. [Google Scholar] [CrossRef]
- Guo, X.; Cao, J.; Peng, Y.; Liu, R. Incorporation of microencapsulated dodecanol into wood flour/high-density polyethylene composite as a phase change material for thermal energy storage. Mater. Des. 2016, 89, 1325–1334. [Google Scholar] [CrossRef]
- Guo, X.; Zhang, S.; Cao, J. An energy-efficient composite by using expanded graphite stabilized paraffin as phase change material. Compos. Part A Appl. Sci. Manuf. 2018, 107, 83–93. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Mehling, H.; Hiebler, S.; Ziegler, F. Heat transfer enhancement in water when used as PCM in thermal energy storage. Appl. Therm. Eng. 2002, 22, 1141–1151. [Google Scholar] [CrossRef]
- A Khateeb, S.; Farid, M.; Selman, J.; Al-Hallaj, S. Design and simulation of a lithium-ion battery with a phase change material thermal management system for an electric scooter. J. Power Sources 2004, 128, 292–307. [Google Scholar] [CrossRef]
- Py, X.; Olives, R.; Mauran, S. Paraffin/porous-graphite-matrix composite as a high and constant power thermal storage material. Int. J. Heat Mass Transf. 2001, 44, 2727–2737. [Google Scholar] [CrossRef]
- Sedeh, M.M.; Khodadadi, J. Thermal conductivity improvement of phase change materials/graphite foam composites. Carbon 2013, 60, 117–128. [Google Scholar] [CrossRef]
- Li, M. A nano-graphite/paraffin phase change material with high thermal conductivity. Appl. Energy 2013, 106, 25–30. [Google Scholar] [CrossRef]
- Fukai, J.; Hamada, Y.; Morozumi, Y.; Miyatake, O. Effect of carbon-fiber brushes on conductive heat transfer in phase change materials. Int. J. Heat Mass Transf. 2002, 45, 4781–4792. [Google Scholar] [CrossRef]
- Fukai, J.; Kanou, M.; Kodama, Y.; Miyatake, O. Thermal conductivity enhancement of energy storage media using carbon fibers. Energy Convers. Manag. 2000, 41, 1543–1556. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, C.; Hu, S.; Yu, X. The experimental exploration of carbon nanofiber and carbon nanotube additives on thermal behavior of phase change materials. Sol. Energy Mater. Sol. Cells 2011, 95, 1208–1212. [Google Scholar] [CrossRef]
- Fan, L.-W.; Fang, X.; Wang, X.; Zeng, Y.; Xiao, Y.-Q.; Yu, Z.-T.; Xu, X.; Hu, Y.-C.; Cen, K. Effects of various carbon nanofillers on the thermal conductivity and energy storage properties of paraffin-based nanocomposite phase change materials. Appl. Energy 2013, 110, 163–172. [Google Scholar] [CrossRef]
- Parameshwaran, R.; Jayavel, R.; Kalaiselvam, S. Study on thermal properties of organic ester phase-change material embedded with silver nanoparticles. J. Therm. Anal. Calorim. 2013, 114, 845–858. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J.; Yan, J. Enhanced thermal conductivity and thermal performance of form-stable composite phase change materials by using β-Aluminum nitride. Appl. Energy 2009, 86, 1196–1200. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Yu, Q.; Cao, G.; Sun, X.; Yang, R.; Zhang, Q.; Liu, F.; Di, X.; Li, J.; et al. Low-cost, three-dimension, high thermal conductivity, carbonized wood-based composite phase change materials for thermal energy storage. Energy 2018, 159, 929–936. [Google Scholar] [CrossRef]
- Li, J.; Xue, P.; Ding, W.; Han, J.; Sun, G. Micro-encapsulated paraffin/high-density polyethylene/wood flour composite as form-stable phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells 2009, 93, 1761–1767. [Google Scholar] [CrossRef]
- Singh, R.; Sadeghi, S.; Shabani, B. Thermal Conductivity Enhancement of Phase Change Materials for Low-Temperature Thermal Energy Storage Applications. Energies 2018, 12, 75. [Google Scholar] [CrossRef] [Green Version]
- Cunha, S.; De Aguiar, J.L.B. Phase change materials and energy efficiency of buildings: A review of knowledge. J. Energy Storage 2020, 27, 101083. [Google Scholar] [CrossRef]
- Madad, A.; Mouhib, T.; Mouhsen, A. Phase Change Materials for Building Applications: A Thorough Review and New Perspectives. Buildings 2018, 8, 63. [Google Scholar] [CrossRef] [Green Version]
- Frigione, M.; Lettieri, M.; Sarcinella, A. Phase Change Materials for Energy Efficiency in Buildings and Their Use in Mortars. Materials 2019, 12, 1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathis, D.; Blanchet, P.; Lagière, P.; Landry, V. Performance of Wood-Based Panels Integrated with a Bio-Based Phase Change Material: A Full-Scale Experiment in a Cold Climate with Timber-Frame Huts. Energies 2018, 11, 3093. [Google Scholar] [CrossRef] [Green Version]
- Bribian, I.Z.; Uson, J.A.A.; Scarpellini, S. Life cycle assessment in buildings: State-of-the-art and simplified LCA methodology as a complement for building certification. Build. Environ. 2009, 44, 2510–2520. [Google Scholar] [CrossRef]
- Zabalza, B.; Valero, C.A.; Aranda, U.A. Life cycle assessment of building materials Comparative analysis of energy and environmental impacts and evaluation of the eco–efficiency improvement potential. Build. Environ. 2011, 46, 1133–1140. [Google Scholar] [CrossRef]
- Chau, C.K.; Leung, T.; Ng, W. A review on Life Cycle Assessment, Life Cycle Energy Assessment and Life Cycle Carbon Emissions Assessment on buildings. Appl. Energy 2015, 143, 395–413. [Google Scholar] [CrossRef]
- Kovacic, I.; Zoller, V. Building life cycle optimization tools for early design phases. Energy 2015, 92, 409–419. [Google Scholar] [CrossRef]
- Kylili, A.; Fokaides, P.A. Life Cycle Assessment (LCA) of Phase Change Materials (PCMs) for building applications: A review. J. Build. Eng. 2016, 6, 133–143. [Google Scholar] [CrossRef]
- De Gracia, A.; Rincón, L.; Castell, A.; Jimenez, M.; Boer, D.; Medrano, M.; Cabeza, L.F. Life Cycle Assessment of the inclusion of phase change materials (PCM) in experimental buildings. Energy Build. 2010, 42, 1517–1523. [Google Scholar] [CrossRef]
- Menoufi, K.; Castell, A.; Farid, M.; Boer, D.; Cabeza, L.F. Life Cycle Assessment of experimental cubicles including PCM manufactured from natural resources (esters): A theoretical study. Renew. Energy 2013, 51, 398–403. [Google Scholar] [CrossRef] [Green Version]
- Menoufi, K.; Castell, A.; Navarro, L.; Pérez, G.; Boer, D.; Cabeza, L.F. Evaluation of the environmental impact of experimental cubicles using Life Cycle Assessment: A highlight on the manufacturing phase. Appl. Energy 2012, 92, 534–544. [Google Scholar] [CrossRef]
- Castell, A.; Menoufi, K.; De Gracia, A.; Rincón, L.; Boer, D.; Cabeza, L.F. Life Cycle Assessment of alveolar brick construction system incorporating phase change materials (PCMs). Appl. Energy 2013, 101, 600–608. [Google Scholar] [CrossRef]
- Serrano, S.; Barreneche, C.; Rincón, L.; Boer, D.; Cabeza, L.F. Stabilized rammed earth incorporating PCM: Optimization and improvement of thermal properties and Life Cycle Assessment. Energy Procedia 2012, 30, 461–470. [Google Scholar] [CrossRef] [Green Version]
- Serrano, S.; Barreneche, C.; Rincón, L.; Boer, D.; Cabeza, L.F. Optimization of three new compositions of stabilized rammed earth incorporating PCM: Thermal properties characterization and LCA. Constr. Build. Mater. 2013, 47, 872–878. [Google Scholar] [CrossRef]
- Uson, J.A.A.; Ferreira, G.A.F.; López-Sabirón, A.M.; Toledo, M.D.M.; Bribian, I.Z. Phase change material applications in buildings: An environmental assessment for some Spanish climate severities. Sci. Total. Environ. 2013, 444, 16–25. [Google Scholar] [CrossRef]
- De Gracia, A.; Navarro, L.; Castell, A.; Boer, D.; Cabeza, L.F. Life cycle assessment of a ventilated facade with PCM in its air chamber. Sol. Energy 2014, 104, 115–123. [Google Scholar] [CrossRef]
- Fabiani, C.; Pisello, A.L.; Barbanera, M.; Cabeza, L.F. Palm oil-based bio-PCM for energy efficient building applications: Multipurpose thermal investigation and life cycle assessment. J. Energy Storage 2020, 28, 101129. [Google Scholar] [CrossRef]
- Konstantinidou, C.A.; Lang, W.; Papadopoulos, A.M.; Santamouris, M. Life cycle and life cycle cost implications of integrated phase change materials in office buildings. Int. J. Energy Res. 2018, 43, 150–166. [Google Scholar] [CrossRef]
- Noël, J.A.; Allred, P.M.; White, M.A. Life cycle assessment of two biologically produced phase change materials and their related products. Int. J. Life Cycle Assess. 2014, 20, 367–376. [Google Scholar] [CrossRef]
- Panayiotou, G.; Kalogirou, S.; Tassou, S.A. Evaluation of the application of Phase Change Materials (PCM) on the envelope of a typical dwelling in the Mediterranean region. Renew. Energy 2016, 97, 24–32. [Google Scholar] [CrossRef] [Green Version]
- Heidari, M.D.; Mathis, D.; Blanchet, P.; Amor, B. Streamlined Life Cycle Assessment of an Innovative Bio-Based Material in Construction: A Case Study of a Phase Change Material Panel. Forests 2019, 10, 160. [Google Scholar] [CrossRef] [Green Version]



| BPCM | Melting T (°C) | Freezing T (°C) | Latent Heat of Fusion (J/g) |
|---|---|---|---|
| Caprylic acid | 16 | 144 | |
| Capric acid (CA) | 32 | 25 | 150 |
| BPCM | Proportion | Melting T (°C) | Freezing T (°C) | Latent Heat of Fusion (J/g) |
|---|---|---|---|---|
| CA:LA | 67:33 | 22.8 | - | 154.16 |
| CA:LA | 64:36 | 19.62 | - | 149.95 |
| CA:LA | 70:30 | 21.09 | - | 124 |
| CA:LA | 45:55 | 21 | - | 143 |
| CA:MA | 74:26 | 22.16 | 21.18 | 154.83 |
| CA:MA | 70:30 | 21.79 | - | 123.62 |
| CA:PA | 76.5:23.5 | 21.85 | - | 173.16 |
| CA:SA | 83:17 | 25.39 | 25.2 | 188.15 |
| CA:SA | 70:30 | 23.4 | - | 104.9 |
| BPCM | Melting T (°C) | Freezing T (°C) | Latent Heat of Fusion (J/g) |
|---|---|---|---|
| Butyl stearate | 19–24 | - | 130–140 |
| Dimethyl sabacate | 21 | - | 120–135 |
| Isopropyl stearate | 22.12 | 21.99 | 113 |
| Vinyl stearate | 27 | 29 | 122 |
| Propyl palmitate | 19 | - | 186 |
| BPCM | Proportion | Melting T (°C) | Freezing T (°C) | Latent Heat of Fusion (J/g) |
|---|---|---|---|---|
| Emerest 2325 (butyl stearate + butyl palmitate) | 49:48 | 17–21 | - | 138–140 |
| Emerest 2326 (butyl stearate + butyl palmitate) | 50:48 | 18–22 | - | 140 |
| Methyl palmitate + Methyl stearate | 93:7 | 23 | 22 | 180 |
| Methyl palmitate + Methyl stearate | 95:5 | 26 | 23 | 180 |
| Methyl palmitate + Methyl stearate | 86:14 | 23.9 | 23.8 | 220 |
| Methyl stearate + cetyl stearate | 91:9 | 22.2 | 21.8 | 180 |
| Methyl stearate + cetyl palmitate | 91:9 | 28.2 | 27.9 | 189 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nazari, M.; Jebrane, M.; Terziev, N. Bio-Based Phase Change Materials Incorporated in Lignocellulose Matrix for Energy Storage in Buildings—A Review. Energies 2020, 13, 3065. https://doi.org/10.3390/en13123065
Nazari M, Jebrane M, Terziev N. Bio-Based Phase Change Materials Incorporated in Lignocellulose Matrix for Energy Storage in Buildings—A Review. Energies. 2020; 13(12):3065. https://doi.org/10.3390/en13123065
Chicago/Turabian StyleNazari, Meysam, Mohamed Jebrane, and Nasko Terziev. 2020. "Bio-Based Phase Change Materials Incorporated in Lignocellulose Matrix for Energy Storage in Buildings—A Review" Energies 13, no. 12: 3065. https://doi.org/10.3390/en13123065





