Efficient Stabilization of Mono and Hybrid Nanofluids
Abstract
:1. Introduction
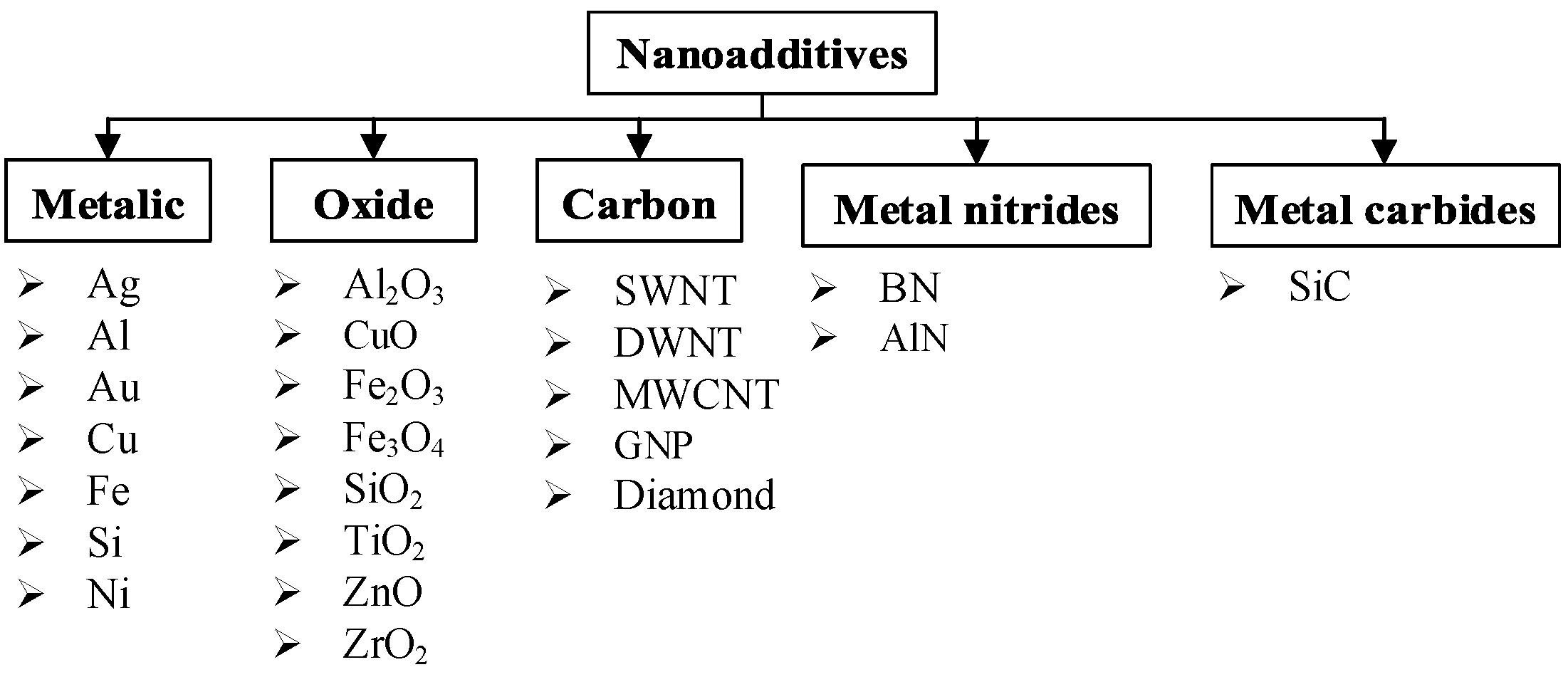
1.1. Types of Conventional Nanoadditives
1.2. Nanocomposites
1.3. Ferrofluids
1.4. Mono vs. Hybrid Nanofluids: Future Challenges
- conventional models specified for mono nanofluids do not apply in the case of hybrid nanofluids; what’s more there are no unequivocal experimental results or agreement regarding available models and characteristics among researchers involved in this subject,
- the relative viscosity and density of a hybrid nanofluid is directly proportional to the concentration of nanoparticles and inversely proportional to the temperature,
- thermal conductivity and heat capacity increase with temperature,
- with rising temperature and concentration of nanoparticles, the thermal properties improve until the critical point is reached—a visible deterioration of nanofluid thermal properties is observed beyond this point,
- the thermal conductivity ratio, viscosity, density, heat capacity, pressure drop and friction factor of a hybrid nanofluid are slightly higher than for the base fluid and mono nanofluid, and they grow directly proportionally to the concentration of nanoparticles,
- dispersion of nanoparticles in the base fluid is a problem frequently during stabilisation, and suspension stability time is relatively short (up to 60 days [35]),
- Brownian motion of nanoparticles and micro-convection effect, clustering and pH values strongly affect the thermal parameters of hybrid nanofluids (which is very rarely discussed in the literature) [36],
2. Nanofluids and Hybrid Nanofluid Symbol Suggestions
| Nanoparticle (concentration, vol, %/ average nanoparticle size, nm/ nanoparticle shape)/Base fluid/Stabilization method used (i.e., two-step or more with precise sonication time and frequency data and so on.) |
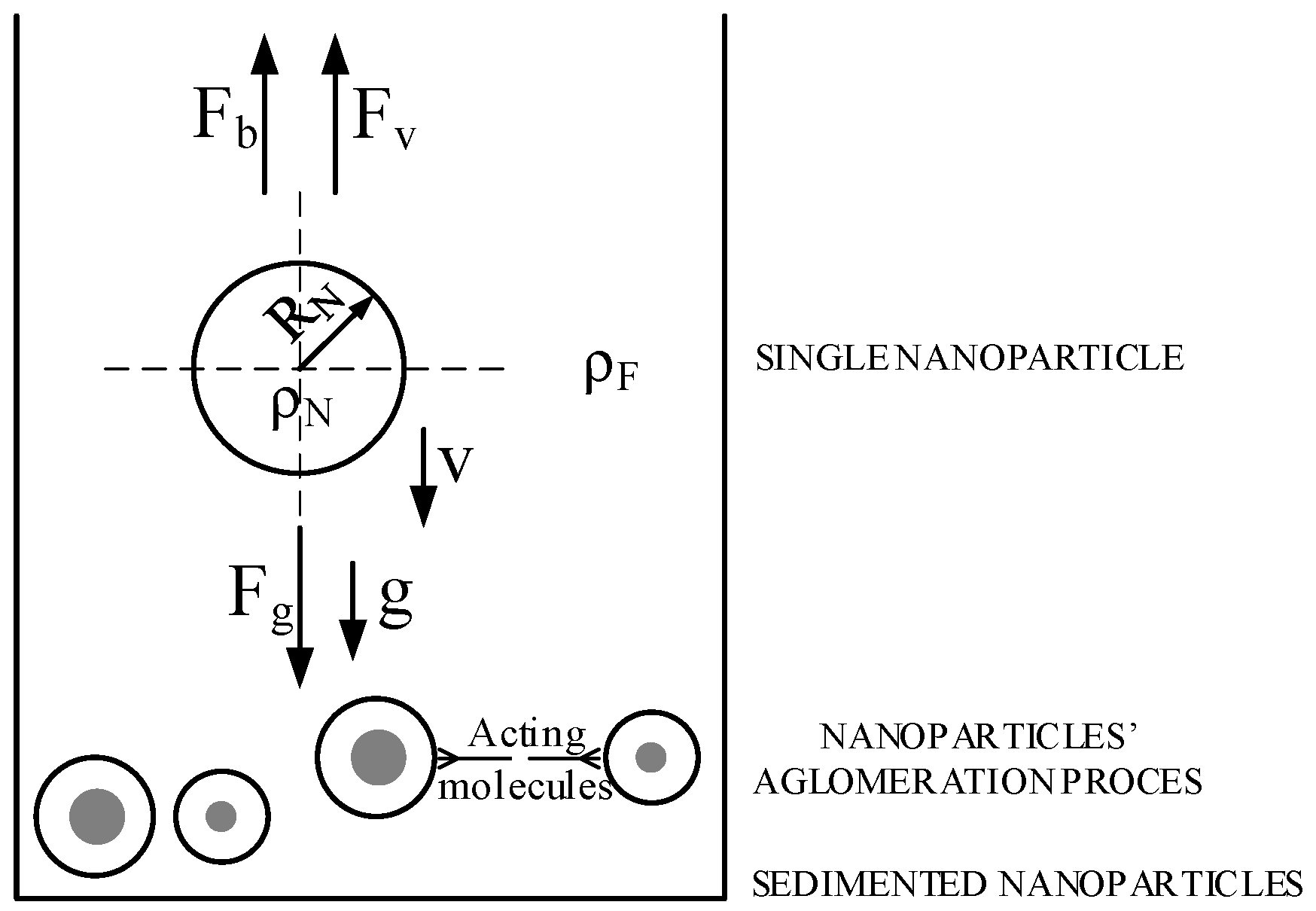
3. Mechanisms of Nanofluids Stability
4. Basic and Most Common Methods of Nanofluid Preparation and Stabilization
4.1. Preparation
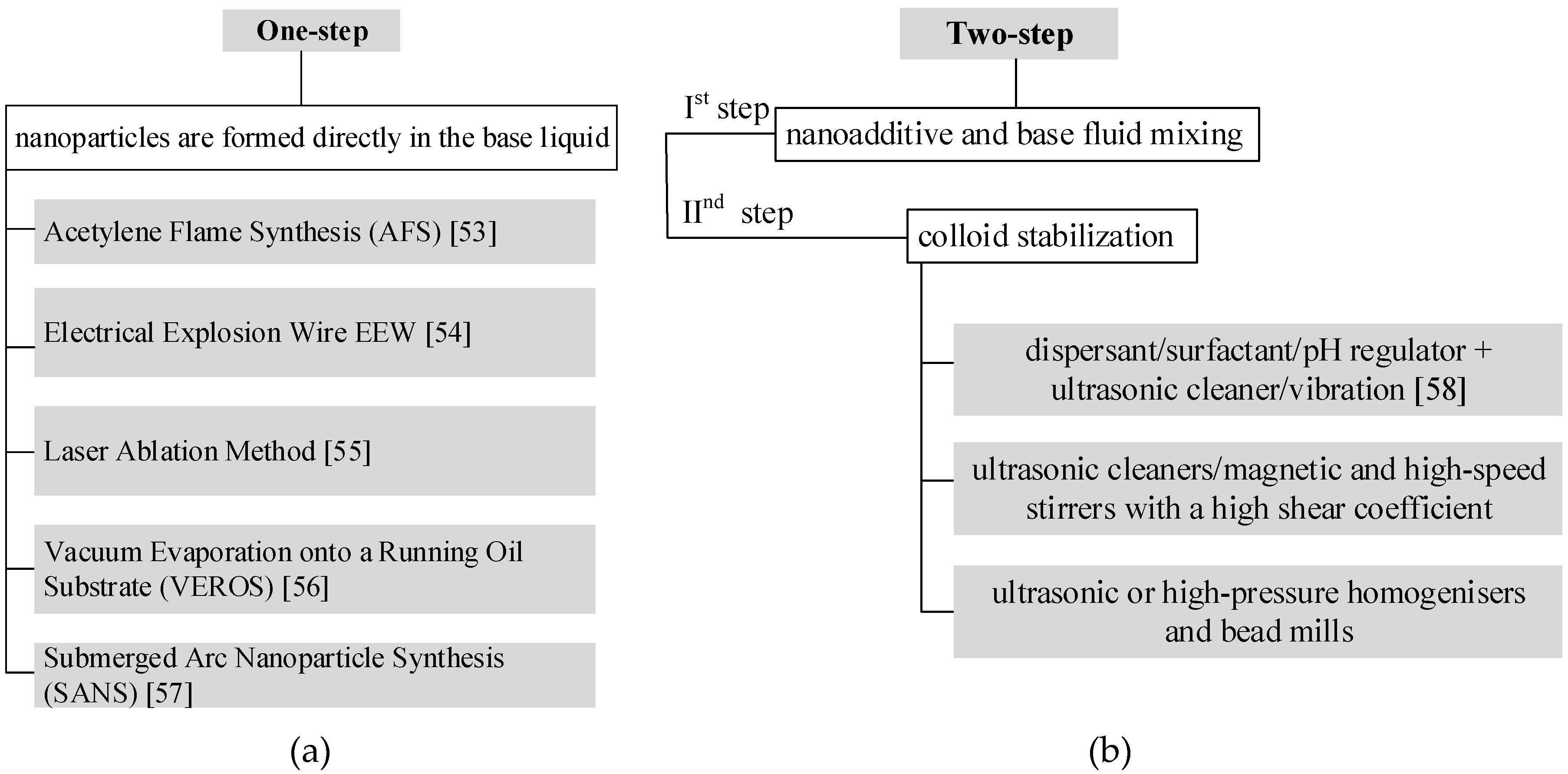
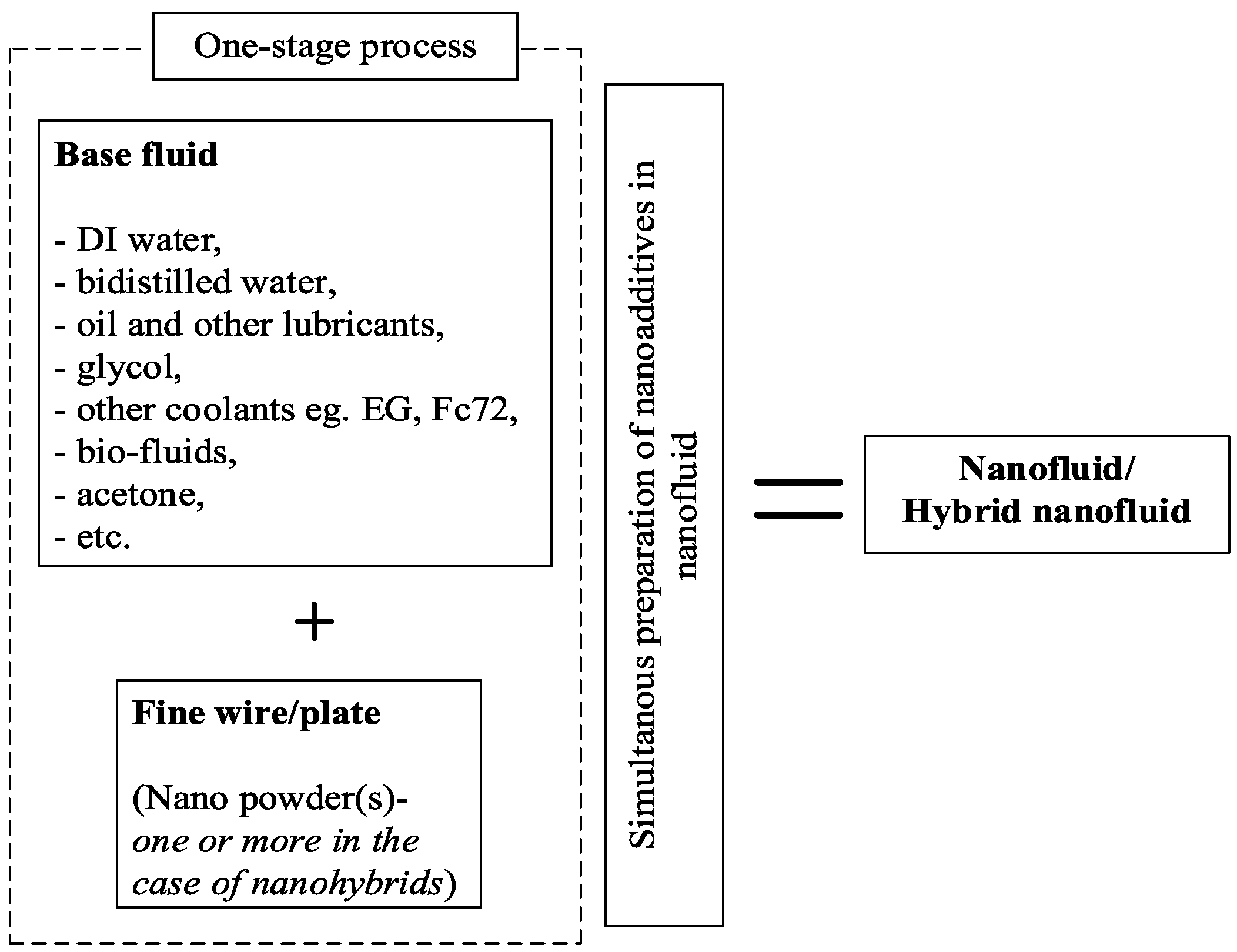
4.1.1. One-Step Method
4.1.2. Two-Step Method
4.1.3. Other Methods
4.2. Stabilization/Nanofluids Stability Increasers
4.2.1. Surfactants
4.2.2. Ultrasonication, Homogenization, Milling
4.2.3. pH Regulators
4.2.4. Steric Stabilization—Chemical Surface Alteration
5. The Quality Assessment of the Nanofluid Preparation
- (1)
- Zeta potential analysis—stability characteristics of the suspension.
- (2)
- Vibrating sample magnetometry (VSM)method—the magnetism characteristics of the suspension.
5.1. Zeta Potential Analysis
5.2. VSM Method
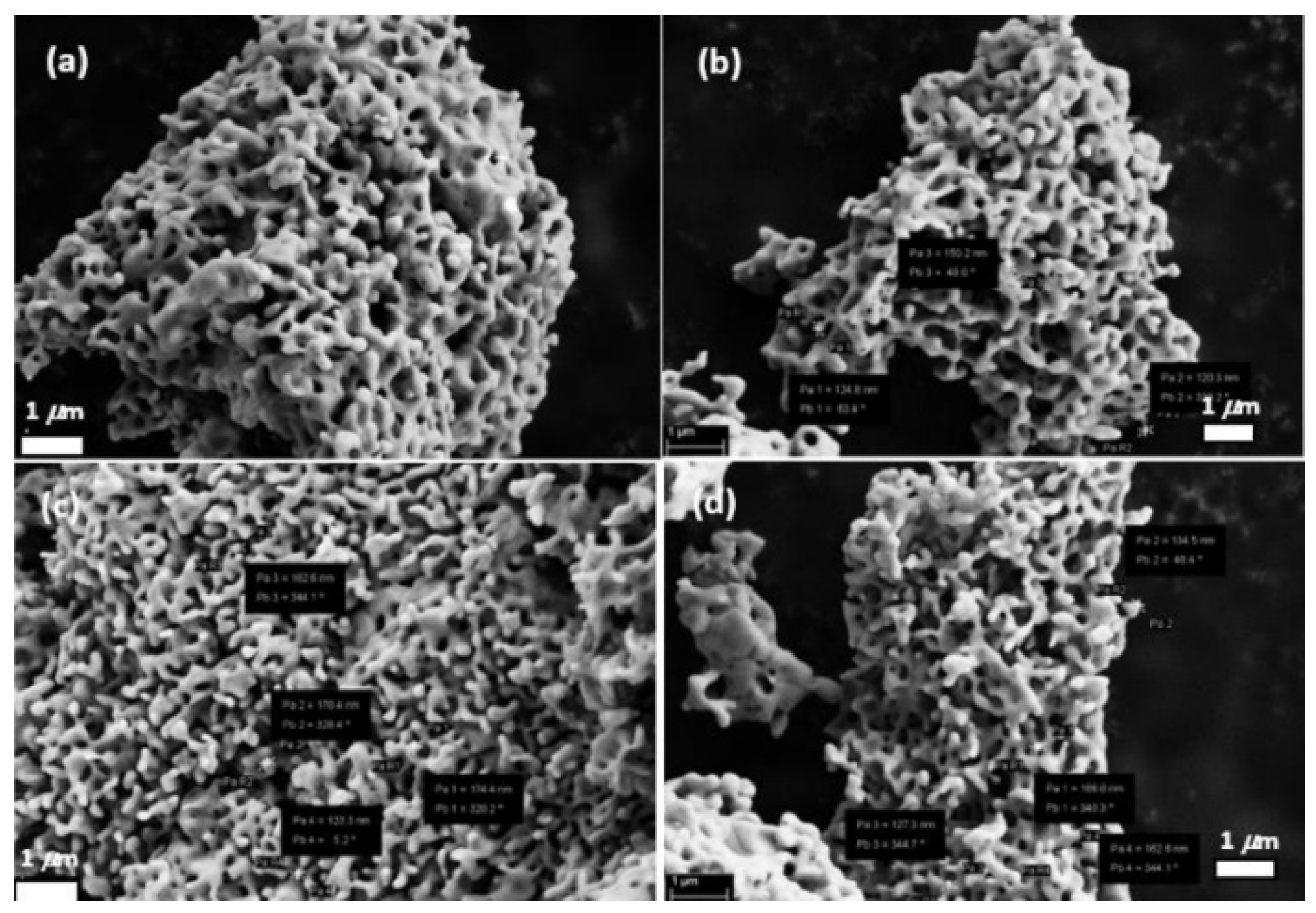
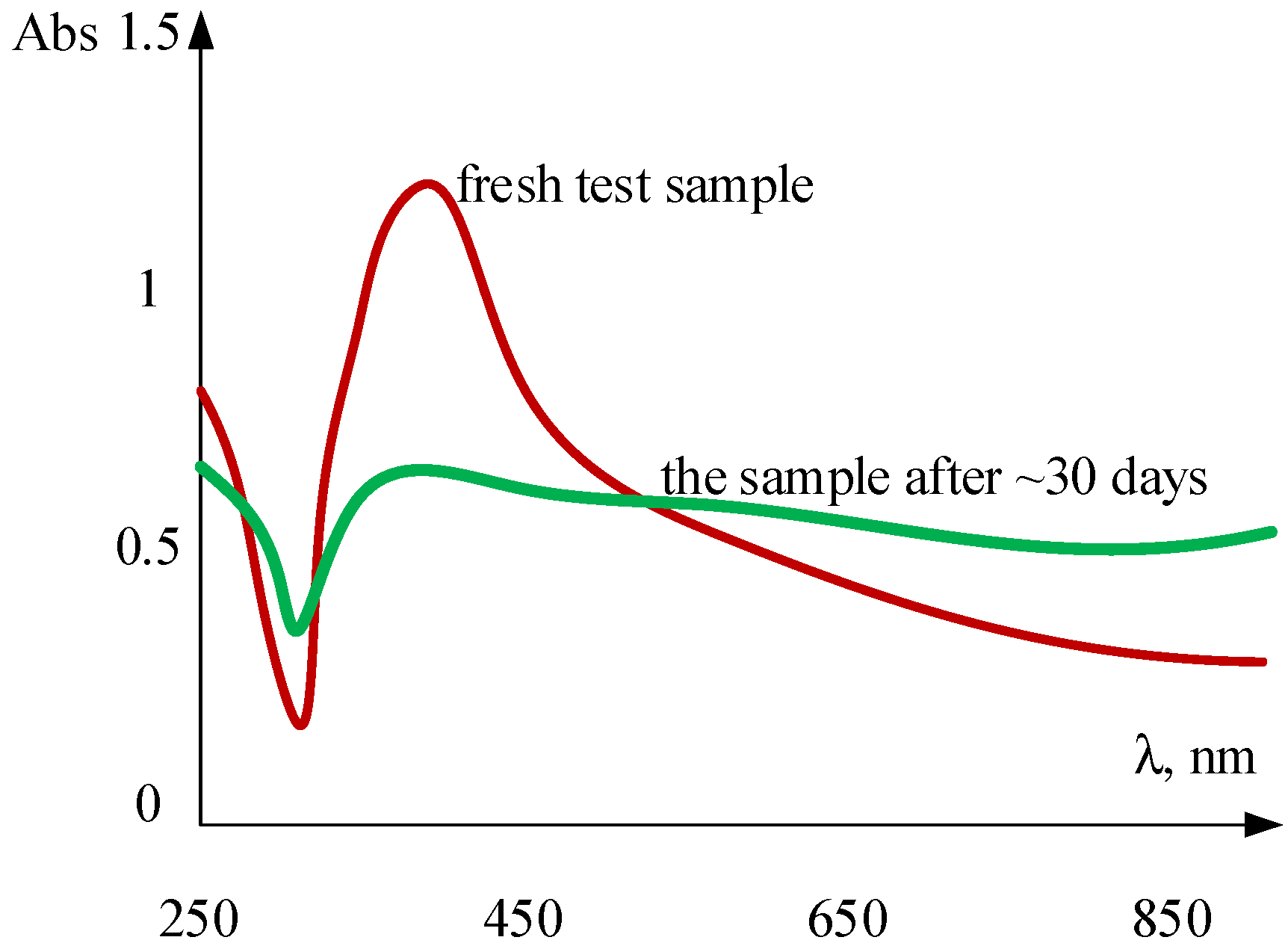
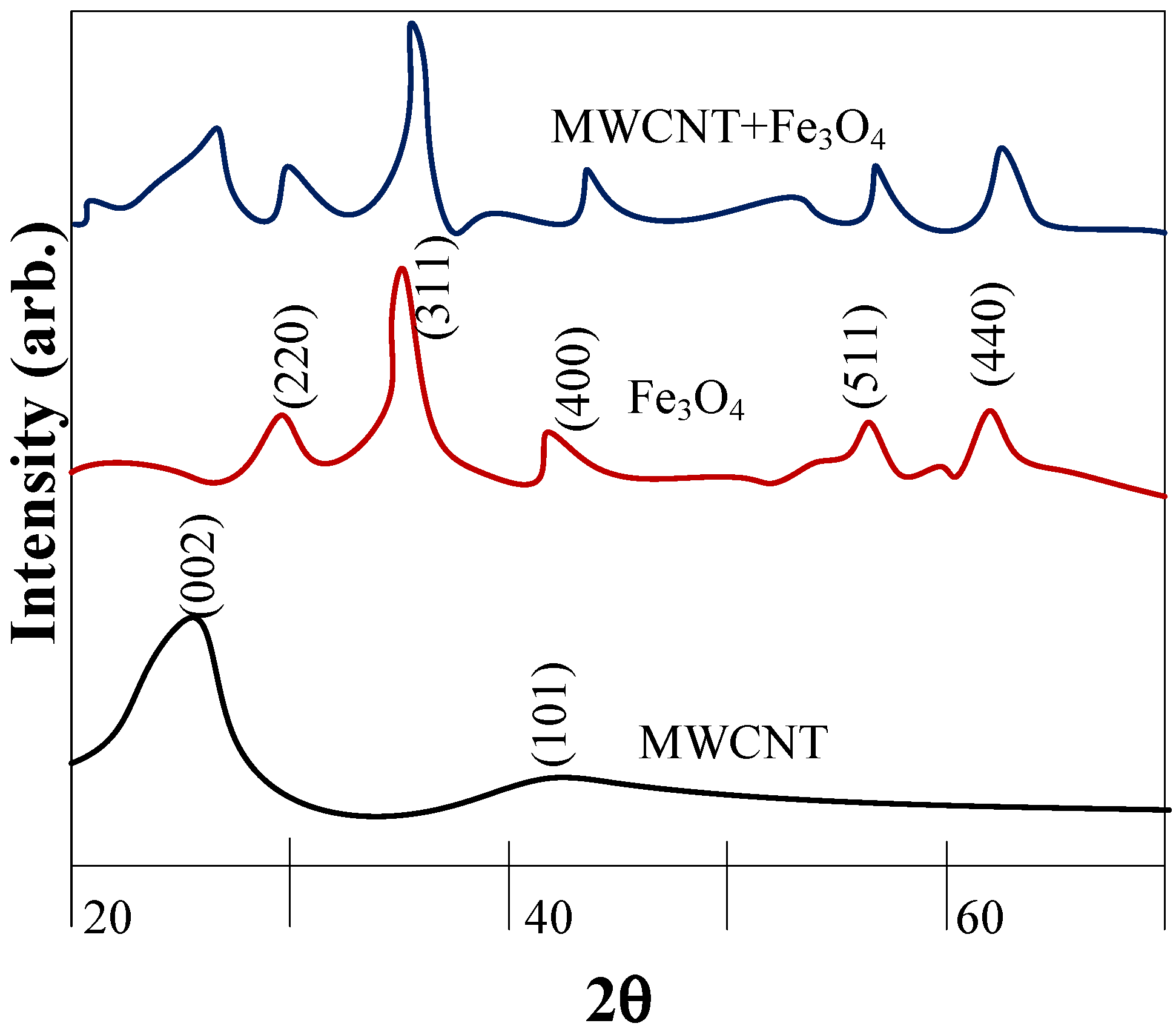
6. Nanoparticle Size Analysis
- (1)
- Dynamic light scattering (DLS) distribution method;
- (2)
- Scanning electron microscopy (SEM) and transmission electron microscopy (TEM0 images;
- (3)
- The absorption spectrum change in time (UV-Visible, use of spectrometer);
- (4)
- X-ray diffraction (XRD)—to check molecules’ crystalline structure.
7. Heat Transfer Enhancement
8. Conclusions
- selection of a proper nanofluid stabilization technique,
- quality assessment of nanofluid preparation with different and available methods,
- thermal conductivity models and enhancement of heat transfer rate,
- new mono and hybrid nanofluids symbology suggestions,
- future challenges and problems need to be solved with nanofluids as the working medium.
Funding
Conflicts of Interest
Nomenclature
| Greeks Latter | |
| α | is the lattice parameter of the crystal lattice |
| βpw | peak width |
| β | the ratio of the nanolayer thickness to the original particle radius (β = h/r) |
| γ | interfacial thermal conductivity ratio |
| δ | standard deviation |
| ε | dielectric constant of the solvent |
| ε0 | vacuum permittivity |
| ζ | zeta potential |
| θ | Bragg’s angle |
| κ | a function of the ionic concentration |
| λ | radiation wavelength |
| µ | dynamic viscosity coefficient |
| ν | kinematic viscosity coefficient |
| ρ | density |
| φ | concentration |
| Symbols | |
| A | Hamaker constant |
| Bip | nanoparticle Biot number |
| Ab | absorbance |
| cp | specific heat capacity |
| D | diffusion constant |
| De | an average crystal size |
| d | diameter |
| dhkl | lattice parameter of the synthezied nanoadditives |
| f | friction factor |
| FN | potential energy |
| FA | potential Van der Waals attraction |
| FR | energy of the repulsive electrostatic interaction respectively |
| Fb, Fg, Fν | hydrostatic, gravity and internal friction forces respectively |
| g | gravitational acceleration. |
| h | thickness of solid-like layer (r+h—equivalent particle radius) |
| h, k, l | Miller’s indicators |
| I | radiation intensity |
| k | thermal conductivity |
| kB | the Boltzmann constant |
| m | mass |
| n | particle number per volume |
| Pr | the Prandtl number of the base fluid |
| R, r | radius |
| Re | the Brownian–Reynolds number |
| Rp-f | interfacial thermal resistance between nanoparticles and different fluids |
| T | temperature |
| t | time |
| V | volume |
| v | velocity |
| x | distance between the surfaces |
| Z | zeta potential |
| Abbreviations (major) | |
| BN | noron nitride |
| CMC | carboxymethylcellulosum |
| CNT | carbon nanotubes |
| CMNC | ceramic matrix nano composites |
| CTAB | cetyl trimethyl ammonium bromide |
| DDDW | double distilled and deionised water |
| DI | deionized |
| DLS | dynamic light scattering |
| DTAB | dodecyl trimethyl ammonium bromide |
| DWNT | double-wall nanotubes |
| EG | ethylene glycol |
| GNP | graphene nano platelets |
| IEP | isoelectric point |
| MFP | mean free path (of molecules) |
| MMNC | metal matrix nano composites |
| MWCNT | multi-wall carbon nanotubes |
| NaDDBS | sodium dodecylbenzenesulfonate |
| PDI | polydispersity index |
| PMNC | polymer matrix nano composites |
| PVP | polyvinyl pyrrolidone |
| SDBS | sodium dodecyl benzene sulfonate |
| SDS | dodecyl sulfate |
| SEM | scanning electron microscopy |
| SOCT | sodium octanoate |
| SWNT | single-wall nanotubes |
| TEM | transmission electron microscopy |
| TCR | thermal conductivity ratio |
| TCE | thermal conductivity enhancement |
| XRD | x-ray diffraction |
| VSM | vibrating sample magnetometry |
| W | water |
| Indexes | |
| c | cluster |
| eff | effective |
| F/f/bf | base fluid |
| N/nf/ hnf | nanofluid/ hybrid nanofluid |
| p | (nano)particles |
References
- Rostami, S.; Shahsavar, A.; Kefayati, G.; Shahsavar Goldanlou, A. Energy and exergy analysis of using turbulator in a parabolic trough solar collector filled with mesoporous silica modified with copper nanoparticles hybrid nanofluid. Energies 2020, 13, 2946. [Google Scholar] [CrossRef]
- Taniguchi, N. On the basic concept of “nano-technology”. In Proceedings of the International Conference on Production Engineering, Tokyo, Japan, 26–29 August 1974; Volume 5, Part II. pp. 8–23. [Google Scholar]
- Maxwell, J.C. Electricity and Magnetism; Clarendon Press: Oxford, UK, 1873. [Google Scholar]
- Bozorth, R.M. Ferromagnetism; Wiley-IEEE Press: New York, NY, USA, 1951; Van Nostrand. [Google Scholar]
- Akoh, H.; Tsukasaki, Y.; Yatsuya, S.; Tasaki, A. Magnetic properties of ferromagnetic ultrafine particles prepared. J. Cryst. Growth 1978, 45, 495–500. [Google Scholar] [CrossRef]
- Duncan, M.A.; Rouvray, D.H. Microclusters. Sci. Am. 1989, 261, 110–115. [Google Scholar] [CrossRef]
- Siegel, R.W.; Eastman, J.A. A small revolution creates materials I atomic building block at a time. Logos 1993, 11, 2–7. [Google Scholar]
- Hill, P.G.; Witting, H.; Demetri, E.P. Condensation of metal vapors during rapid expansion. J. Heat Transf. 1963, 85, 303–317. [Google Scholar] [CrossRef]
- Andres, R.P.; Bowles, R.S.; Kolstad, J.J.; Calo, J.M. Generation of molecular clusters of controlled size. Surf. Sci. 1981, 106, 117–124. [Google Scholar] [CrossRef]
- Brown, D.P.; Chung, J.N.; Crowe, C.T. A numerical simulation of nanocluster formation in supersonic expansion flows. Micromechanical Syst. 1992, 40, 211–225. [Google Scholar]
- Choi, U.S.; Eastman, J.A. Enhancing thermal conductivity of fluids with nanoparticles, Developments and Applications of Non-Newtonian Flows. J. Heat Transf. 1995, 66, 99–105. [Google Scholar]
- Khoshvaght-Aliabadi, M.; Sahamiyan, M. Performance of nanofluid flow in corrugated minichannels heat sink (CMCHS). Energy Convers. Manag. 2016, 108, 297–308. [Google Scholar] [CrossRef]
- Wen, D.; Ding, Y. Effect of particle migration on heat transfer in suspensions of nanoparticles flowing through minichannels. Microfluid Nanofluid 2005, 1, 183–189. [Google Scholar] [CrossRef]
- Qureshi, Z.A.; Ali, H.M.; Khushnood, S. Recent advances on thermal conductivity enhancement of phase change materials for energy storage system: A review. Int. J. Heat Mass Transf. 2018, 127, 838–856. [Google Scholar] [CrossRef]
- Orzechowski, T.; Stokowiec, K. Quasi-stationary phase change heat transfer on a fin. FM15—Experimental Fluid Mechanics. In EPJ Web of Conferences; Dancova, P., Vesely, M., Eds.; EPJ Web of Conferences Series; EPD Sciences: Paris, France, 2016; Volume 114, p. 02086. [Google Scholar] [CrossRef] [Green Version]
- Radomska, E.; Mika, L.; Sztekler, K. The impact of additives on the main properties of phase change materials. Energies 2020, 13, 3064. [Google Scholar] [CrossRef]
- Benkovičová, M.; Végsö, K.; Šiffalovič, P.; Jergel, M.; Majková, E.; Luby, S.; Šatka, A. Preparation of sterically stabilized gold nanoparticles for plasmonic applications. Chem. Pap. 2013, 67, 1225–1230. [Google Scholar] [CrossRef]
- Suhaimi, N.S.; Md Din, M.F.; Rahman, A.R.A.; Hamid, M.H.A.; Amin, N.A.M.; Zamri, W.F.H.W.; Wang, J. Optimum electrical and dielectric performance of multi-walled carbon nanotubes doped disposed transformer oil. Energies 2020, 13, 3181. [Google Scholar] [CrossRef]
- Sekar, A.D.; Jayabalan, T.; Muthukumar, H.; Chandrasekaran, N.I.; Mohamed, S.N.; Matheswaran, M. Enhancing power generation and treatment of dairy waste water in microbial fuel cell using Cu-doped iron oxide nanoparticles decorated anode. Energy 2019, 172, 173–180. [Google Scholar] [CrossRef]
- Sahiner, N.; Seven, F. The use of superporous p(AAc (acrylic acid)) cryogels as support for Co and Ni nanoparticle preparation and as reactor in H2 production from sodium borohydride hydrolysis. Energy 2014, 71, 170–179. [Google Scholar] [CrossRef]
- Karimi-Nazarabad, M.; Goharshadi, E.K.; Entezari, M.H.; Nancarrow, P. Rheological properties of the nanofluids of tungsten oxide nanoparticles in ethylene glycol and glycerol. Microfluid Nanofluid 2015, 19, 1191–1202. [Google Scholar] [CrossRef]
- Babar, H.; Ali, H.M. Towards hybrid nanofluids: Preparation, thermophysical properties, applications, and challenges. J. Mol. Liq. 2019, 281, 598–633. [Google Scholar] [CrossRef]
- Madhesh, D.; Parameshwaran, R.; Kalaiselvam, S. Experimental investigation on convective heat transfer and rheological characteristics of Cu-TiO2 hybrid nanofluids. Exp. Therm. Fluid Sci. 2014, 52, 104–115. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Y.; Liu, H.; Yin, Y.; Liang, J. Preparation and Application of Magnetic Nano-Solid Acid Catalyst Fe3O4-PDA-SO3H. Energies 2020, 13, 1484. [Google Scholar] [CrossRef] [Green Version]
- Mączka, M.; Gągor, A.; Macalik, B.; Pikul, A.; Ptak, M.; Hanuza, J. Order-disorder transition and weak ferromagnetism in the perovskite metal formate frameworks of [(CH3)(2)NH2][M(HCOO)(3)] and [(CH3)(2)ND2][M(HCOO)(3)] (M = Ni, Mn). Inorg. Chem. 2014, 53, 457–467. [Google Scholar] [CrossRef]
- Sheikholeslami, M.; Ganji, D.D. Ferrohydrodynamic and magnetohydrodynamic effects on ferrofluid flow and convective heat transfer. Energy 2014, 75, 400–410. [Google Scholar] [CrossRef]
- Abadeh, A.; Mohammadi, M.; Passandideh-Fard, M. Experimental investigation on heat transfer enhancement for a ferrofluid in ahelically coiled pipe under constant magnetic field. J. Therm. Anal. Calorim. 2018, 135, 1069–1079. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Magnetic fluids. Annu. Rev. Fluid Mech. 1987, 19, 437–461. [Google Scholar] [CrossRef]
- Hayat, T.; Nadeem, S. Heat transfer enhancement with Ag-CuO/water hybrid nanofluid. Results Phys. 2017, 7, 2317–2324. [Google Scholar] [CrossRef]
- Esfe, M.H.; Alirezaie, A.; Rejvani, M. An applicable study on the thermal conductivity of SWCNT-MgO hybryd nanofluid and price-performance analysis for energy management. Appl. Therm. Eng. 2017, 111, 1202–1210. [Google Scholar] [CrossRef]
- Marciak-Kozłowska, J.; Kozłowski, M.; Mucha, Z. Time and energy scales for thermal properties of nanoparticles. Mater. Sci. Forum 2002, 384–385, 75–78. [Google Scholar] [CrossRef]
- Wciślik, S. A simple economic and heat transfer analysis of the nanoparticles use. Chem. Pap. 2017, 11696, 1–7. [Google Scholar] [CrossRef]
- Sarkar, J.; Ghosh, P.; Adil, A. A review on hybrid nanofluids: Recent research, development and applications. Renew. Sustain. Energy Rev. 2015, 43, 164–177. [Google Scholar] [CrossRef]
- Babu, J.A.R.; Kumar, K.K.; Rao, S.S. State-of-art review on hybrid nanofluids. Renew. Sustain. Energy Rev. 2017, 77, 551–565. [Google Scholar] [CrossRef]
- Sundar, L.S.; Singh, M.K.; Sousa, A.C.M. Enhanced heat transfer and friction factor of MWCNT-Fe3O4/water hybrid nanofluids. Int. Commun. Heat Mass Transf. 2014, 52, 73–83. [Google Scholar] [CrossRef]
- Wu, S.; Ortiz, C.R. Experimental investigation of the effect of magnetic field on vapour absorption with LiBr-H2O nanofluid. Energy 2020, 193, 116640. [Google Scholar] [CrossRef]
- Xiao, X.; Jia, H.; Wen, D.; Zhao, X. Thermal performance analysis of a solar energy storage unit encapsulated with HITEC salt/copper foam/nanoparticles composite. Energy 2020, 192, 116593. [Google Scholar] [CrossRef]
- Lee, J.W.; Kang, Y.T. CO2 absorption enhancement by Al2O3 nanoparticles in NaCl aqueous solution. Energy 2020, 53, 206–211. [Google Scholar] [CrossRef]
- Vajjha, R.S.; Das, D.K. Experimental determination of thermal conductivity of three nanofluids and development of new correlations. Int. J. Heat Mass Transf. 2009, 52, 4675–4682. [Google Scholar] [CrossRef]
- Zhou, Y.; Zheng, S. Multi-level uncertainty optimisation on phase change materials integrated renewable systems with hybrid ventilations and active cooling. Energy 2020, 202, 117747. [Google Scholar] [CrossRef]
- Passandideh-Fard, A.A.M.; Maghrebi, M.J.; Mohammadi, M. Stability and magnetization of Fe3O4/water nanofluid preparation characteristics using Taguchi metod. J. Therm. Anal. Calorim. 2018, 135, 1323–1334. [Google Scholar] [CrossRef]
- Devendiran, D.K.; Amirtham, V.A. A review on preparation, characterization, properties and applications of nanofluids. Renew. Sustain. Energy Rev. 2016, 60, 21–40. [Google Scholar] [CrossRef]
- Guo, S.; Dong, S.; Wang, E. Gold/platinum hybrid nanoparticles supported on multiwalled carbon nanotube /silica coaxial nanocables: Preparation and application as electrocatalysts for oxygen reduction. J. Phys. Chem. C 2008, 112, 2389–2393. [Google Scholar] [CrossRef]
- Pavlenko, A.; Koshlak, H.; Slowak, A. Stability of multiphase liquid media. IOP C. Ser. Earth Env. 2019, 227, 1–11. [Google Scholar] [CrossRef]
- Khan, S.I.U.; Alzahrani, E.; Khan, U.; Zeb, N.; Zeb, A. On mixed convection squeezing flow of nanofluids. Energies 2020, 13, 3138. [Google Scholar] [CrossRef]
- Dey, D.; Kumar, P.; Samantaray, S. A review of nanofluid preparation, stability, and thermo-physical properties. Heat Transf. Asian Res. 2017, 46, 1413–1442. [Google Scholar] [CrossRef]
- Levine, S.; Dube, G.P. Interaction between two hydrophobic colloidal particles, using the approximate Debye-Huckel theory. I. General properties. Trans. Faraday Soc. 1940, 35, 1125–1141. [Google Scholar] [CrossRef]
- Singh, P.K.; Khandelwal, D.; Sidhant, C.; Shubham, A.; Priyanshu, N.; Rasu, N.G. Nanofluid heat transfer mechanism and thermo-physical properties: A review. IJMET 2017, 8, 156–164. Available online: http://www.iaeme.com/ijmet/issues.asp?JType=IJMET&VType=8&IType=11 (accessed on 15 July 2020).
- Cieslinski, J.T.; Ronewicz, K.; Smolen, S. Measurement of temperature-dependent viscosity and thermal conductivity of alumina and titania thermal oil nanofluids. Arch. Thermodyn. 2015, 36, 35–47. [Google Scholar] [CrossRef] [Green Version]
- Hussein, A.M. Thermal performance and thermal properties of hybrid nanofluid laminar flow in a double pipe heat exchanger. Exp. Therm. Fluid Sci. 2017, 88, 37–45. [Google Scholar] [CrossRef]
- Prakash, V.; Rai, B.; Tyagai, V.K.; Niyogi, U.K. Dispersion and characterizations of nanofluids prepared with CuO and CNT nanoparticles. J. Indian Chem. Soc. 2015, 92, 1245–1251. [Google Scholar]
- Lamas, B.; Abreu, B.; Fonseca, A.; Martins, N.; Oliveira, M. Assessing colloidal stability of long term MWCNTs based nanofluids. J. Colloid Interface Sci. 2012, 381, 17–23. [Google Scholar] [CrossRef]
- Hung, Y.H.; Wang, W.P.; Hsu, Y.C.; Teng, T.P. Performance evaluation of an air-cooled heat exchange system for hybrid nanofluids. Exp. Therm. Fluid Sci. 2017, 81, 43–55. [Google Scholar] [CrossRef]
- Aberoumand, S.; Jafarimoghaddam, A. Tungsten (III) oxide (WO3)—Silver/transformer oil hybrid nanofluid: Preparation, stability, thermal conductivity and dielectric strength. Alex. Eng. J. 2016, 57, 169–174. [Google Scholar] [CrossRef]
- Tran, P.X.; Soong, Y. Preparation of nanofluids using laser ablation in liquid technique. In Proceedings of the ASME Applied Mechanics and Material Conference 2007, Austin, TX, USA, 3–7 June 2007. [Google Scholar]
- Akoh, H.; Tsukasaki, Y.; Yatsuya, S.; Tasaki, A. Magnetic properties of ferromagnetic ultrafine particles prepared by vacuum evaporation on running oil substrate. J. Cryst. Growth 1978, 45, 495–500. [Google Scholar] [CrossRef]
- Loa, C.H.; Tsunga, T.T.; Lin, H.M. Preparation of silver nanofluid by the submerged arc nanoparticle synthesis system (SANSS). J. Alloys Compd. 2007, 434–435, 659–662. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, K.S.; Jang, S.P.; Lee, B.H.; Kim, J.H.; Choi, S.U.S.; Choi, C.J. Effective viscosities and thermal conductivities of aqueous nanofluids containing low volume concentrations of Al2O3 nanoparticles. Int. J. Heat Mass Transf. 2008, 5, 2651–2656. [Google Scholar] [CrossRef]
- Zhu, H.; Lin, Y.; Yin, Y. A novel one-step chemical method for preparation of copper nanofluids. Colloid Interface Sci. 2004, 277, 100–103. [Google Scholar] [CrossRef]
- Wagener, M.; Murty, B.S.; Gunther, B. Preparation of metal nanosuspensions by high pressure DC sputtering on running liquids. Nanocrystalline Nanocomposite Mater. II 1997, 457, 149–154. [Google Scholar] [CrossRef]
- Munkhbayar, B.; Tanshen, M.R.; Jeoun, J.; Chung, H.; Jeong, H. Surfactant-free dispersion of silver nanoparticles into MWCNT-aqueous nanofluids prepared by one-step technique and their thermal characteristics. Ceram. Int. 2013, 39, 6415–6425. [Google Scholar] [CrossRef]
- Mukherjee, S.; Paria, S. Preparation and Stability of Nanofluids—A Review. J. Mech. Civ. Eng. 2013, 9, 63–69. [Google Scholar] [CrossRef]
- Mali, S.; Pise, A.; Acharya, A. Review on flow boiling heat transfer enhancement with nanofluids. IOSR J. Mech. Civ. Eng. 2014, 11, 43–48. [Google Scholar] [CrossRef]
- Wang, X.Q.; Mujumdar, A.S. A review on nanofluids part II: Experiments and applications. Braz. J. Chem. Eng. 2008, 25, 631–648. [Google Scholar] [CrossRef]
- Syam, S.L.; Venkata, R.E.; Singh, M.K.; DeSousa, A.C.M. Viscosity of low volume concentrations of magnetic Fe3O4 nanoparticles dispersedin ethylene glycol and water mixture. Chem. Phys. Lett. 2012, 554, 236–242. [Google Scholar] [CrossRef]
- Jama, M.; Singh, T.; Gamaleldin, S.M.; Koc, M.; Samara, A.; Isaifan, R.J.; Atieh, M.A. Critical Review on Nanofluids: Preparation, Characterization, and Applications. J. Nanomater. 2016, 2016, 6717624. [Google Scholar] [CrossRef] [Green Version]
- Kadhim, S.; Humud, H.; Abdulmajeed, I.M. Silver nanofluids prepared by pulse exploding wire. AARJMD 2014, 1, 2319–2801. [Google Scholar]
- Cho, C.; Kang, C.; Ha, Y.C.; Jin, T.S.; Rim, G.H. Optimum discharge conditions for smaller particles from Ag wire explosion in liquid media. J. Phys. Soc. Jpn. 2011, 59, 3662–3665. [Google Scholar] [CrossRef]
- Shehata, F.; Abdelhameed, M.; Fathy, A.; Elmahdy, M. Preparation and characteristics of Cu-Al2O3 Nanocomposite. Open J. Met. 2011, 1, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Esfe, M.H.; Esfandeh, S.; Saedodin, S.; Rostamian, H. Experimental evaluation, sensitivity analyzation and ANN modeling of thermal conductivity of ZnO-MWCNT/EG-water hybrid nanofluid for engineering applications. Appl. Therm. Eng. 2017, 125, 673–685. [Google Scholar] [CrossRef]
- Hamid, K.A.; Azmi, W.H.; Nabil, M.F.; Mamat, R. Experimental investigation of nanoparticle mixture ratios on TiO2-SiO2 nanofluids heat transfer performance under turbulent flow. Int. J. Heat Mass Transf. 2018, 118, 617–627. [Google Scholar] [CrossRef]
- Nabil, M.F.; Azmi, W.H.; Hamid, K.A.; Mamat, R.; Hagos, F.Y. An experimental study on the thermal conductivity and dynamic viscosity of TiO2-SiO2 nanofluids in water: Ethylene glycol mixture. Int. Commun. Heat Mass Transf. 2017, 86, 181–189. [Google Scholar] [CrossRef]
- Wei, B.; Zou, C.; Yuan, X.; Li, X. Thermo-physical property evaluation of diathermic oil based hybrid nanofluids for heat transfer applications. Int. J. Heat Mass Transf. 2017, 107, 281–287. [Google Scholar] [CrossRef]
- Qing, S.H.; Rashmi, W.; Khalid, M.; Gupta, T.C.S.M.; Nabipoor, M.; Hajibeigy, M.T. Thermal conductivity and electrical properties of Hybrid SiO2-graphene naphthenic mineral oil nanofluid as potential transformer oil. Mater. Res. Express. 2017, 4, 015504. [Google Scholar] [CrossRef]
- Shehata, F.; Fathy, A.; Abdelhameed, M.; Moustafa, S. Preparation and properties of Al2O3 nanoparticle reinforced copper matrix composites by insitu processing. Mater. Des. 2009, 30, 2756–2762. [Google Scholar] [CrossRef]
- Colangelo, G.; Favale, E.; Miglietta, P.; Milanese, M.; de Risi, A. Thermal conductivity, viscosity and stability of Al2O3-diathermic oil nanofluids for solar energy systems. Energy 2016, 95, 124–136. [Google Scholar] [CrossRef]
- Rabienataj Darzi, A.A.; Farhadi, M.; Sedighi, K.; Shafaghat, R.; Zabihi, K. Experimental investigation of turbulent heat transfer and flow characteristics of SiO2/water nanofluid within helically corrugated tubes. Int. Commun. Heat Mass Transf. 2012, 39, 1425–1434. [Google Scholar] [CrossRef]
- Setia, H.; Gupta, R.; Wanchoo, R. Stability of nanofluids. Mater. Sci. Forum 2013, 757, 139–149. [Google Scholar] [CrossRef]
- Zhou, M.Z.; Xia, G.D.; Li, J.; Chai, L.; Zhou, L.J. Analysis of factors influencing thermal conductivity and viscosity in different kinds of surfactant solutions. Exp. Therm. Fluid Sci. 2012, 36, 22–29. [Google Scholar]
- Sarsam, W.S.; Amiri, A.; Kazi, S.N.; Badarudin, A. Stability and thermo-physical properties of non-covalently functionalized graphene nanoplatelets. Energy Convers. Manag. 2016, 116, 101–111. [Google Scholar] [CrossRef]
- Sarsam, W.S.; Amiri, A.; Zubir, M.N.M.; Yarmand, H.; Kazi, S.N.; Badarudin, A. Stability and thermo-physical properties of water-based nano-fluids containing triethanolamine-treated graphene nanoplatelets with different specific surface. Colloids Surf. A 2016, 500, 17–31. [Google Scholar] [CrossRef]
- Brinkman, H.C. The viscosity of concentrated suspensions and solutions. J. Chem. Phys. 1952, 20, 571–581. [Google Scholar] [CrossRef]
- Chung, S.J.; Leonard, J.P.; Nettleship, I.; Lee, J.K.; Soong, Y.; Martello, D.V.; Chyu, M.K. Characterization of ZnO nanoparticle suspension in water: Effectiveness of ultrasonic dispersion. Powder Tech. 2009, 194, 1–2, 75–80. [Google Scholar] [CrossRef]
- Silambarasan, M.; Manikandan, S.; Rajan, K.S. Viscosity and thermal conductivity of dispersions of sub-micron TiO2 particles in water prepared by stirred bead milling and ultrasonication. Int. J. Heat Mass Transf. 2012, 55, 7991–8002. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Shirazi, S.F.S.; Goodarzi, M.; Amiri, A.; Sarsam, W.S.; Alehashem, M.S.; Dahari, M.; Kazi, S.N. Study of synthesis, stability and thermo-physical properties of graphene nanoplatelet/platinum hybrid nanofluid. Int. Commun. Heat Mass Transf. 2016, 77, 15–21. [Google Scholar] [CrossRef]
- Kwon, D.; Kim, H.; Leong, H. Heat and its effects to chemical mechanical polishing. J. Mater. Process. Technol. 2006, 178, 82–87. [Google Scholar] [CrossRef]
- Yarmand, H.; Gharehkhani, S.; Ahmadi, G.; Shirazi, S.F.S.; Baradaran, S.; Montazer, E.; Zubir, M.N.M.; Alehashem, M.S.; Kazi, S.N.; Dahari, M. Graphene nanoplatelets-silver hybrid nanofluids for enhanced heat transfer. Energy Convers. Manag. 2015, 100, 419–428. [Google Scholar] [CrossRef]
- Zhang, P.; Hong, W.; Wu, J.F.; Liu, G.Z.; Xiao, J.; Chen, Z.B.; Cheng, H.B. Effect of surface modification on the suspension stability and thermal conductivity of carbon nanotubes nanofluids. Energy Procedia 2015, 69, 699–705. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.K.; Harikrishna, P.V.; Sundararajan, T.; Das, S.K. Experimental and numerical investigation into the hydrodynamics of nanofluids in microchannels. Exp. Therm. Fluid Sci. 2012, 42, 174–186. [Google Scholar] [CrossRef]
- Uskokovic, V. Nanotechnologies: What we do not know. Technol. Soc. 2007, 29, 43–61. [Google Scholar] [CrossRef]
- Odenbach, S. Ferrofluids: Magnetically controllable fluids and their applications. In Lecture Notes in Physics; Springer: Bremen, Germany, 2002; Volume 594, pp. 33–58. [Google Scholar] [CrossRef]
- Lau, Z.Y.; Lee, K.C.; Soleimani, H.; Beh, H.G. Experimental study of electromagnetic-assisted rare-earth doped yttrium iron garnet (YIG) nanofluids on wettability and interfacial tension alteration. Energies 2019, 12, 3806. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, R.; Etemad, S.G.; Keshavarzi, E.; Haghshenasfard, M. Investigation of alumina nanofluid stability by UV-vis spectrum. Microfluid Nanofluid 2015, 18, 1023–1030. [Google Scholar] [CrossRef]
- West, J.; Sears, J.; Smith, S.; Carter, M. Photonic sintering—An example: Photonic curing of silver nanoparticles. In Sintering of Advanced Materials—Fundamentals and Processes; Woodhead Publishing: Oxford, UK, 2010; pp. 275–288. [Google Scholar]
- Tiwari, K.A.; Ghosh, P.; Sarkar, J. Investigation of thermal Conductivity and viscosity of Nanofluids. J. Environ. Res. Dev. 2012, 2, 768–777. [Google Scholar]
- Özerinç, S.; Kakaç, S.; Yazicioglu, A.G. Enhanced thermal conductivity of nanofluids: A state-of-the-art review. Microfluid Nanofluid 2010, 8, 145–170. [Google Scholar] [CrossRef]
- Esfe, M.H.; Behbahani, P.M.; Arani, A.A.A.; Sarlak, M.R. Thermal conductivity enhancement of SiO2-MWCNT (85:15%)-EG hybrid nanofluids. J. Therm. Anal. Calorim. 2017, 128, 249–258. [Google Scholar] [CrossRef]
- Toghraie, D.; Chaharsoghi, V.A.; Afrand, M. Measurement of thermal conductivity of ZnO-TiO2/EG hybrid nanofluid. J. Therm. Anal. Calorim. 2016, 125, 527–535. [Google Scholar] [CrossRef]
- Harandi, S.S.; Karimipour, A.; Afrand, M.; Akbari, M.; D’Orazio, A. An experimental study on thermal conductivity of F-MWCNTs-Fe3O4/EG hybrid nanofluid: Effects of temperature and concentration. Int. Commun. Heat Mass Transf. 2016, 76, 171–177. [Google Scholar] [CrossRef] [Green Version]
- Chougule, S.S.; Sahu, S.K. Comparative study on heat transfer enhancement of low volume concentration of al2o3–water and carbon nanotube–water nanofluids in laminar regime using helical screw tape inserts. J. Nanotechnol. Eng. Med. 2013, 4, 040904. [Google Scholar] [CrossRef]
- Prasher, R.; Bhattacharya, P.; Phelan, P.E. Brownian-motion-based convective-conductive model for the effective thermal conductivity of nanofluids. J. Heat Transf. 2006, 128, 588–595. [Google Scholar] [CrossRef]
- Chon, C.H.; Kihm, K.D.; Lee, S.P.; Choi, S.U.S. Empirical correlation finding the role of temperature and particle size for nanofluid (Al2O3) thermal conductivity enhancement. Appl. Phys. Lett. 2005, 87, 153107. [Google Scholar] [CrossRef]
- Yu, W.; Choi, S.U.S. The role of interfacial layers in the enhanced thermal conductivity of nanofluids: A renovated Maxwell model. J. Nanopart. Res. 2003, 5, 167–171. [Google Scholar] [CrossRef]
- Xuan, Y.; Li, Q.; Hu, W. Aggression structure and thermal conductivity of nanofluids. Am. Inst. Chem. Eng. 2003, 49, 1038–1043. [Google Scholar] [CrossRef]
- Hamilton, R.; Crosser, O. Thermal conductivity of heterogeneous two component systems. Ind. Eng. Chem. Fundam. 1962, 125, 187–191. [Google Scholar] [CrossRef]
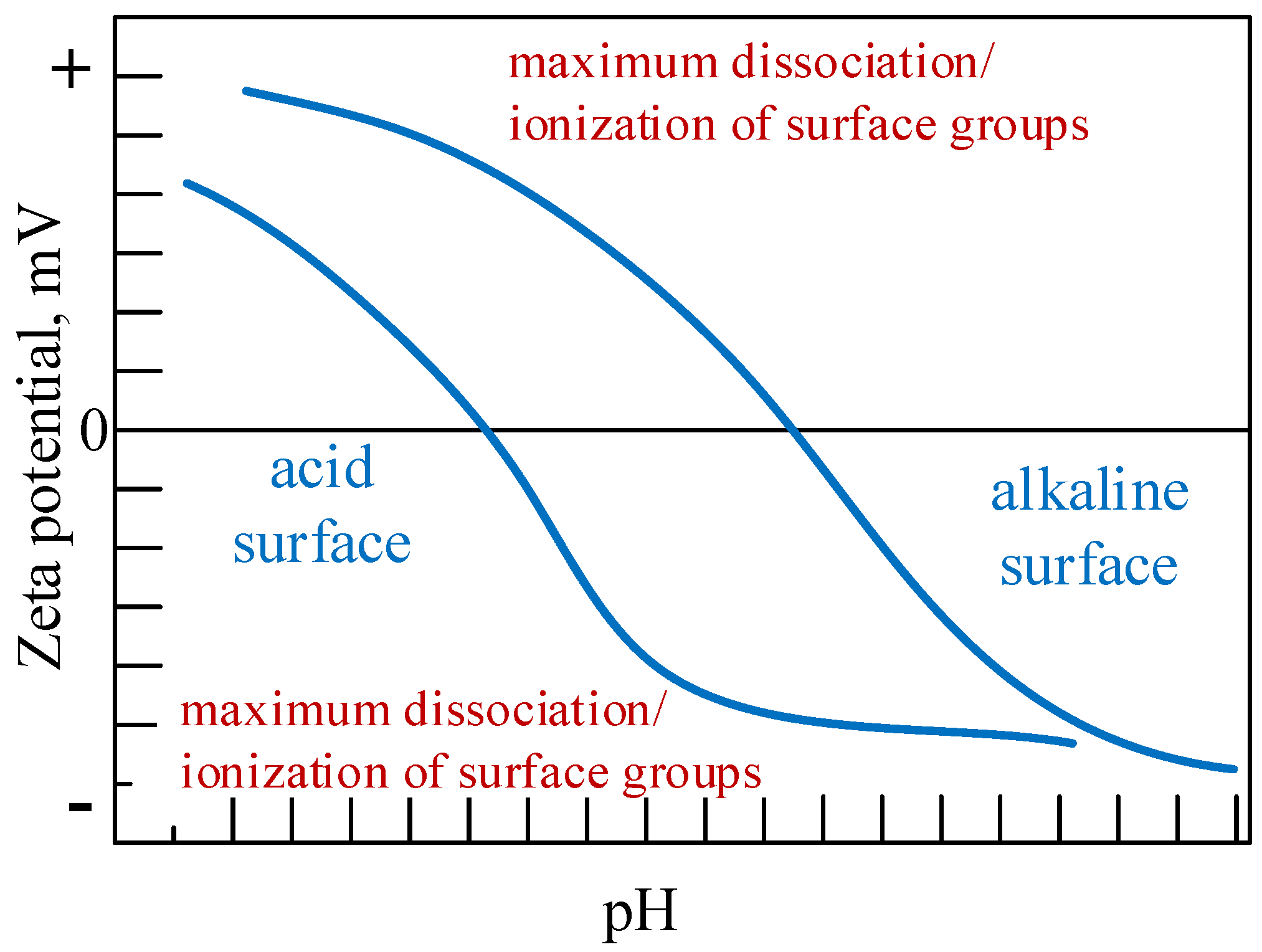
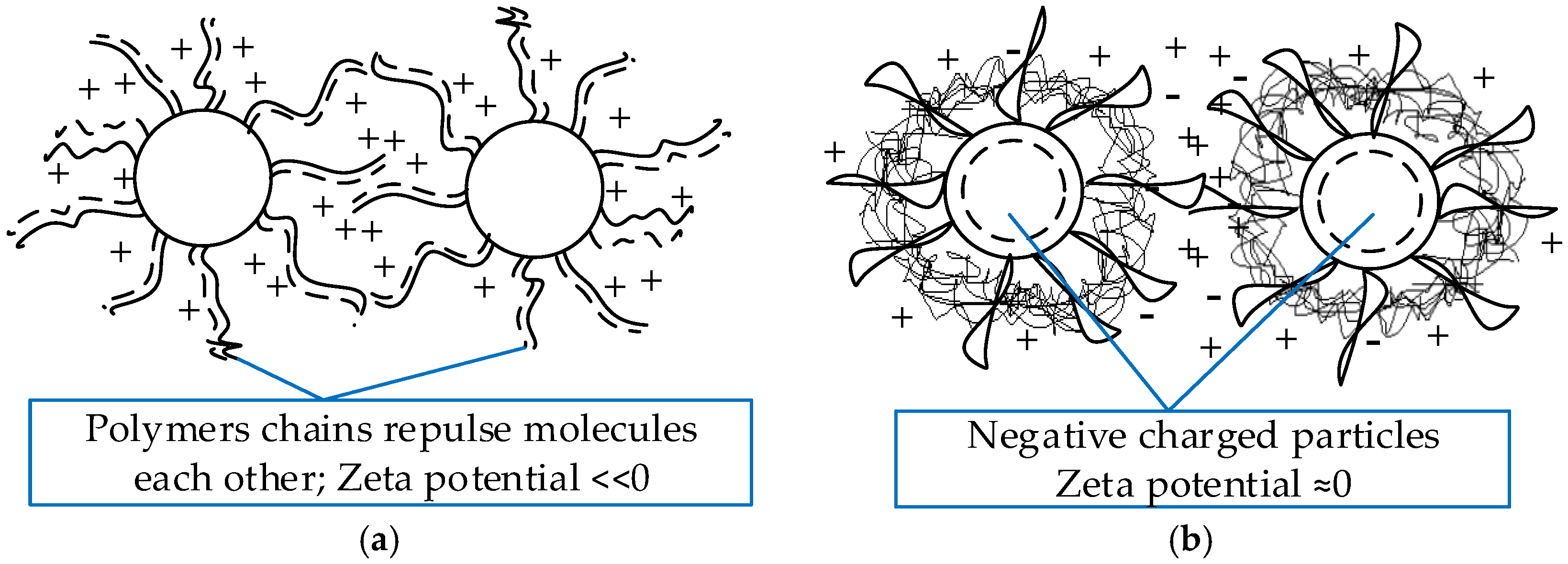
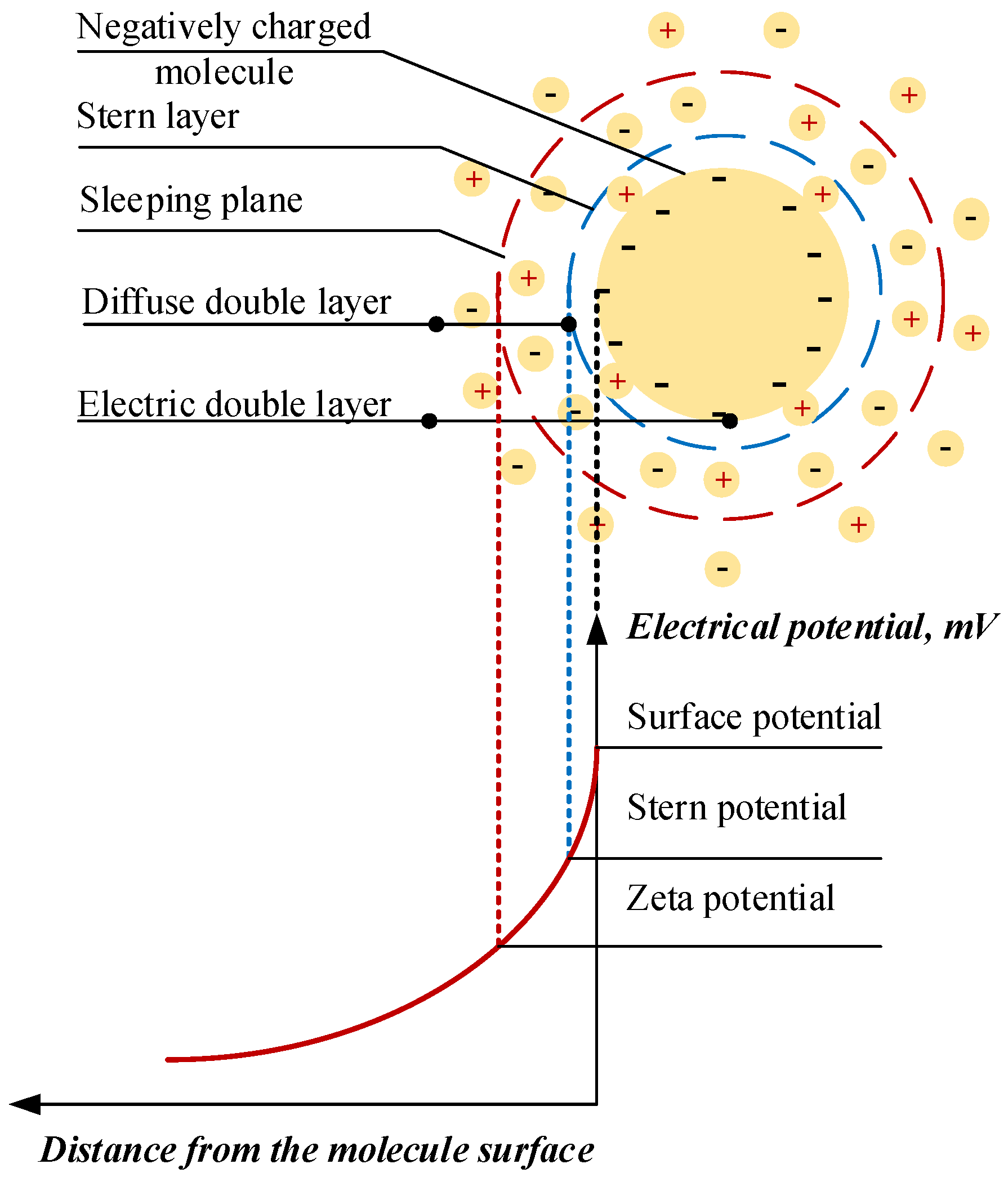
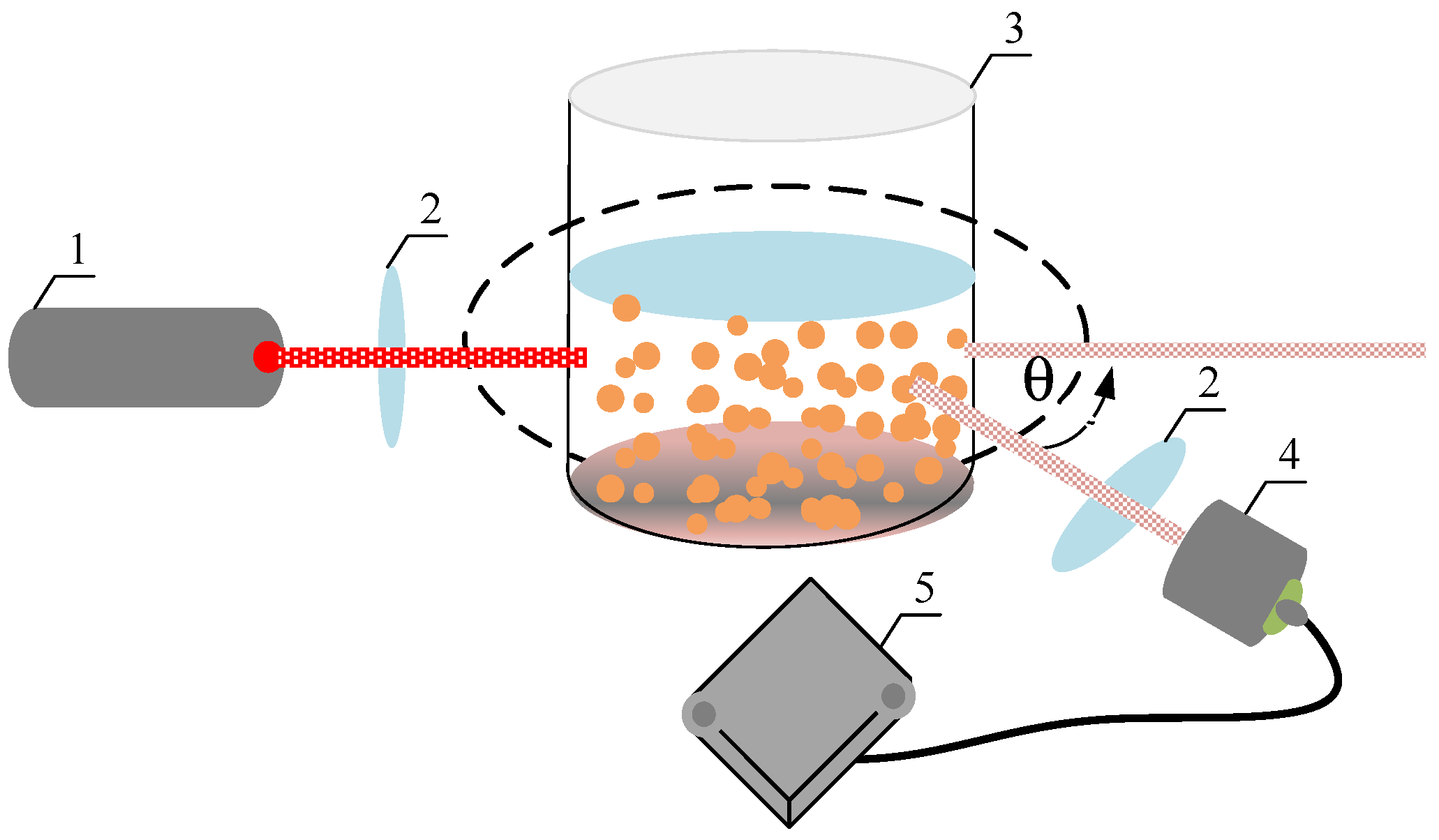
| 1873 | Maxwell [3] proposes the innovative idea of adding solid particles to heat transfer fluids to rise their thermal conductivity thermodynamic parameter. |
| 1881 | Maxwell published academic and experimental papers of the effective thermal conductivity of dispersions with millimetre and micrometer-sized solid particles. |
| 1951 | Development of ferrofluids as intelligent nanofluids (Bozorth [4]) |
| 1959 | Richard Feynman proposes a new theory of quantum electrodynamics and the technical directions of nanoscience were defined. |
| 1974 | Norio Taniguchi presents in Japan the basic concept of ‘nanotechnology‘. |
| 1978 | Suggestions to produce nanophase powders from the vapor phase directly into a flowing low vapor pressure fluid fail due to problems in subsequently separating the dry particles (Akoh et al. [5]). |
| 1986 | Drexlers gives the idea of molecular nanotechnology and claims Feynman theory. |
| 1989–1994 | Advanced fluids for industrial applications—including district heating and cooling systems—are developed by researchers from the Argonne National Laboratory. The need for nanoscale additives to prevent clogging problems in heat exchangers was delineated. |
| Confirmation that the thermal, mechanical, optical, magnetic, and electrical properties of nanoadditives and nanofluids are much better in comparison to those of the typical fluids used in industrial applications. The relatively high surface-area-to-volume ratio of nanoadditives that is due to the high percent of constituent atoms that reside at the grain boundaries was used by material scientists and engineers alike (e.g., Duncan and Rouvray, [6]; Siegel and Estman, [7]). | |
| Development of physical gas-phase condensation or chemical synthesis techniques for the production of nanopowders with average particle sizes in the 10 nm range. | |
| 1963–1992 | Development a third technique for nanophase material generation by condensation of metal vapors during rapid expansion in a supersonic nozzle (e.g., Hill, et al., [8]; Andres, et al., [9]; Brown, et al., [10]). |
| 1995 | Choi and Eastman propose for the first time the practical use of copper nanoparticles dispersed in water [11]. |
| 2000– | Development of mono and hybrid nanofluids for heat transfer applications (problems and techniques of stabilization, coagulation and clustering of multi-sized nanocomposites, heat transfer models, thermal and rheological properties upgrading) and other problems described in the following sections of this review. |
| Nanoparticles Type | Base Fluid | Particle Loading, Vol. % | Particle Size, nm | Dispersion Method (Ultrasonication, h/Magnetic Stirring, h) | Stability Time, Days | Ref. |
|---|---|---|---|---|---|---|
| Al2O3 ZnO CuO | EG/water 60:40 | 1–10 | 53 29, 0, 77.0 29 | 2/- | Not reported | Vajjha and Das 2009 [39] |
| MWCNTs-ZnO | water/EG | 3/2 | 10 | Esfe et al. 2017 [70] | ||
| Aluminum Nitride | EG | 1–4 | 30 | 2.5/- | 60 | Hussein 2017 [50] |
| TiO2/SiO2 TiO2:SiO2 of 20:80, 40:60, 50:50, 60:40, 80:20 | water/EG | 1 | 50/22 | 2/1 | 14 | Hamid et al. 2018 [71] |
| TiO2/SiO2 | water/EG | 1.5/- | 14 | Nabil et al. 2017 [72] | ||
| SiC-TiO2 | diathermic oil | 0.1–1 | 30/10 | 2/0.5 | 10 | Wei et al. 2017 [73] |
| SiO2-graphene | naphthenic mineral oil | 0.01, 0.04, 0.08 | 4/- | 14 | Qing et al. 2017 [74] | |
| MWCNTs-Fe2O3 | water | 0, 0.1, 0.3 | 30/13 | 1/- | 60 | Sundar et al. 2014 [35] |
| Element | Samples, Weight, % |
|---|---|
| YIG * | |
| Y | 34.37 |
| Fe | 29.89 |
| O | 35.73 |
| RE | 0 |
| Researcher | Thermal Conductivity Model | Kind of Mono/Hybrid Naofluid | Maximum Thermal Conductivity Ratio/Thermal Conductivity Increase * | Remarks | |||
|---|---|---|---|---|---|---|---|
| Esfe et al. 2017 [97] | SiO2+MWCNT (85:15%)/EG | 22.2% for T = 50 °C | ϕ = 0.05–1.95 vol.%, T = 30–50 °C | ||||
| Toghraie et al. 2016 [98] | ZnO+TiO2/EG | ϕ = 0–3.5 vol.%, T = 25–50 °C | |||||
| Harandi et al. 2016 [99] | MWCNTs + Fe3O4/EG | 30% (for T = 50 °C, ϕ = 2.3%) | ϕ = 0.1, 0.25, 0.45, 0.8%, 1.25, 1.8 and 2.3 vol.%, T = 25–50 °C | ||||
| Chougule and Sahu 2013 [100] | The model cannot be used for higher particle concertation. | ||||||
| Vajjha and Das 2009 [39] | d = 29–77 nm; T = 298-363 K; ϕ = 1–10 vol.% | Al2O3/EG/W, ZnO/EG/W, CuO/EG/W EG/W: 60:40 | CuO/W: 1.6/60% ZnO/W: 1.49/49% Al2O3/W: 1.65/65% | Nanofluid thermal conductivity is directly proportional to particle concentration and temperature and inversly proportional to nanoparticle diameter | |||
| Prasher et al. 2006 [101] | m = 2.5 ± (15% of 2.5) for H2O based nanofluids, m = 1.6 ± (15% of 1.6) for EG-based nanofluids and m = 1.05 ± (15% of 1.05) for oil-based nanofluids | general H2O, oil and EG— based nanofluids | Convective-conductive model ie. combination of Maxwell-Garnett conduction model | ||||
| Chon et al. 2005 [102] | H2O based nanofluids | Based on Buckingham-Pi theorem with a linear regression scheme; Brownian motion of the nanoparticle is the crucial factor in the nanofluids thermal conductivity enhancement | |||||
| Yu and Choi 2003 [103] | Based on: The model is modified Maxwell model. It is noticeable that nanolayer is higher for smaller nanoparticles (r~h) It is appropriate for h ≤ 5 nm For larger particles, when h > 10 nm (r >> h, β → 0), the nanolayer impact is small and [103] model reduces to the original Maxwell model | 1.0 vol.% Cu/EG | 8× higher value than Maxwell model without taking into account the nanolayer | Authors suggest to insert particles of smaller diameter (<10 nm) instead of adding more particles of higher diameter. | |||
| Xuan et al. 2003 [104] | Cu/W | The model includes the Brownian motion of nanoparticles, which enhances the thermal conductivity of the nanofluid. | |||||
| Hamilton and Crosser 1962 [105] | For spherical particles n = 3 and Hamilton-Crosser model is equals the Maxwell model | ||||||
| Maxwell 1873 [3] | The model does not include the nanolayer | ||||||
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wciślik, S. Efficient Stabilization of Mono and Hybrid Nanofluids. Energies 2020, 13, 3793. https://doi.org/10.3390/en13153793
Wciślik S. Efficient Stabilization of Mono and Hybrid Nanofluids. Energies. 2020; 13(15):3793. https://doi.org/10.3390/en13153793
Chicago/Turabian StyleWciślik, Sylwia. 2020. "Efficient Stabilization of Mono and Hybrid Nanofluids" Energies 13, no. 15: 3793. https://doi.org/10.3390/en13153793
APA StyleWciślik, S. (2020). Efficient Stabilization of Mono and Hybrid Nanofluids. Energies, 13(15), 3793. https://doi.org/10.3390/en13153793








