Steam Explosion Pretreatment of Beechwood. Part 2: Quantification of Cellulase Inhibitors and Their Effect on Avicel Hydrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biomass Feedstock
2.2. Steam Explosion Pretreatment
2.3. Enzymatic Hydrolysis
2.4. Multiple Linear Regression
2.5. Analytical Methods
3. Results and Discussion
3.1. Inhibitor Quantification
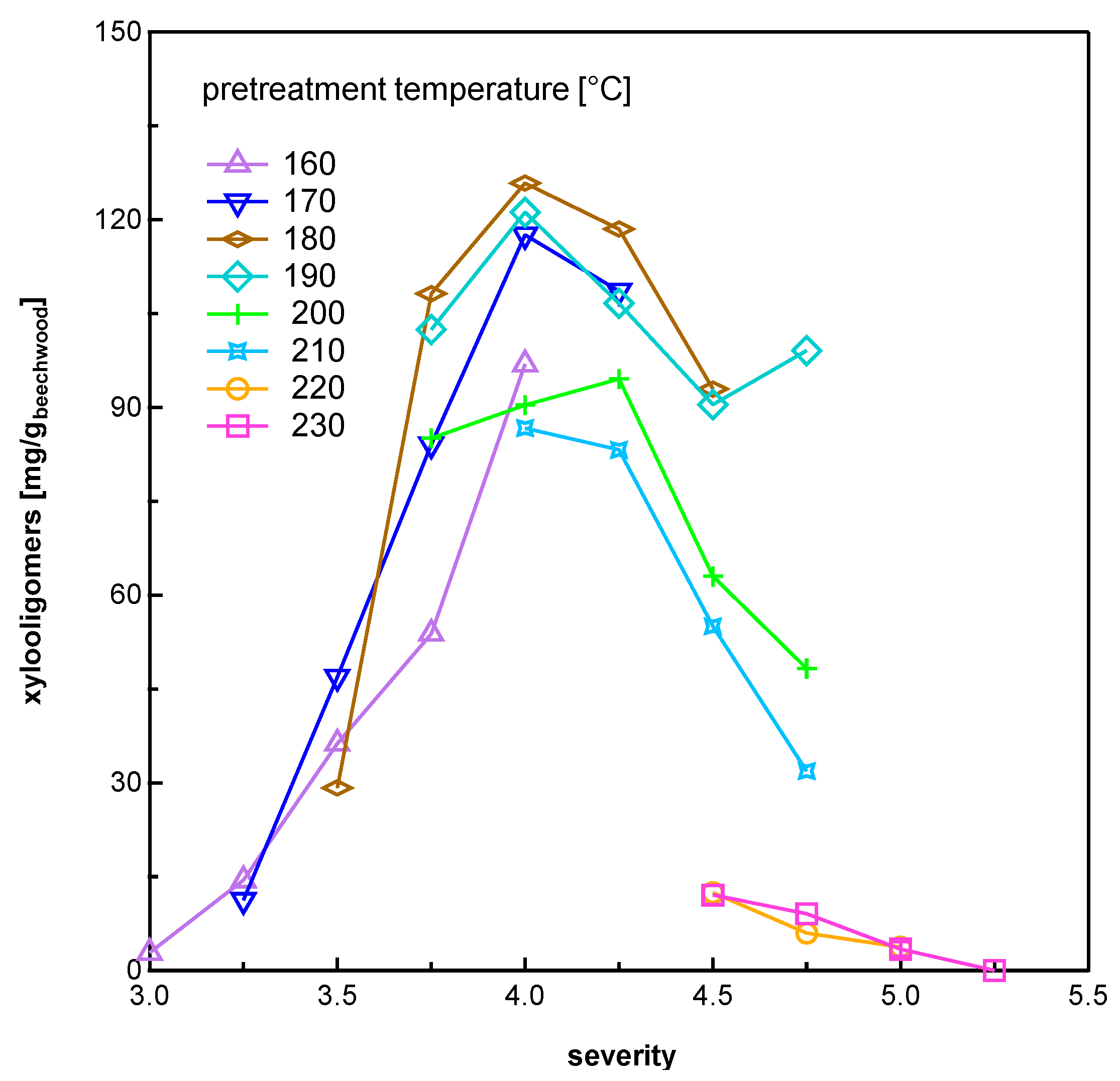
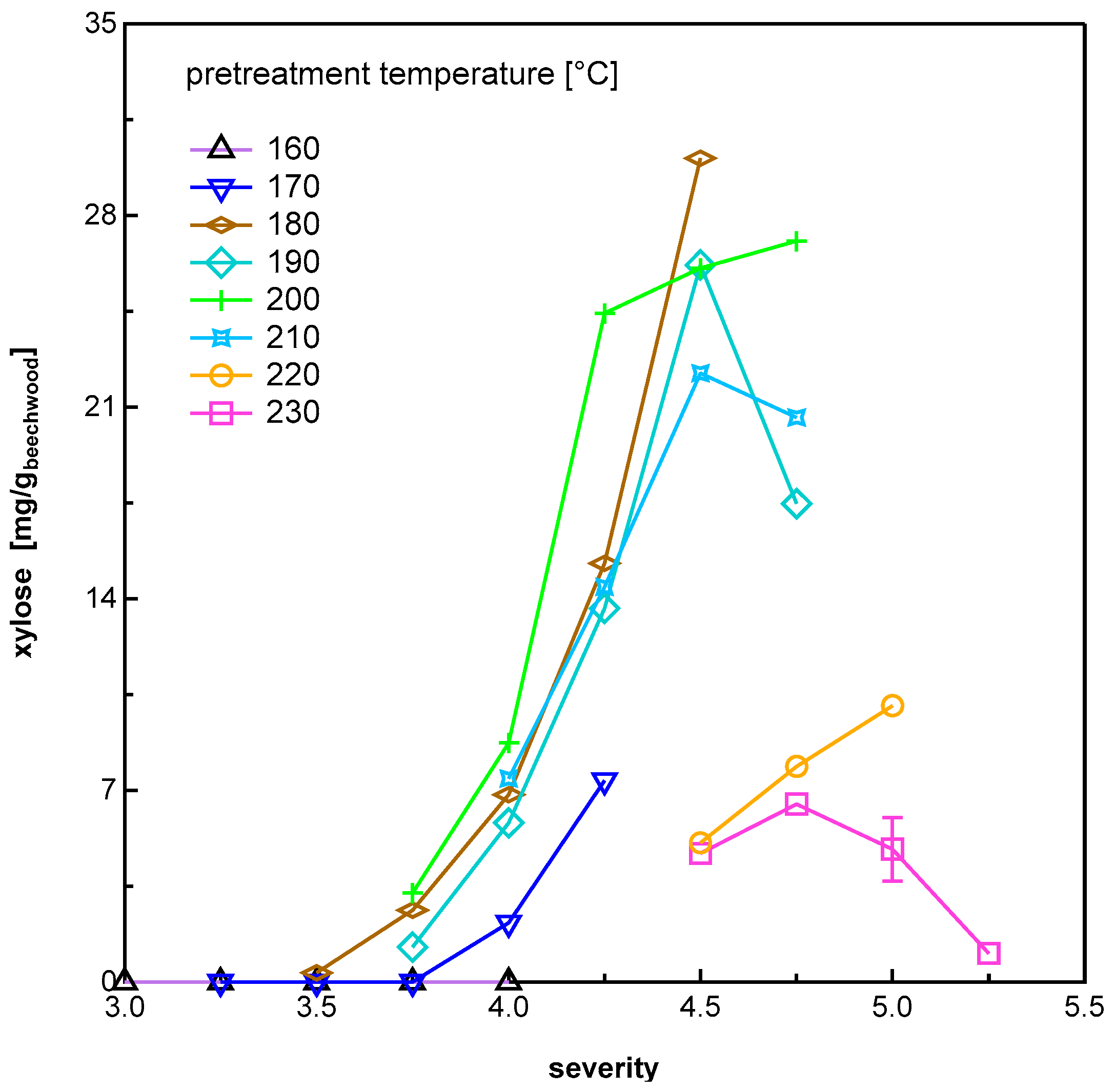
3.1.1. Pentose Sugars
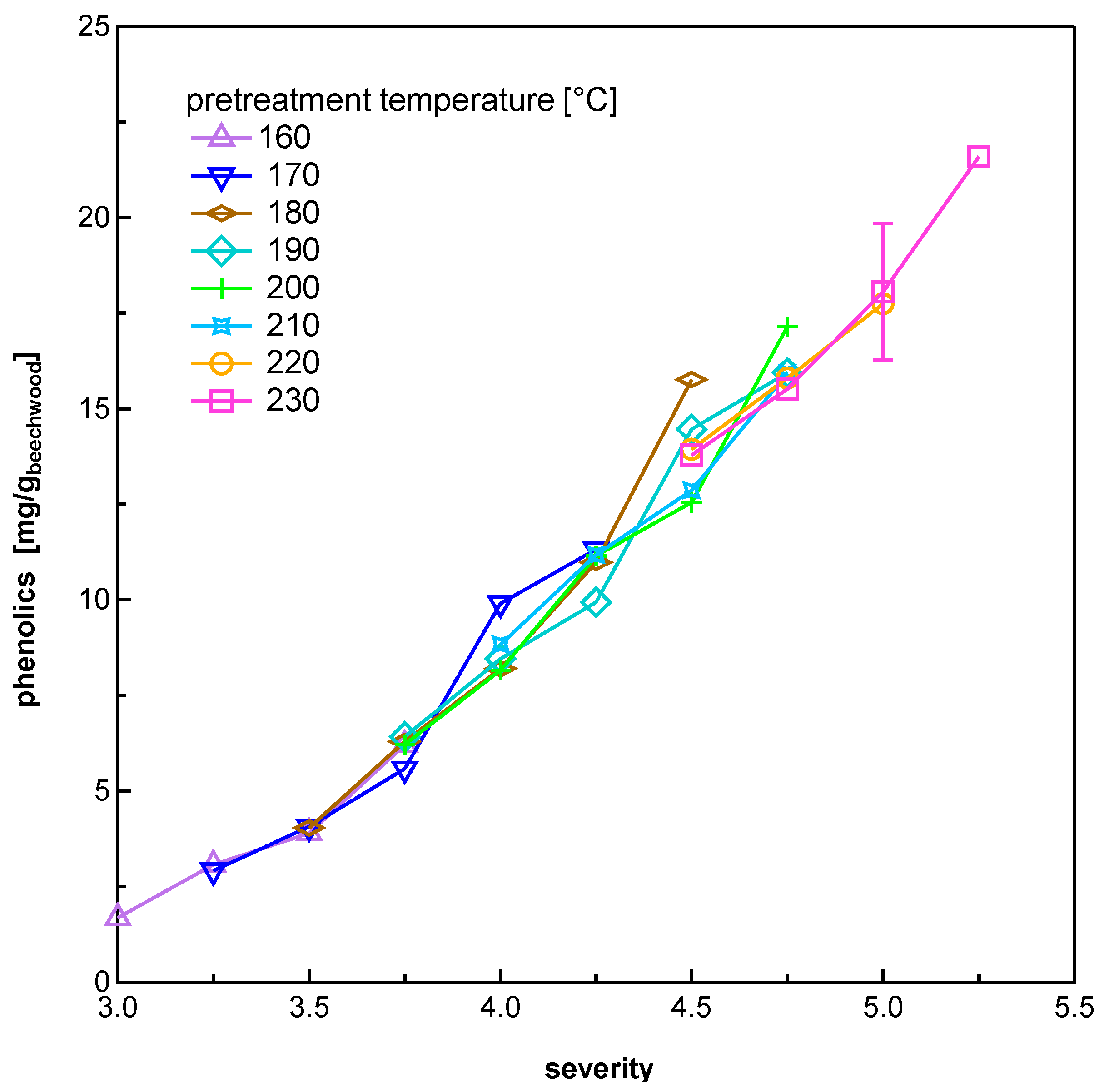
3.1.2. Phenolics
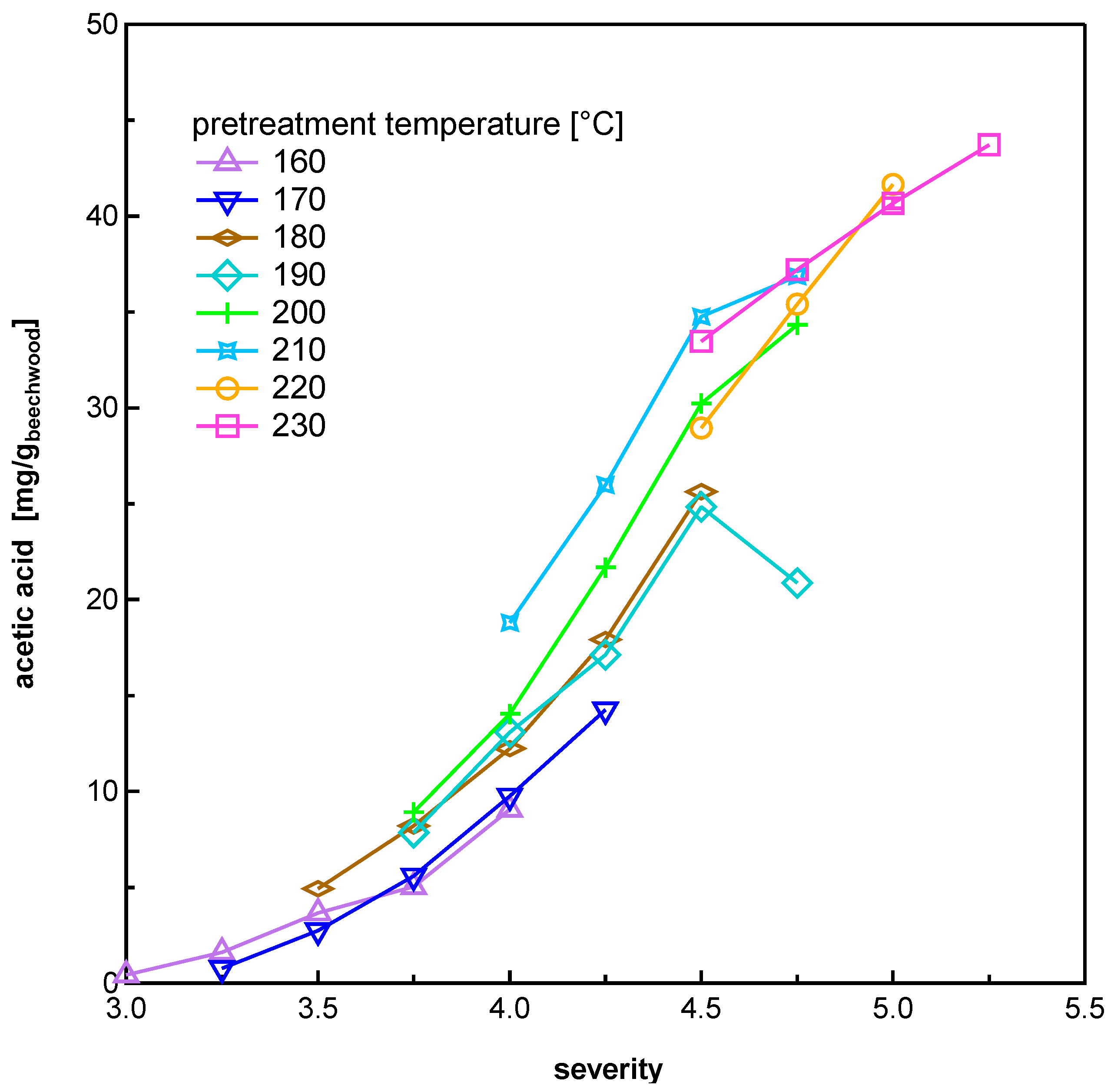
3.1.3. Organic Acids
3.1.4. Furan Aldehydes
3.2. Inhibition of Enzymatic Hydrolysis
3.3. Relation between Inhibitor Concentrations and Extent of Enzyme Inhibition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ragauskas, A.J.; Williams, C.K.; Davison, B.H.; Britovsek, G.; Cairney, J.; Eckert, C.A.; Frederick, W.J., Jr.; Hallett, J.P.; Leak, D.J.; Liotta, C.L.; et al. The path forward for biofuels and biomaterials. Science 2006, 311, 484–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandel, A.K.; Garlapati, V.K.; Singh, A.K.; Antunes, F.A.F.; da Silva, S.S. The path forward for lignocellulose biorefineries: Bottlenecks, solutions, and perspective on commercialization. Bioresour. Technol. 2018, 264, 370–381. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, F.; Jungmeier, G.; Wellisch, M.; Willke, T.; Skiadas, I.; Van Ree, R.; de Jong, E. Toward a common classification approach for biorefinery systems. Biofuels Bioprod. Biorefin. 2009, 3, 534–546. [Google Scholar] [CrossRef]
- Yang, B.; Wyman, C.E. Pretreatment: The key to unlocking low-cost cellulosic ethanol. Biofuels Bioprod. Biorefin. 2008, 2, 26–40. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Kumari, D.; Singh, R. Pretreatment of lignocellulosic wastes for biofuel production: A critical review. Renew. Sustain. Energy Rev. 2018, 90, 877–891. [Google Scholar] [CrossRef]
- Kim, D.; Orrego, D.; Ximenes, E.A.; Ladisch, M.R. Cellulose conversion of corn pericarp without pretreatment. Bioresour. Technol. 2017, 245, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, A.C.; Ximenes, E.; Kim, Y.; Ladisch, M.R. Lignin-Enzyme Interactions in the Hydrolysis of Lignocellulosic Biomass. Trends Biotechnol. 2019, 37, 518–531. [Google Scholar] [CrossRef]
- Jonsson, L.J.; Alriksson, B.; Nilvebrant, N.-O. Bioconversion of lignocellulose: Inhibitors and detoxification. Biotechnol. Biofuels 2013, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Jonsson, L.J.; Martin, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, H.; Sorensen, H.R.; Meyer, A.S. Formation of degradation compounds from lignocellulosic biomass in the biorefinery: Sugar reaction mechanisms. Carbohydr. Res. 2014, 385, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Jagtap, S.S.; Bedekar, A.A.; Bhatia, R.K.; Patel, A.K.; Pant, D.; Rajesh Banu, J.; Rao, C.V.; Kim, Y.-G.; Yang, Y.-H. Recent developments in pretreatment technologies on lignocellulosic biomass: Effect of key parameters, technological improvements, and challenges. Bioresour. Technol. 2020, 300, 122724. [Google Scholar] [CrossRef] [PubMed]
- Geddes, C.C.; Nieves, I.U.; Ingram, L.O. Advances in ethanol production. Curr. Opin. Biotechnol. 2011, 22, 312–319. [Google Scholar] [CrossRef] [Green Version]
- Djioleu, A.; Carrier, D.J. Statistical approach for the identification of cellulolytic enzyme inhibitors using switchgrass dilute acid prehydrolyzates as a model system. ACS Sustain. Chem. Eng. 2018, 6, 3443–3452. [Google Scholar] [CrossRef]
- Zhai, R.; Hu, J.; Saddler, J.N. Understanding the slowdown of whole slurry hydrolysis of steam pretreated lignocellulosic woody biomass catalyzed by an up-to-date enzyme cocktail. Sustain. Energy Fuels 2018, 2, 1048–1056. [Google Scholar] [CrossRef]
- García-Aparicio, P.; Ballesteros, I.; González, A.; Oliva, J.M.; Ballesteros, M.; Negro, J. Effect of inhibitors released during steam-explosion pretreatment of barley straw on enzymatic hydrolysis. Appl. Biochem. Biotech. 2006, 129, 278–288. [Google Scholar] [CrossRef]
- Kim, Y.; Ximenes, E.; Mosier, N.S.; Ladisch, M.R. Soluble inhibitors/deactivators of cellulase enzymes from lignocellulosic biomass. Enzym. Microb. Technol. 2011, 48, 408–415. [Google Scholar] [CrossRef] [PubMed]
- Michelin, M.; Ximenes, E.; de Moraes, M.D.; Ladisch, M.R. Effect of phenolic compounds from pretreated sugarcane bagasse on cellulolytic and hemicellulolytic activities. Bioresour. Technol. 2016, 199, 275–278. [Google Scholar] [CrossRef]
- Zhai, R.; Hu, J.; Saddler, J.N. Extent of enzyme inhibition by phenolics derived from pretreated biomass is significantly influenced by the size and carbonyl group content of the phenolics. ACS Sustain. Chem. Eng. 2018, 6, 3823–3829. [Google Scholar] [CrossRef]
- Chen, X.; Zhai, R.; Li, Y.; Yuan, X.; Liu, Z.-H.; Jin, M. Understanding the structural characteristics of water-soluble phenolic compounds from four pretreatments of corn stover and their inhibitory effects on enzymatic hydrolysis and fermentation. Biotechnol. Biofuels 2020, 13, 44. [Google Scholar] [CrossRef]
- McMillan, J.D.; Jennings, E.W.; Mohagheghi, A.; Zuccarello, M. Comparative performance of precommercial cellulases hydrolyzing pretreated corn stover. Biotechnol. Biofuels 2011, 4, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhai, R.; Hu, J.; Saddler, J.N. What are the major components in steam pretreated lignocellulosic biomass that inhibit the efficacy of cellulase enzyme mixtures? ACS Sustain. Chem. Eng. 2016, 4, 3429–3436. [Google Scholar] [CrossRef]
- Ladeira Ázar, R.I.S.; Morgan, T.; Dos Santos, A.C.F.; de Aquino Ximenes, E.; Ladisch, M.R.; Guimarães, V.M. Deactivation and activation of lignocellulose degrading enzymes in the presence of laccase. Enzym. Microb. Technol. 2018, 109, 25–30. [Google Scholar] [CrossRef]
- Balan, R.; Antczak, A.; Brethauer, S.; Zielenkiewicz, T.; Studer, M.H. Steam explosion pretreatment of beechwood. Part 1: Comparison of the enzymatic hydrolysis of washed solids and whole pretreatment slurry at different solid loadings. Energies 2020, 13, 3653. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass: Laboratory Analytical Procedure (LAP); Technical Report NREL/TP-510-42618; National Renewable Energy Laboratory: Golden, CO, USA, 2012. [Google Scholar]
- Overend, R.P.; Chornet, E.; Gascoigne, J.A. Fractionation of lignocellulosics by steam-aqueous pretreatments [and discussion]. Philos. Trans. R. Soc. A 1987, 321, 523–536. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Sugars, Byproducts, and Degradation Products in Liquid Fraction Process Samples: Laboratory Analytical Procedure (LAP); Technical Report NREL/TP-510-42623; National Renewable Energy Laboratory: Golden, CO, USA, 2008. [Google Scholar]
- Kapu, N.S.; Piddocke, M.; Saddler, J.J.N. High gravity and high cell density mitigate some of the fermentation inhibitory effects of softwood hydrolysates. AMB Express 2013, 3, 15. [Google Scholar] [CrossRef] [Green Version]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [Green Version]
- Qing, Q.; Yang, B.; Wyman, C.E. Bioresource Technology Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour. Technol. 2010, 101, 9624–9630. [Google Scholar] [CrossRef]
- Kim, Y.; Kreke, T.; Mosier, N.S.; Ladisch, M.R. Severity factor coefficients for subcritical liquid hot water pretreatment of hardwood chips. Biotechnol. Bioeng. 2014, 111, 254–263. [Google Scholar] [CrossRef]
- Nitsos, C.K.; Matis, K.A.; Triantafyllidis, K.S. Optimization of hydrothermal pretreatment of lignocellulosic biomass in the bioethanol production process. ChemSusChem 2013, 6, 110–122. [Google Scholar] [CrossRef]
- Kim, Y.; Kreke, T.; Hendrickson, R.; Parenti, J.; Ladisch, M.R. Fractionation of cellulase and fermentation inhibitors from steam pretreated mixed hardwood. Bioresour. Technol. 2013, 135, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Inhibition of cellulases by phenols. Enzym. Microb. Tech. 2010, 46, 170–176. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Hirata, S.; Fujimoto, S.; Ibrahim, I.; Hassan, M.A. Soluble inhibitors generated during hydrothermal pretreatment of oil palm mesocarp fiber suppressed the catalytic activity of Acremonium cellulase. Bioresour. Technol. 2016, 200, 541–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladeira Ázar, R.I.S.; Bordignon-Junior, S.E.; Laufer, C.; Specht, J.; Ferrier, D.; Kim, D. Effect of Lignin Content on Cellulolytic Saccharification of Liquid Hot Water Pretreated Sugarcane Bagasse. Molecules 2020, 25, 623. [Google Scholar] [CrossRef] [Green Version]
- Pereira Ramos, L. The chemistry involved in the steam treatment of lignocellulosic materials. Quím. Nova 2003, 26, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Klinke, H.B.; Thomsen, A.B.; Ahring, B.K. Potential inhibitors from wet oxidation of wheat straw and their effect on growth and ethanol production by Thermoanaerobacter mathranii. Appl. Microbiol. Biotechnol. 2001, 57, 631–638. [Google Scholar] [CrossRef]
- Ximenes, E.; Kim, Y.; Mosier, N.; Dien, B.; Ladisch, M. Deactivation of cellulases by phenols. Enzyme Microb. Tech. 2011, 48, 54–60. [Google Scholar] [CrossRef]
- Kont, R.; Kurasin, M.; Teugjas, H.; Valjamae, P. Strong cellulase inhibitors from the hydrothermal pretreatment of wheat straw. Biotechnol. Biofuels 2013, 6, 135. [Google Scholar] [CrossRef] [Green Version]
- Panagiotou, G.; Olsson, L. Effect of compounds released during pretreatment of wheat straw on microbial growth and enzymatic hydrolysis rates. Biotechnol. Bioeng. 2007, 96, 250–258. [Google Scholar] [CrossRef]
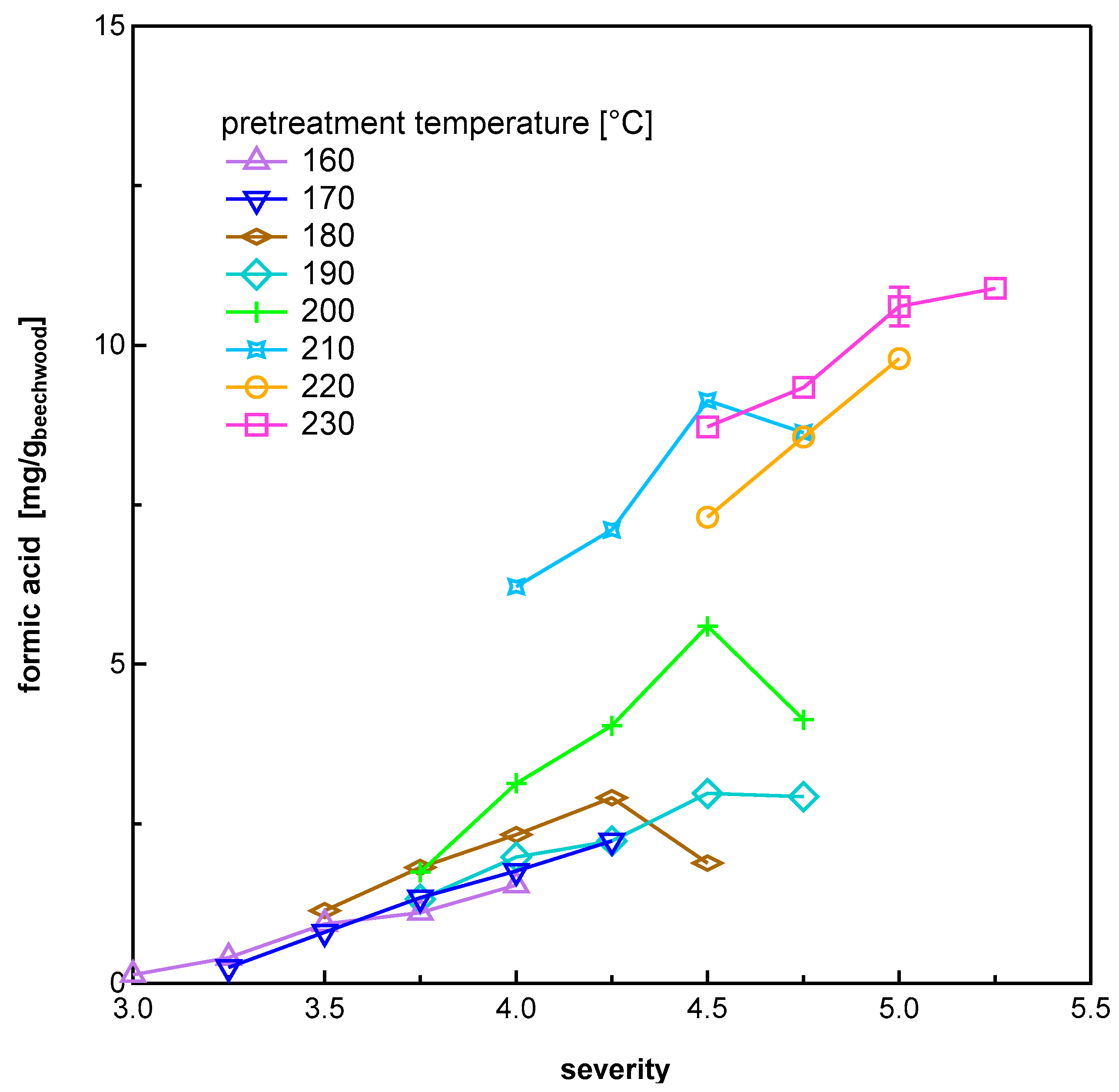

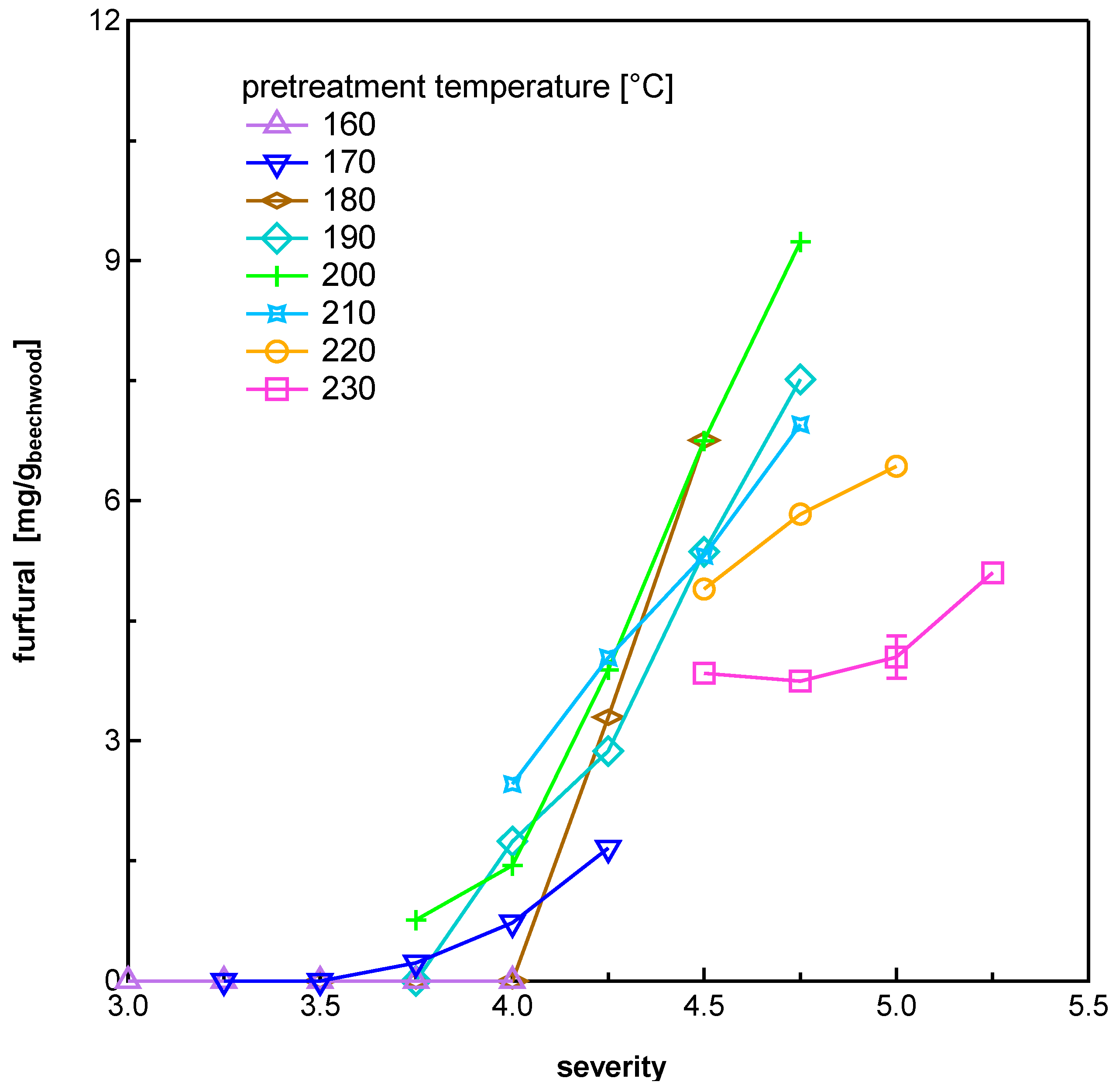

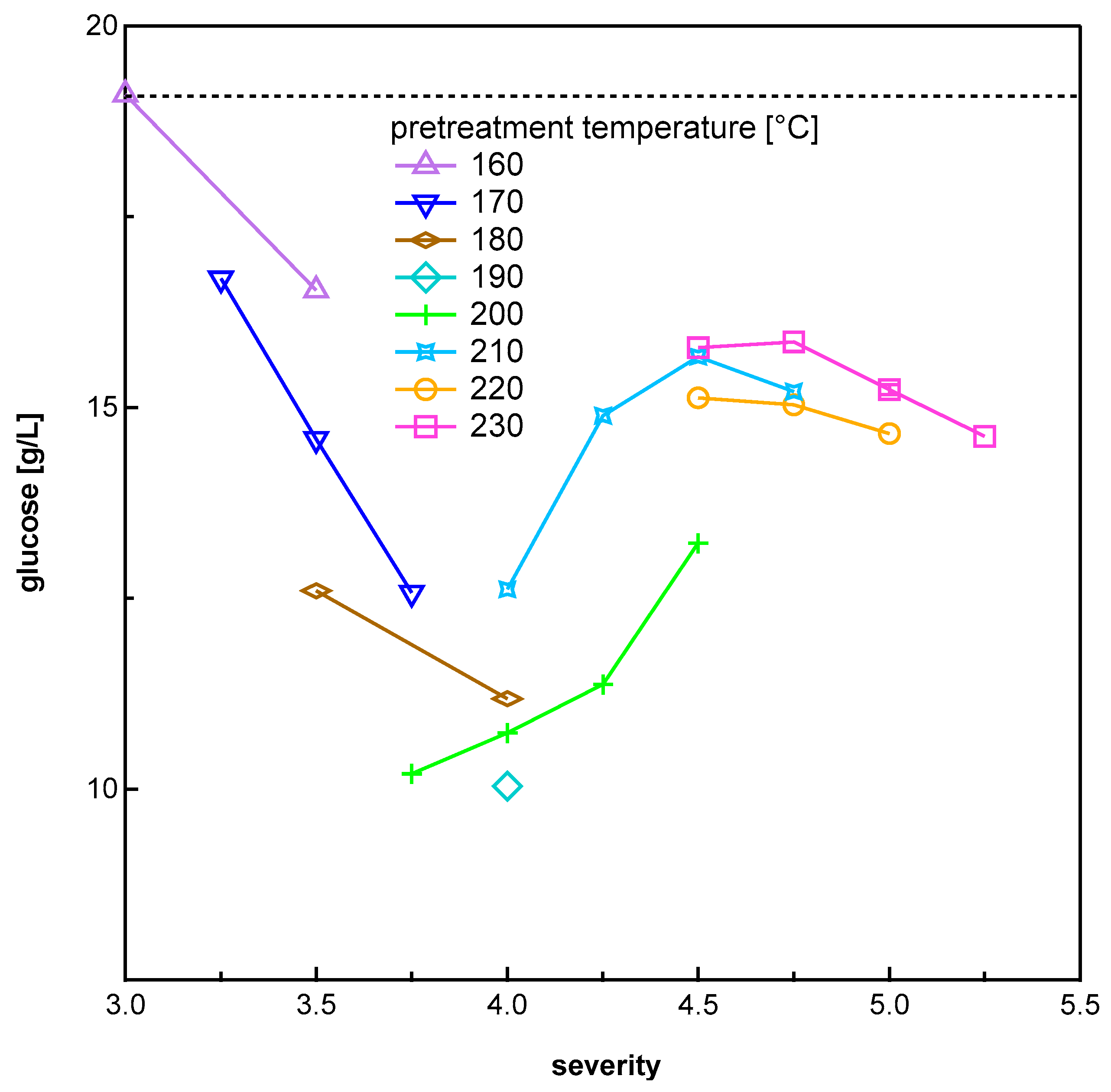

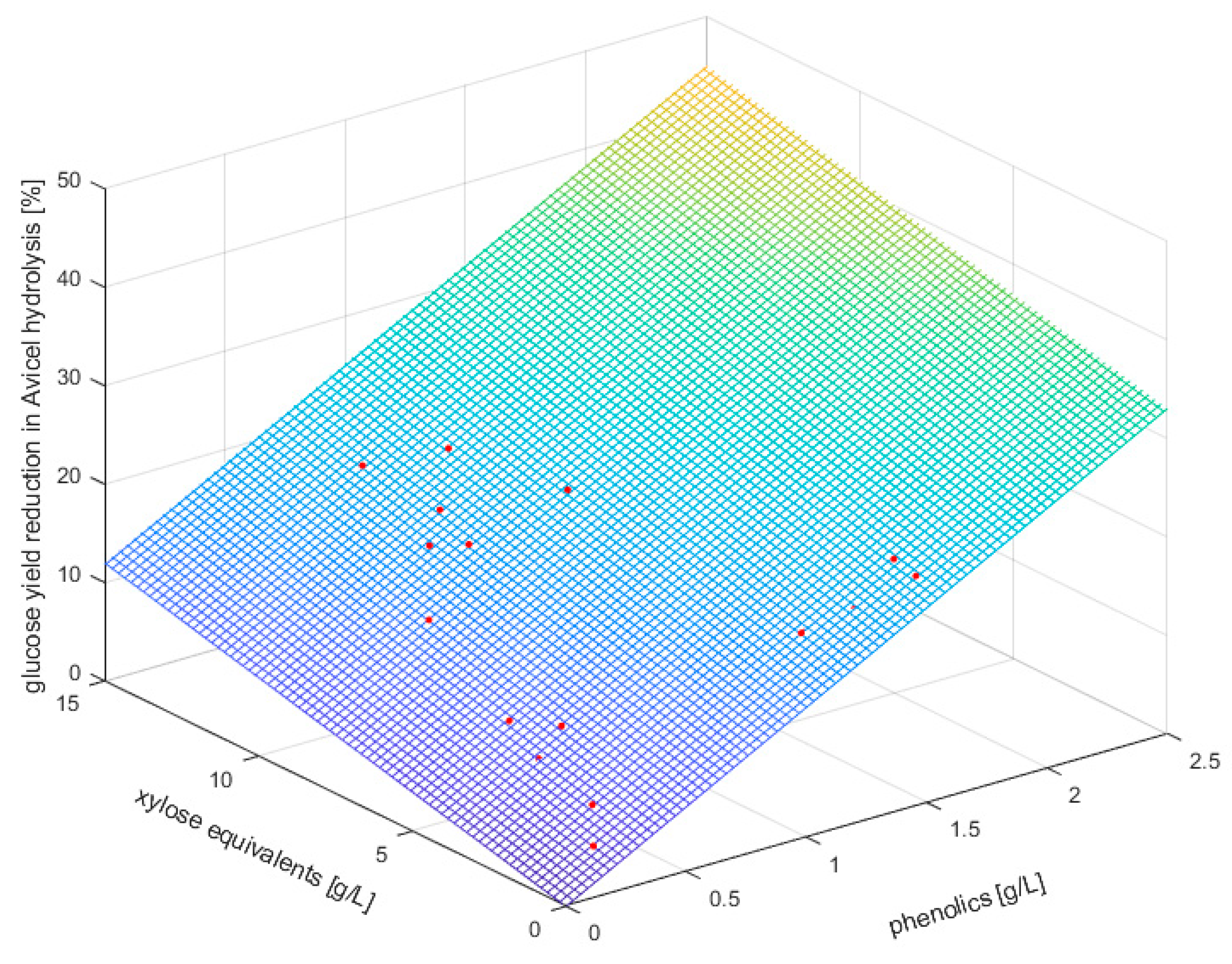
| Temperature [°C] | Pretreatment Severity log R0 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | 3.25 | 3.5 | 3.75 | 4 | 4.25 | 4.5 | 4.75 | 5 | 5.25 | |
| 160 | 17.1 | 30.4 | 54.1 | 96.2 | 171.2 | - | - | - | - | - |
| 170 | - | 15.5 | 27.5 | 48.9 | 86.9 | 154.5 | - | - | - | - |
| 180 | - | - | 13.9 | 24.8 | 44.1 | 78.4 | 139.5 | - | - | - |
| 190 | - | - | - | 12.6 | 22.4 | 39.8 | 70.8 | 125.9 | - | - |
| 200 | - | - | - | 6.4 | 11.4 | 20.2 | 35.9 | 63.9 | - | |
| 210 | - | - | - | - | 5.8 | 10.3 | 18.2 | 32.4 | 57.7 | - |
| 220 | - | - | - | - | 2.9 | 5.2 | 9.3 | 16.5 | 29.3 | - |
| 230 | - | - | - | - | - | 2.6 | 4.7 | 8.4 | 14.9 | 26.4 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brethauer, S.; Antczak, A.; Balan, R.; Zielenkiewicz, T.; Studer, M.H. Steam Explosion Pretreatment of Beechwood. Part 2: Quantification of Cellulase Inhibitors and Their Effect on Avicel Hydrolysis. Energies 2020, 13, 3638. https://doi.org/10.3390/en13143638
Brethauer S, Antczak A, Balan R, Zielenkiewicz T, Studer MH. Steam Explosion Pretreatment of Beechwood. Part 2: Quantification of Cellulase Inhibitors and Their Effect on Avicel Hydrolysis. Energies. 2020; 13(14):3638. https://doi.org/10.3390/en13143638
Chicago/Turabian StyleBrethauer, Simone, Andrzej Antczak, Robert Balan, Tomasz Zielenkiewicz, and Michael H. Studer. 2020. "Steam Explosion Pretreatment of Beechwood. Part 2: Quantification of Cellulase Inhibitors and Their Effect on Avicel Hydrolysis" Energies 13, no. 14: 3638. https://doi.org/10.3390/en13143638
APA StyleBrethauer, S., Antczak, A., Balan, R., Zielenkiewicz, T., & Studer, M. H. (2020). Steam Explosion Pretreatment of Beechwood. Part 2: Quantification of Cellulase Inhibitors and Their Effect on Avicel Hydrolysis. Energies, 13(14), 3638. https://doi.org/10.3390/en13143638





