The Effect of Hydrogen Addition on Low-Temperature Combustion of Light Hydrocarbons and Alcohols
Abstract
:1. Introduction
2. Materials and Methods
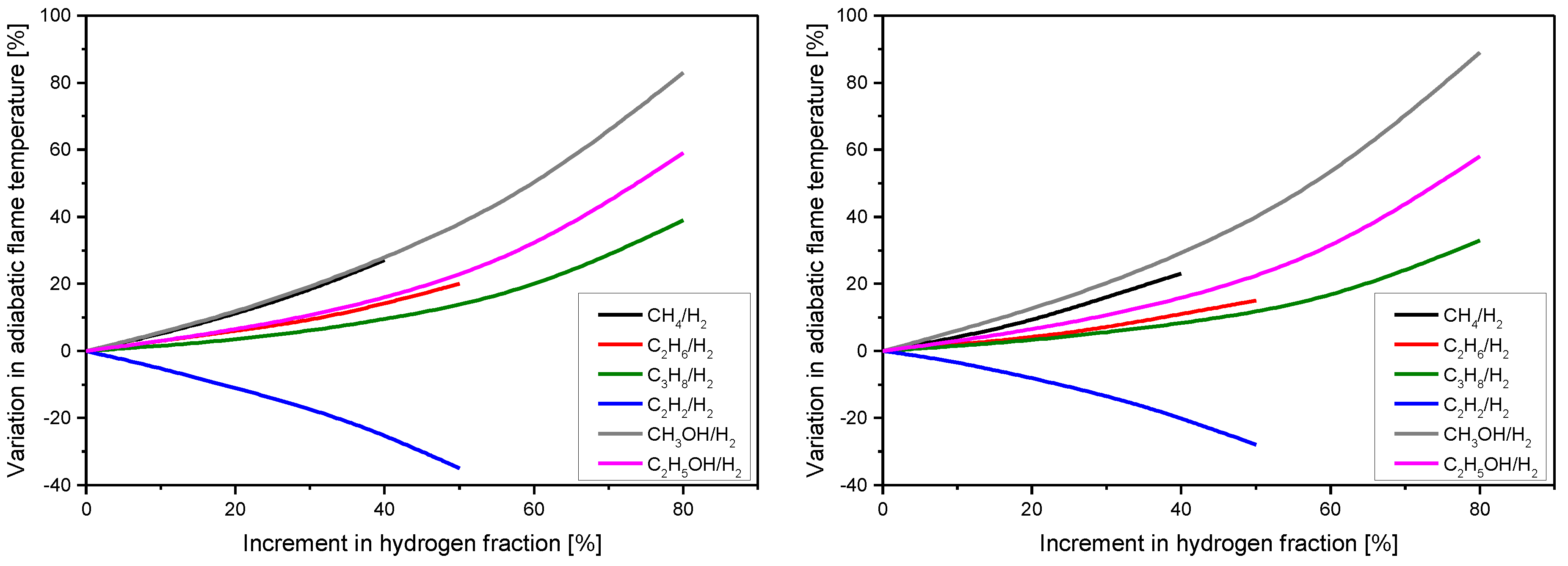
3. Results
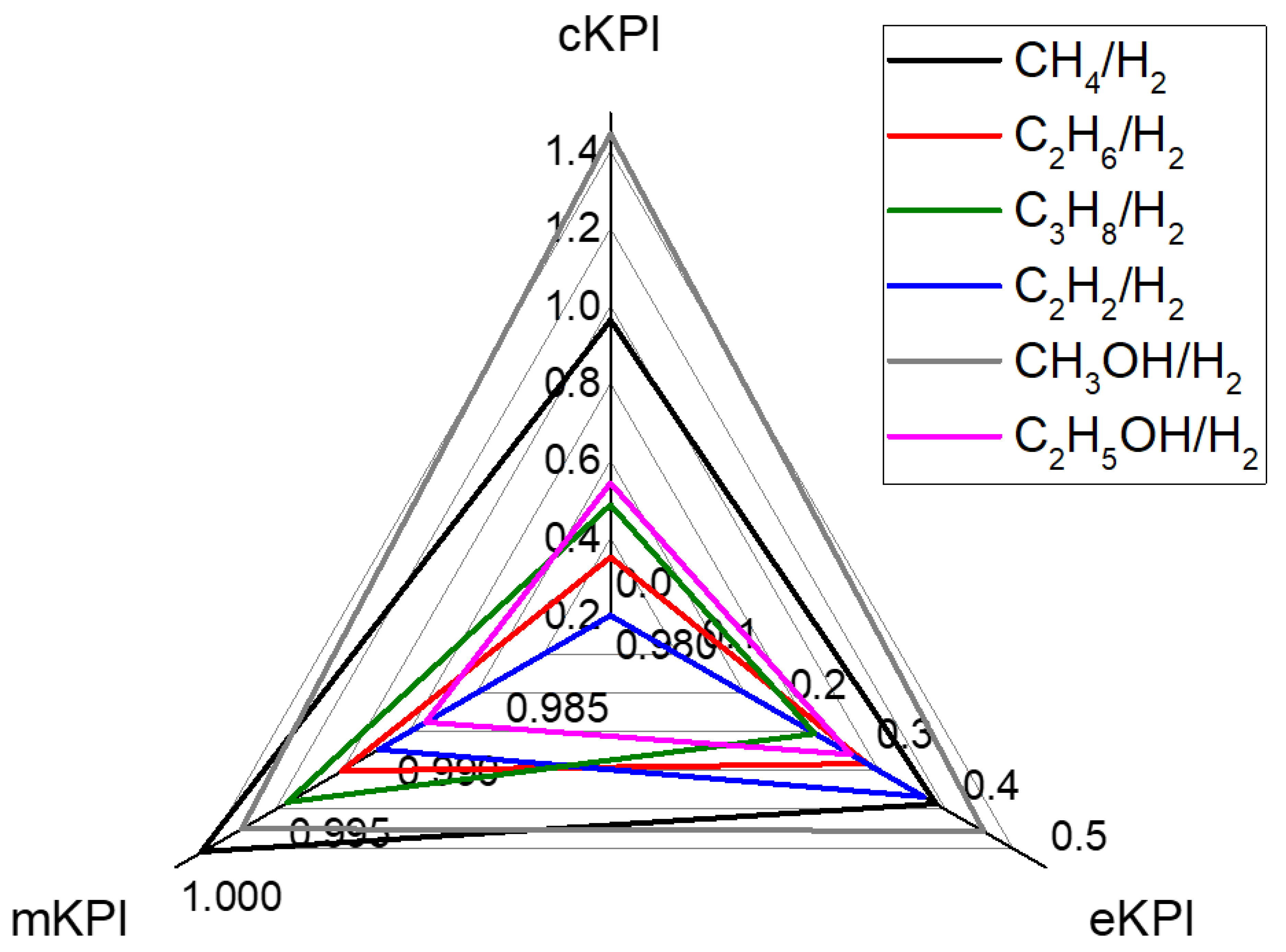
Key Performance Indicators
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- An, Y.; Jaasim, M.; Vallinayagam, R.; Vedharaj, S.; Im, H.G.; Johansson, B. Numerical simulation of combustion and soot under partially premixed combustion of low-octane gasoline. Fuel 2018, 211, 420–431. [Google Scholar] [CrossRef]
- Shi, Z.; Lee, C.-F.; Wu, H.; Wu, Y.; Zhang, L.; Liu, F. Optical diagnostics of low-temperature ignition and combustion characteristics of diesel/kerosene blends under cold-start conditions. Appl. Energy 2019, 251, 113307. [Google Scholar] [CrossRef]
- Dincer, I.; Acar, C. Review and evaluation of hydrogen production methods for better sustainability. Int. J. Hydrogen Energy 2015, 40, 11094–11111. [Google Scholar] [CrossRef]
- van Blarigan, P.; Keller, J. A hydrogen fuelled internal combustion engine designed for single speed/power operation. Int. J. Hydrogen Energy 1998, 23, 603–609. [Google Scholar] [CrossRef]
- Akansu, S.O.; Dulger, Z.; Kahraman, N.; Veziroǧlu, T.N. Internal combustion engines fueled by natural gas—hydrogen mixtures. Int. J. Hydrogen Energy 2004, 29, 1527–1539. [Google Scholar] [CrossRef]
- Dong, C.; Zhou, Q.; Zhang, X.; Zhao, Q.; Xu, T. Experimental study on the laminar flame speed of hydrogen/natural gas/air mixtures. Front. Chem. Eng. China 2010, 4, 417–422. [Google Scholar] [CrossRef]
- Katoch, A.; Milaa-Merino, A.; Kumar, S. Measurement of laminar burning velocity of ethanol-air mixtures at elevated temperatures. Fuel 2018, 231, 37–44. [Google Scholar] [CrossRef]
- Liu, Y.; Lenze, B.; Leuckel, W. Investigation of the laminar and turbulent burning velocities of premixed lean and rich flames of CH 4-H 2-air mixtures. In Dynamics of Deflagrations and Reactive Systems: Flames; American Institute of Aeronautics and Astronautics, Inc.: Reston, VA, USA, 1991; pp. 259–274. [Google Scholar]
- Salzano, E.; Pio, G.; Ricca, A.; Palma, V. The effect of a hydrogen addition to the premixed flame structure of light alkanes. Fuel 2018, 234, 1064–1070. [Google Scholar] [CrossRef]
- Law, C.K.; Kwon, O. Effects of hydrocarbon substitution on atmospheric hydrogen–air flame propagation. Int. J. Hydrogen Energy 2004, 29, 867–879. [Google Scholar] [CrossRef]
- Nilsson, E.J.; van Sprang, A.; Larfeldt, J.; Konnov, A.A. The comparative and combined effects of hydrogen addition on the laminar burning velocities of methane and its blends with ethane and propane. Fuel 2017, 189, 369–376. [Google Scholar] [CrossRef]
- Dong, Y.; Vagelopoulos, C.M.; Spedding, G.R.; Egolfopoulos, F.N. Measurement of laminar flame speeds through digital particle image velocimetry: Mixtures of methane and ethane with hydrogen, oxygen, nitrogen, and helium. Proc. Combust. Inst. 2002, 29, 1419–1426. [Google Scholar] [CrossRef]
- Tang, C.; Huang, Z.; Jin, C.; He, J.; Wang, J.; Wang, X.; Miao, H. Laminar burning velocities and combustion characteristics of propane–hydrogen–air premixed flames. Int. J. Hydrogen Energy 2008, 33, 4906–4914. [Google Scholar] [CrossRef]
- Milton, B.; Keck, J. Laminar burning velocities in stoichiometric hydrogen and hydrogen hydrocarbon gas mixtures. Combust. Flame 1984, 58, 13–22. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Zeng, K.; Liu, B.; Wang, Q.; Jiang, D. Measurements of laminar burning velocities for natural gas–hydrogen–air mixtures. Combust. Flame 2006, 146, 302–311. [Google Scholar] [CrossRef]
- Coppens, F.; de Ruyck, J.; Konnov, A.A. The effects of composition on burning velocity and nitric oxide formation in laminar premixed flames of CH4+ H2+ O2+ N2. Combust. Flame 2007, 149, 409–417. [Google Scholar] [CrossRef]
- Hermanns, R.; Konnov, A.A.; Bastiaans, R.; de Goey, L.; Lucka, K.; Köhne, H. Effects of temperature and composition on the laminar burning velocity of CH4+ H2+ O2+ N2 flames. Fuel 2010, 89, 114–121. [Google Scholar] [CrossRef]
- Hu, E.; Huang, Z.; He, J.; Jin, C.; Zheng, J. Experimental and numerical study on laminar burning characteristics of premixed methane–hydrogen–air flames. Int. J. Hydrogen Energy 2009, 34, 4876–4888. [Google Scholar] [CrossRef]
- Christensen, M. Laminar Burning Velocity and Development of a Chemical Kinetic Model for Small Oxygenated Fuels. Ph.D. Thesis, Lund University, Lund, Sweden, 2016. [Google Scholar]
- Wu, F.; Kelley, A.P.; Zhu, D.; Law, C.K. Further study on effects of hydrogen addition on laminar flame speeds of fuel-air mixtures. In Proceedings of the 7th US National Technical Meeting of the Combustion Institute, Atlanta, GA, USA, 20–23 March 2011; pp. 1336–1353. [Google Scholar]
- Sepman, A.; Mokhov, A.; Levinsky, H. The effects of hydrogen addition on NO formation in atmospheric-pressure, fuel-rich-premixed, burner-stabilized methane, ethane and propane flames. Int. J. Hydrogen Energy 2011, 36, 4474–4481. [Google Scholar] [CrossRef]
- Yu, G.; Law, C.K.; Wu, C. Laminar flame speeds of hydrocarbon+ air mixtures with hydrogen addition. Combust. Flame 1986, 63, 339–347. [Google Scholar] [CrossRef] [Green Version]
- Kariuki, J.; Balachandran, R. Experimental investigation of dynamics of premixed acetylene–air flames in a micro-combustor. Exp. Therm. Fluid Sci. 2010, 34, 330–337. [Google Scholar] [CrossRef]
- Balki, M.K.; Sayin, C. The effect of compression ratio on the performance, emissions and combustion of an SI (spark ignition) engine fueled with pure ethanol, methanol and unleaded gasoline. Energy 2014, 71, 194–201. [Google Scholar] [CrossRef]
- Chen, H.; Su, X.; He, J.; Xie, B. Investigation on combustion and emission characteristics of a common rail diesel engine fueled with diesel/n-pentanol/methanol blends. Energy 2019, 167, 297–311. [Google Scholar] [CrossRef]
- Wei, L.; Yao, C.; Han, G.; Pan, W. Effects of methanol to diesel ratio and diesel injection timing on combustion, performance and emissions of a methanol port premixed diesel engine. Energy 2016, 95, 223–232. [Google Scholar] [CrossRef]
- Gong, C.; Liu, Z.; Su, H.; Chen, Y.; Li, J.; Liu, F. Effect of injection strategy on cold start firing, combustion and emissions of a LPG/methanol dual-fuel spark-ignition engine. Energy 2019, 178, 126–133. [Google Scholar] [CrossRef]
- Gong, C.; Liu, J.; Peng, L.; Liu, F. Numerical study of effect of injection and ignition timings on combustion and unregulated emissions of DISI methanol engine during cold start. Renew. Energy 2017, 112, 457–465. [Google Scholar] [CrossRef]
- Hu, Z.; Zhang, X. Study on laminar combustion characteristic of low calorific value gas blended with hydrogen in a constant volume combustion bomb. Int. J. Hydrogen Energy 2019, 44, 487–493. [Google Scholar] [CrossRef]
- Xiao, P.; Lee, C.-f.; Wu, H.; Liu, F. Effects of hydrogen addition on the laminar methanol-air flame under different initial temperatures. Renew. Energy 2020, 154, 209–222. [Google Scholar] [CrossRef]
- Li, G.; Zhang, Z.; Liang, J.; Dong, F.; Li, Y.; Gao, X. Effects of hydrogen addition on the premixed laminar-flames of ethanol–air gaseous mixtures: An experimental study. Int. J. Hydrogen Energy 2012, 37, 4490–4501. [Google Scholar] [CrossRef]
- Xiao, P.; Lee, C.-f.; Wu, H.; Akram, M.Z.; Liu, F. Impacts of hydrogen-addition on methanol-air laminar burning coupled with pressures variation effects. Energy 2019, 187, 115997. [Google Scholar] [CrossRef]
- Korakianitis, T.; Namasivayam, A.; Crookes, R. Natural-gas fueled spark-ignition (SI) and compression-ignition (CI) engine performance and emissions. Prog. Energy Combust. Sci. 2011, 37, 89–112. [Google Scholar] [CrossRef]
- Shin, B.; Cho, Y.; Han, D.; Song, S.; Chun, K.M. Hydrogen effects on NOx emissions and brake thermal efficiency in a diesel engine under low-temperature and heavy-EGR conditions. Int. J. Hydrogen Energy 2011, 36, 6281–6291. [Google Scholar] [CrossRef]
- Saravanan, N.; Nagarajan, G. An experimental investigation of hydrogen-enriched air induction in a diesel engine system. Int. J. Hydrogen Energy 2008, 33, 1769–1775. [Google Scholar] [CrossRef]
- Catapano, F.; di Iorio, S.; Magno, A.; Sementa, P.; Vaglieco, B. A comprehensive analysis of the effect of ethanol, methane and methane-hydrogen blend on the combustion process in a PFI (port fuel injection) engine. Energy 2015, 88, 101–110. [Google Scholar] [CrossRef]
- Kee, R.J.; Grcar, J.F.; Smooke, M.D.; Miller, J.A.; Meeks, E. PREMIX: A Fortran Program for Modeling Steady Laminar One-dimensional Premixed Flames. Sandia National Laboratories Report. Sandia Reaction Design, 11436 Sorrento Valley Road, San Diego. 1985. Available online: https://www.researchgate.net/profile/Ellen_Meeks/publication/260388319_PREMIX_a_fortran_program_for_modeling_steady_laminar_one-dimensional_premixed_flames/links/53fe0de20cf23bb019bd249d/PREMIX-a-fortran-program-for-modeling-steady-laminar-one-dimensional-premixed-flames.pdf (accessed on 24 July 2020).
- Rogg, B. RUN-1DL: The Cambridge universal laminar flame code. In Technical Report CUED/A-THERMO/TR39; Department of Engineering, University of Cambridge: Cambridge, UK, 1991. [Google Scholar]
- Bowman, C.; Frenklach, M.; Gardiner, W.; Smith, G. The GRI 3.0 Chemical Kinetic Mechanism; Gas Research Institute: Berkeley, CA, USA, 1999. [Google Scholar]
- Sher, E.; Refael, S. A simplified reaction scheme for the combustion of hydrogen enriched methane/air flame. Combust. Sci. Technol. 1988, 59, 371–389. [Google Scholar] [CrossRef]
- Kunioshi, N.; Fukutani, S. Fuel mixing effects on propagation of premixed flames. II. Hydrogen+ methane flames. Bull. Chem. Soc. Jpn. 1992, 65, 2573–2577. [Google Scholar] [CrossRef]
- Hynes, R.; Mackie, J.; Masri, A. Sample Probe Measurements on a Hydrogen− Ethane− Air− 2-H-Heptafluoropropane Flame. Energy Fuels 1999, 13, 485–492. [Google Scholar] [CrossRef]
- Qin, Z.; Lissianski, V.V.; Yang, H.; Gardiner, W.C.; Davis, S.G.; Wang, H. Combustion chemistry of propane: A case study of detailed reaction mechanism optimization. Proc. Combust. Inst. 2000, 28, 1663–1669. [Google Scholar] [CrossRef]
- Al-Hamamre, Z.; Yamin, J. The effect of hydrogen addition on premixed laminar acetylene–hydrogen–air and ethanol–hydrogen–air flames. Int. J. Hydrogen Energy 2013, 38, 7499–7509. [Google Scholar] [CrossRef]
- Burke, U.; Metcalfe, W.K.; Burke, S.M.; Heufer, K.A.; Dagaut, P.; Curran, H.J. A detailed chemical kinetic modeling, ignition delay time and jet-stirred reactor study of methanol oxidation. Combust. Flame 2016, 165, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Gong, C.; Li, Z.; Li, D.; Liu, J.; Si, X.; Yu, J.; Huang, W.; Liu, F.; Han, Y. Numerical investigation of hydrogen addition effects on methanol-air mixtures combustion in premixed laminar flames under lean burn conditions. Renew. Energy 2018, 127, 56–63. [Google Scholar] [CrossRef]
- Gong, C.; Li, D.; Li, Z.; Liu, F. Numerical study on combustion and emission in a DISI methanol engine with hydrogen addition. Int. J. Hydrogen Energy 2016, 41, 647–655. [Google Scholar] [CrossRef]
- Ji, C.; Yang, J.; Liu, X.; Zhang, B.; Wang, S.; Gao, B. A quasi-dimensional model for combustion performance prediction of an SI hydrogen-enriched methanol engine. Int. J. Hydrogen Energy 2016, 41, 17676–17686. [Google Scholar] [CrossRef]
- Pio, G.; Barba, D.; Palma, V.; Salzano, E. A numerical study on the effect of temperature and composition on the flammability of methane–hydrogen sulfide mixtures. Combust. Sci. Technol. 2019, 191, 1541–1557. [Google Scholar] [CrossRef]
- Pio, G.; Salzano, E. Evaluation of safety parameters of light alkenes by means of detailed kinetic models. Process. Saf. Environ. Prot. 2018, 119, 131–137. [Google Scholar] [CrossRef]
- Pio, G.; Salzano, E. Flammability parameters of liquified natural gas. J. Loss Prev. Process. Ind. 2018, 56, 424–429. [Google Scholar] [CrossRef]
- Burcat, A.; Ruscic, B. Third Millenium Ideal Gas and Condensed Phase Thermochemical Database for Combustion (with Update from Active Thermochemical Tables); Argonne National Lab. (ANL): Argonne, IL, USA, 2005. [Google Scholar]
- Pio, G.; Palma, V.; Salzano, E. Comparison and validation of detailed kinetic models for the oxidation of light alkenes. Ind. Eng. Chem. Res. 2018, 57, 7130–7135. [Google Scholar] [CrossRef]
- Goodwin, D. An open-source, extensible software suite for CVD process simulation. Chem. Vap. Depos. Xvi Eurocvd 2003, 14, 2003–2008. [Google Scholar]
- Pio, G.; Salzano, E. The effect of ultra-low temperature on the flammability limits of a methane/air/diluent mixtures. J. Hazard. Mater. 2019, 362, 224–229. [Google Scholar] [CrossRef]
- Pio, G.; Salzano, E. Implementation of gas-phase kinetic model for the optimization of the ethylene oxide production. Chem. Eng. Sci. 2020, 212, 115331. [Google Scholar] [CrossRef]
- Sreedharan, P. Recent estimates of energy efficiency potential in the USA. Energy Effic. 2013, 6, 433–445. [Google Scholar] [CrossRef]
- Kido, H.; Huang, S.; Tanoue, K.; Nitta, T. Improving the combustion performance of lean hydrocarbon mixtures by hydrogen addition. JSAE Rev. 1994, 15, 165–170. [Google Scholar] [CrossRef]
- Teng, F. The Effect of Hydrogen Concentration on the Flame Stability and Laminar Burning Velocity of Hydrogen-Hydrocarbon-Carbon Dioxide Mixtures; University of Sheffield: Sheffield, UK, 2014. [Google Scholar]
- Battin-Leclerc, F. Detailed chemical kinetic models for the low-temperature combustion of hydrocarbons with application to gasoline and diesel fuel surrogates. Prog. Energy Combust. Sci. 2008, 34, 440–498. [Google Scholar] [CrossRef] [Green Version]
- Curran, H.J. Developing detailed chemical kinetic mechanisms for fuel combustion. Proc. Combust. Inst. 2019, 37, 57–81. [Google Scholar] [CrossRef]
- Sarathy, S.M.; Vranckx, S.; Yasunaga, K.; Mehl, M.; Oßwald, P.; Metcalfe, W.K.; Westbrook, C.K.; Pitz, W.J.; Kohse-Höinghaus, K.; Fernandes, R.X.; et al. A comprehensive chemical kinetic combustion model for the four butanol isomers. Combust. Flame 2012, 159, 2028–2055. [Google Scholar] [CrossRef]
- Warnatz, J. The structure of laminar alkane-, alkene-, and acetylene flames. Symp. (Int.) Combust. 1981, 18, 369–384. [Google Scholar] [CrossRef]
- Yalamanchili, S.; Sirignano, W.; Seiser, R.; Seshadri, K. Reduced methanol kinetic mechanisms for combustion applications Combust. Flame 2005, 142, 258–265. [Google Scholar] [CrossRef]
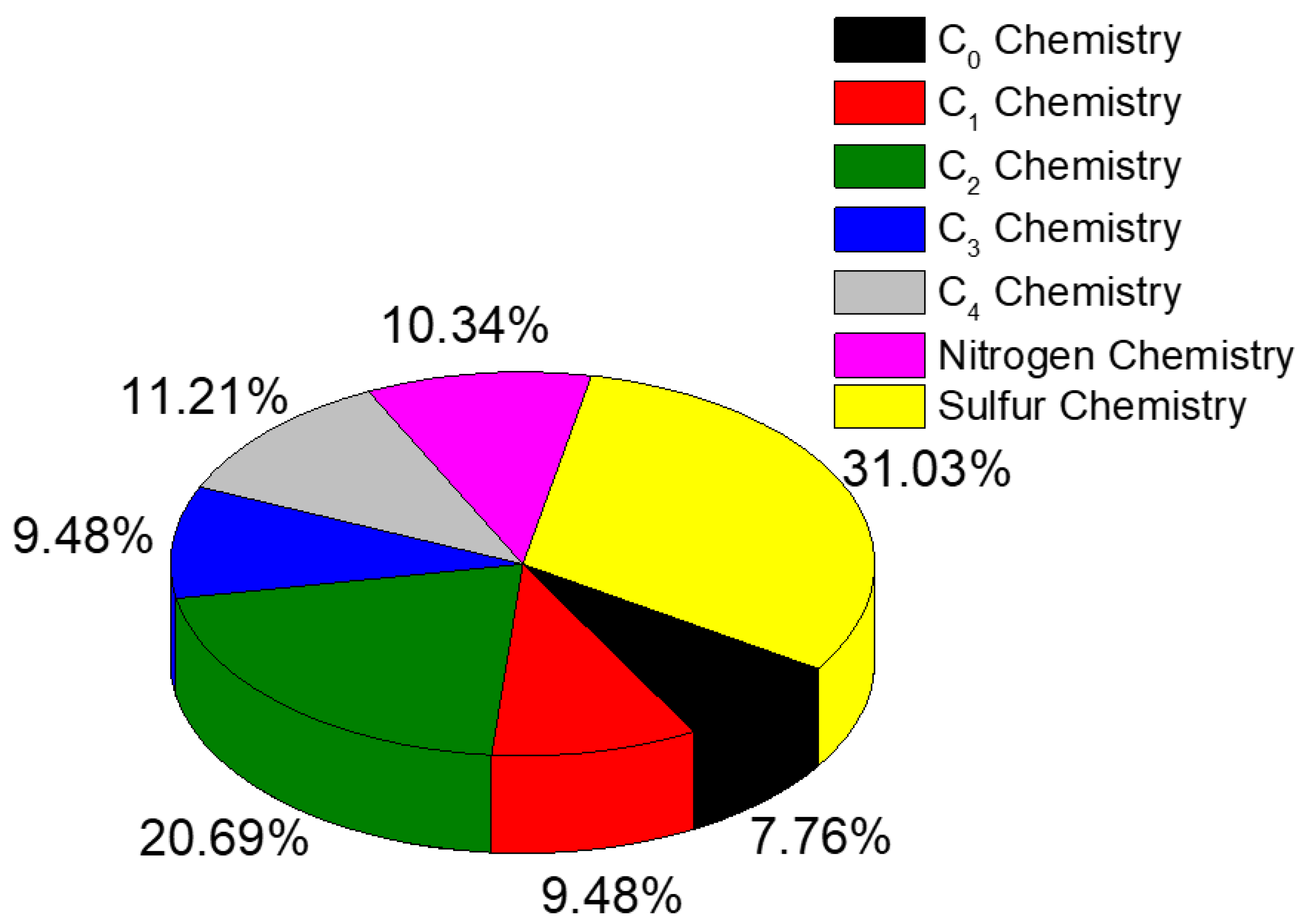
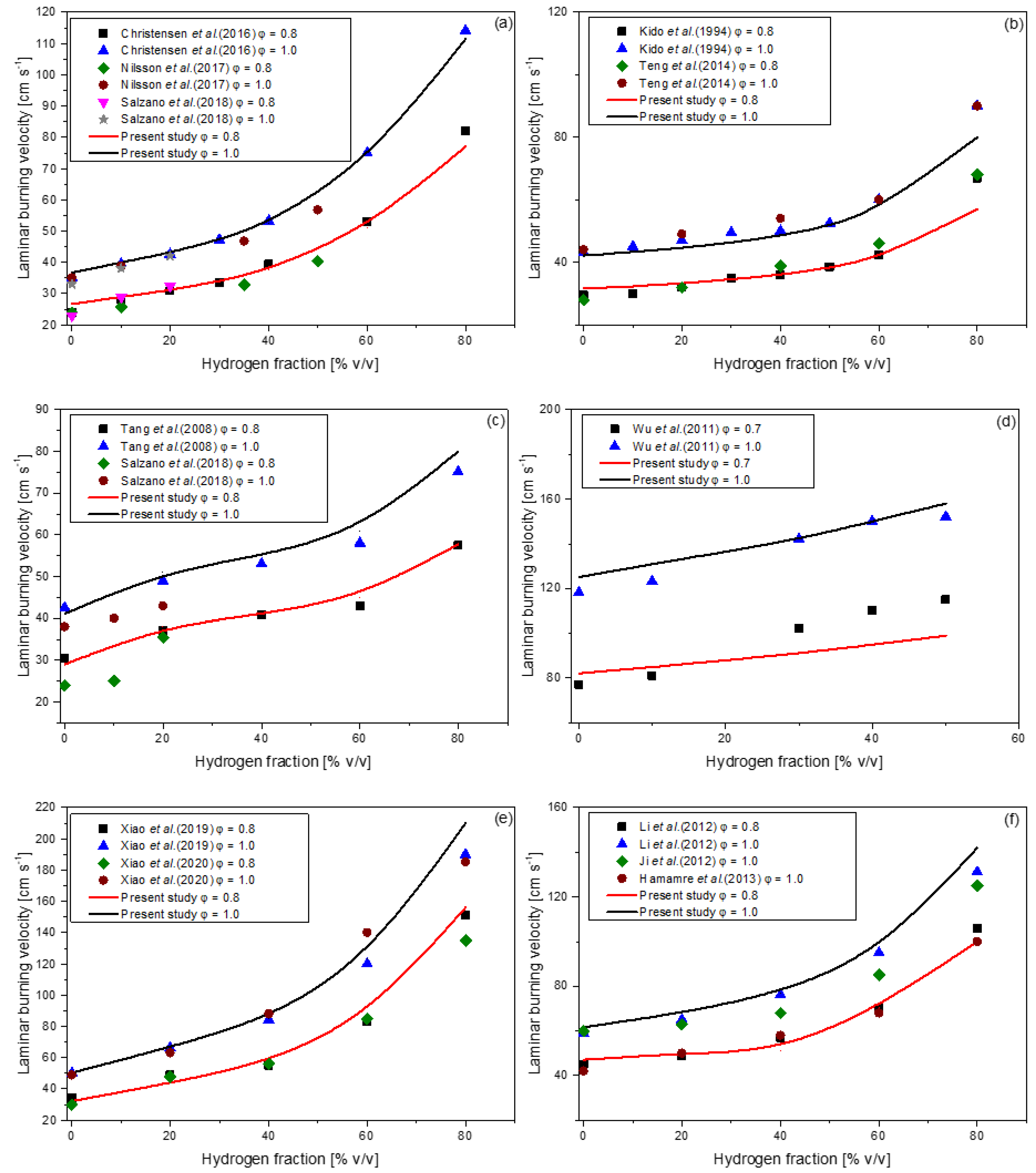
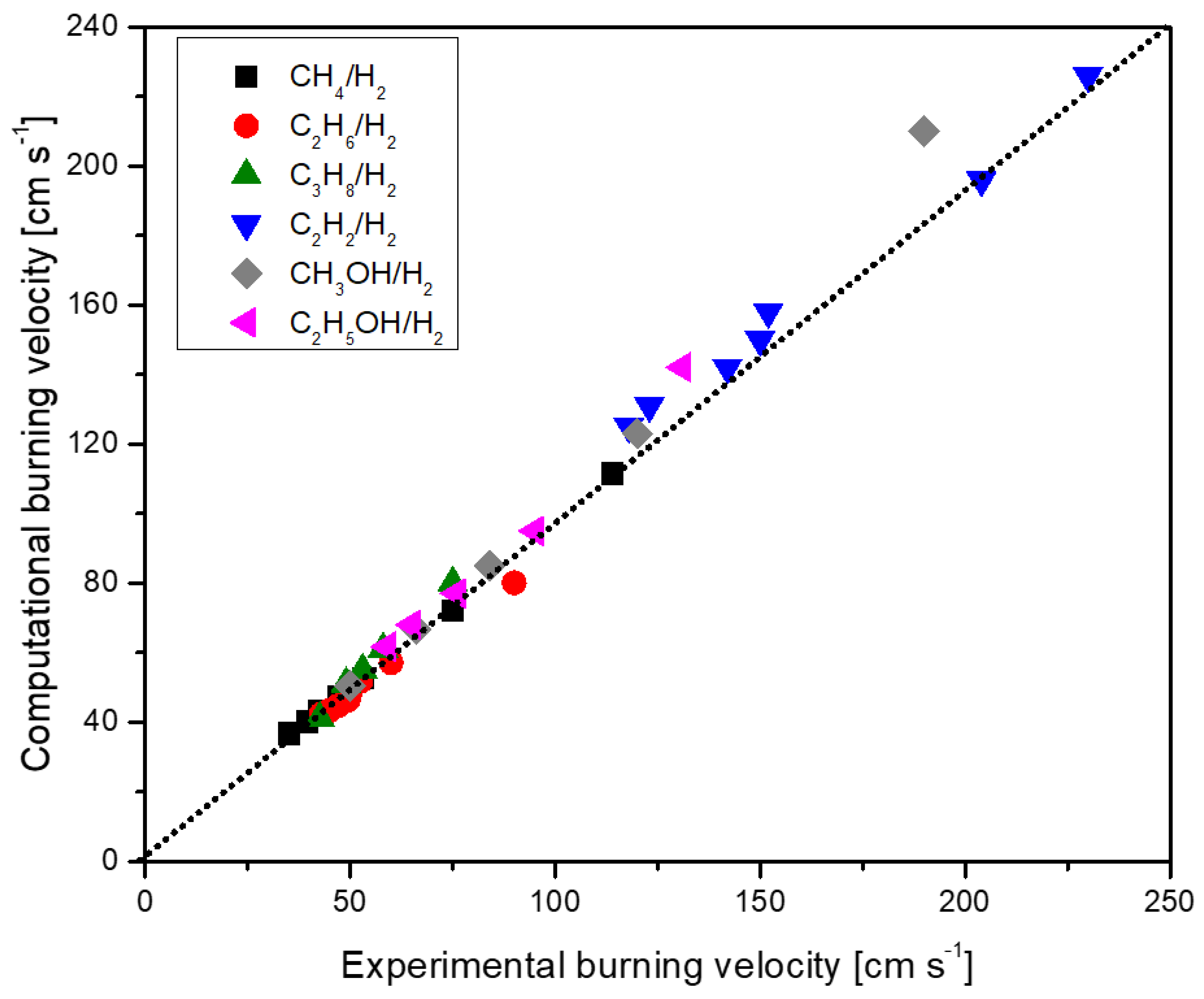
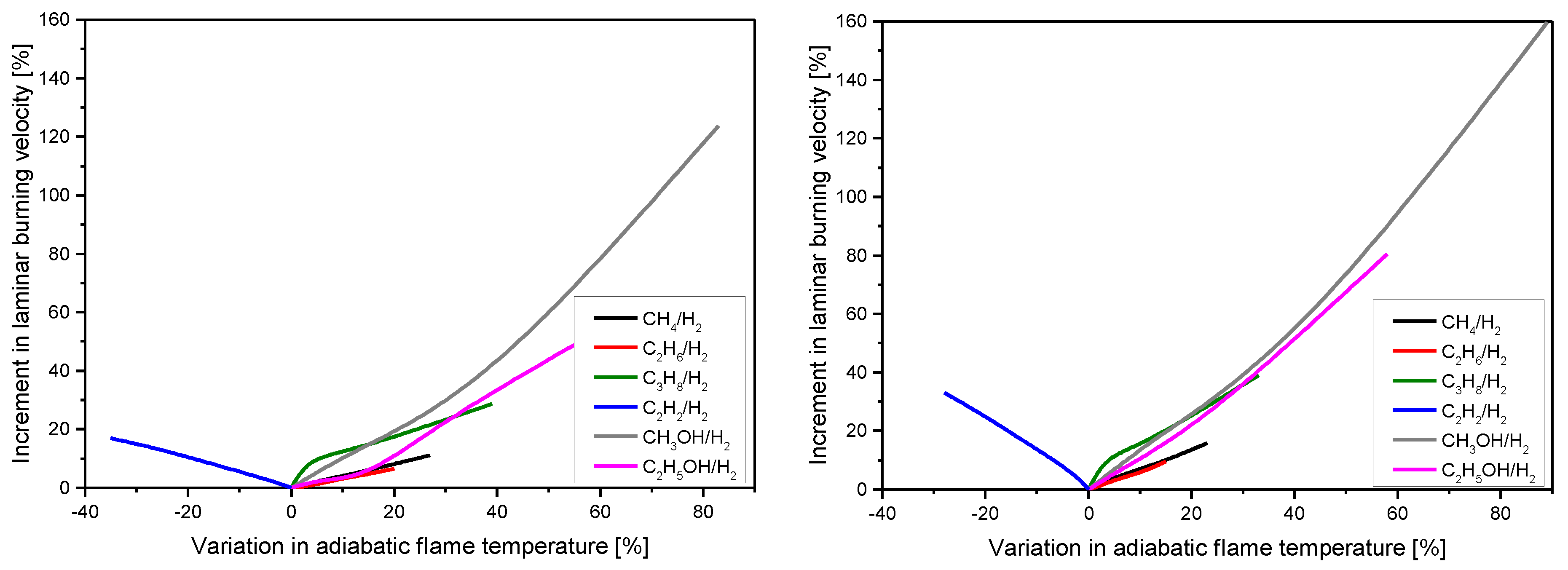


| Author (s) | Fuel Composition | Equivalence Ratio (-) | Technique |
|---|---|---|---|
| Liu et al. [8] | CH4 + H2 | 0.6–2.2 | Combustion burner |
| Salzano et al. [9] | CH4 + H2 | 0.6–2.0 | Heat flux burner |
| C3H8 + H2 | |||
| Law and Kwon [10] | CH4 + H2 | 1.0 | Combustion chamber |
| C3H8 + H2 | |||
| Nilsson et al. [11] | CH4 + H2 | 0.6–1.7 | Heat flux burner |
| C2H6 + H2 | |||
| C3H8 + H2 | |||
| CH4 + C2H6 + H2 | |||
| CH4 + C3H8+ H2 | |||
| CH4 + C2H6 + C3H8 + H2 | |||
| Dong et al. [12] | C2H6 + H2 | 0.6 | Counterflow burner |
| Tang et al. [13] | C3H8 + H2 | 0.6–1.6 | Combustion bomb |
| Milton and Keck [14] | CH4 + H2 | 1.0 | Combustion bomb |
| C3H8 + H2 | |||
| Huang et al. [15] | CH4 + C2H6 + C3H8 + H2 | 0.6–1.4 | Combustion bomb |
| Coppens [16] | CH4 + C2H6 + C3H8 + H2 | 0.7–1.4 | Heat flux burner |
| Hermanns et al. [17] | CH4 + H2 | 0.6–1.5 | Heat flux burner |
| Hu et al. [18] | CH4 + H2 | 0.6–1.3 | Combustion chamber |
| Christensen and Moah [19] | CH4 + H2 | 0.6–1.7 | Heat flux burner |
| Wu et al. [20] | C2H6 + H2 | 0.7, 1.0, 1.6 | Spherical flame |
| Sepman et al. [21] | C2H6 + H2 | 1.3 | Heat flux burner |
| C3H8 + H2 | |||
| Yu et al. [22] | CH4 + H2 | 0.5–1.4 | Counterflow chamber |
| C3H8 + H2 |
| Author (s) | Fuel Composition | Equivalence Ratio (-) | Technique |
|---|---|---|---|
| Milton and Keck [14] | C2H2 + H2 | 1.0 | Combustion chamber |
| Wu et al. [20] | C2H2 + H2 | 0.7; 1.0; 1.6 | spherical flame |
| Xiao et al. [30] | CH3OH + H2 | 0.8; 1.0: 1.2 | Combustion chamber |
| Li et al. [2012] [31] | C2H5OH + H2 | 0.6–1.6 | Spherical flame |
| Peng et al. [32] | C2H5OH + H2 | 0.8; 1.0; 1.2 | Combustion burner |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mosisa Wako, F.; Pio, G.; Salzano, E. The Effect of Hydrogen Addition on Low-Temperature Combustion of Light Hydrocarbons and Alcohols. Energies 2020, 13, 3808. https://doi.org/10.3390/en13153808
Mosisa Wako F, Pio G, Salzano E. The Effect of Hydrogen Addition on Low-Temperature Combustion of Light Hydrocarbons and Alcohols. Energies. 2020; 13(15):3808. https://doi.org/10.3390/en13153808
Chicago/Turabian StyleMosisa Wako, Fekadu, Gianmaria Pio, and Ernesto Salzano. 2020. "The Effect of Hydrogen Addition on Low-Temperature Combustion of Light Hydrocarbons and Alcohols" Energies 13, no. 15: 3808. https://doi.org/10.3390/en13153808
APA StyleMosisa Wako, F., Pio, G., & Salzano, E. (2020). The Effect of Hydrogen Addition on Low-Temperature Combustion of Light Hydrocarbons and Alcohols. Energies, 13(15), 3808. https://doi.org/10.3390/en13153808






