Effect of Oxygen for Enhancing the Gas Storage Performance of Activated Green Carbon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Carbonization
2.2. Chemical Activation and Neutralization
2.3. Characterization
3. Results and Discussion
3.1. Structural Investigation With XRD, and Raman Spectroscopy
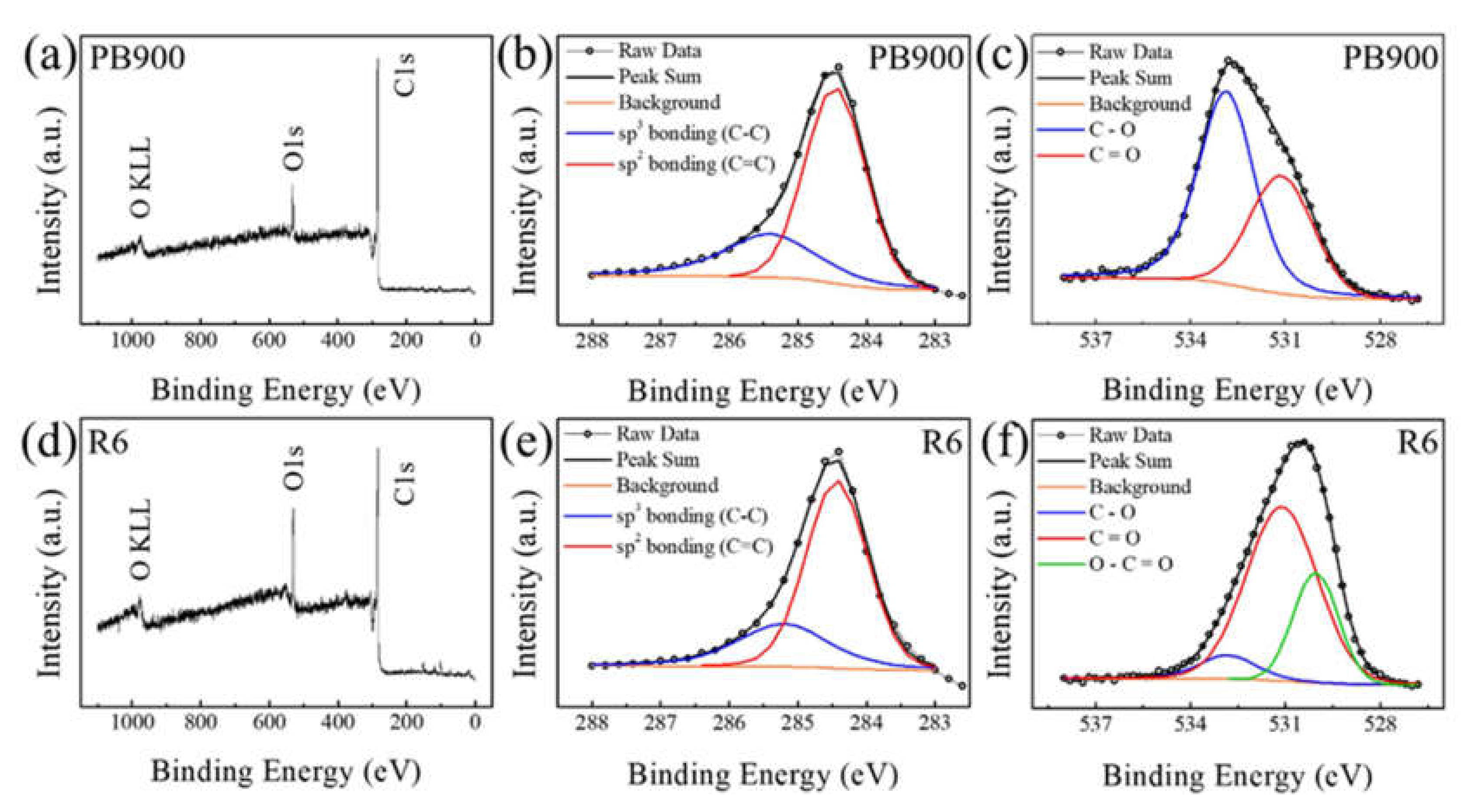
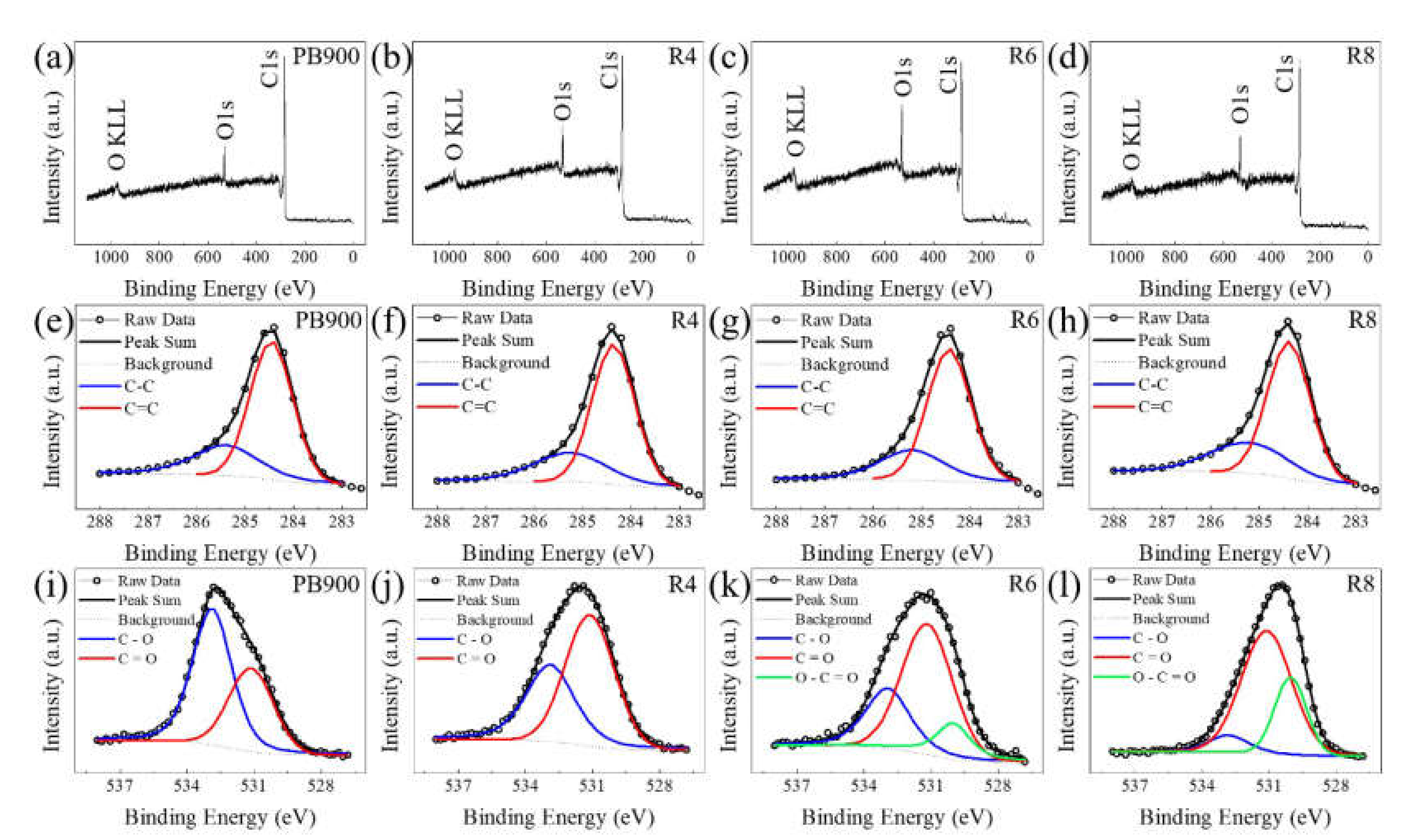
3.2. X-ray Photoelectron Study before and after Chemical Activation
3.3. Surface Structure and Gas Adsorption Using BET Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- US DOE. The Fuel Cell Technologies Office Multi-Year Research, Development, and Demonstration Plan; Technical Report; US Department of Energy: Washington, DC, USA, 2016. [Google Scholar]
- Elam, C.C. IEA Agreement on the Production and Utilization of Hydrogen; USA Annual Report; National Renewable Energy Laboratory: Golden, CO, USA, 1996. [Google Scholar]
- Satyapal, S.; Petrovic, J.; Read, C.; Thomas, G.; Ordaz, G. The US Department of Energy’s National Hydrogen Storage Project: Progress towards meeting hydrogen-powered vehicle requirements. Catal. Today 2007, 120, 246–256. [Google Scholar] [CrossRef] [Green Version]
- Hao, G.P.; Li, W.C.; Lu, A.H. Novel porous solids for carbon dioxide capture. J. Mater. Chem. 2011, 21, 6447–6451. [Google Scholar] [CrossRef]
- Rashidi, N.A.; Yusup, S. An overview of activated carbons utilization for the post-combustion carbon dioxide capture. J. CO2 Util. 2016, 13, 1–16. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [Green Version]
- Lozano-Castello, D.; Calo, J.M.; Cazorla-Amoros, D.; Linares-Solano, A. Carbon activation with KOH as explored by temperature programmed techniques, and the effects of hydrogen. Carbon 2007, 45, 2529–2536. [Google Scholar] [CrossRef]
- Teng, H.; Hsu, L.Y. High-porosity carbons prepared from bituminous coal with potassium hydroxide activation. Ind. Eng. Chem. Res. 1999, 38, 2947–2953. [Google Scholar] [CrossRef]
- Ströbel, R.; Garche, J.; Moseley, P.T.; Jörissen, L.; Wolf, G. Hydrogen storage by carbon materials. J. Power Sources 2006, 159, 781–801. [Google Scholar] [CrossRef]
- Nijkamp, M.G.; Raaymakers, J.E.M.J.; Van Dillen, A.J.; De Jong, K.P. Hydrogen storage using physisorption–materials demands. Appl. Phys. A 2001, 72, 619–623. [Google Scholar] [CrossRef] [Green Version]
- Dillon, A.C.; Heben, M.J. Hydrogen storage using carbon adsorbents: Past, present and future. Appl. Phys. A 2001, 72, 133–142. [Google Scholar] [CrossRef]
- Patel, H.A.; Byun, J.; Yavuz, C.T. Carbon dioxide capture adsorbents: Chemistry and methods. ChemSusChem 2017, 10, 1303–1317. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. Chemsuschem Chem. Sustain. Energy Mater. 2009, 2, 796–854. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Al-Jumialy, A.S.M.; Fuertes, A.B.; Mokaya, R. Optimization of the pore structure of biomass-based carbons in relation to their use for CO2 capture under low-and high-pressure regimes. ACS Appl. Mater. Interfaces 2018, 10, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Lakhi, K.S.; Sil, S.; Bhosale, S.V.; Kim, I.; Albahily, K.; Vinu, A. Biomass derived porous carbon for CO2 capture. Carbon 2019, 148, 164–186. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Li, W.C.; Lu, A.H. Designed porous carbon materials for efficient CO2 adsorption and separation. New Carbon Mater. 2015, 30, 481–501. [Google Scholar] [CrossRef]
- Sreńscek-Nazzal, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.J.; Michalkiewicz, B. Comparison of optimized isotherm models and error functions for carbon dioxide adsorption on activated carbon. J. Chem. Eng. Data 2015, 60, 3148–3158. [Google Scholar] [CrossRef]
- Sreńscek-Nazzal, J.; Narkiewicz, U.; Morawski, A.W.; Wróbel, R.; Gęsikiewicz-Puchalska, A.; Michalkiewicz, B. Modification of Commercial Activated Carbons for CO2 Adsorption. Acta Phys. Pol. A 2016, 129, 394–401. [Google Scholar] [CrossRef]
- Sreńscek-Nazzal, J.; Kiełbasa, K. Advances in modification of commercial activated carbon for enhancement of CO2 capture. Appl. Surf. Sci. 2019, 494, 137–151. [Google Scholar] [CrossRef]
- Casco, M.E.; Martínez-Escandell, M.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. Effect of the porous structure in carbon materials for CO2 capture at atmospheric and high-pressure. Carbon 2014, 67, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Jimenez, V.; Ramírez-Lucas, A.; Diaz, J.A.; Sanchez, P.; Romero, A. CO2 capture in different carbon materials. Environ. Sci. Technol. 2012, 46, 7407–7414. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, Z.; Zhu, Y. Porous carbon-based materials for hydrogen storage: Advancement and challenges. J. Mater. Chem. A 2013, 1, 9365–9381. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.; Wang, T. Enhanced hydrogen storage performance of three-dimensional hierarchical porous graphene with nickel nanoparticles. Int. J. Hydrog. Energy 2018, 43, 11120–11131. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, C.; Cheng, L.; Wahab, M.A.; Cui, J.; Zou, J.; Zhu, M.; Yao, X. Destabilization of Mg–H bonding through nano-interfacial confinement by unsaturated carbon for hydrogen desorption from MgH2. Phys. Chem. Chem. Phys. 2013, 15, 5814–5820. [Google Scholar] [CrossRef] [Green Version]
- Zlotea, C.; Oumellal, Y.; Hwang, S.J.; Ghimbeu, C.M.; de Jongh, P.E.; Latroche, M. Ultrasmall MgH2 nanoparticles embedded in an ordered microporous carbon exhibiting rapid hydrogen sorption kinetics. J. Phys. Chem. C 2015, 119, 18091–18098. [Google Scholar] [CrossRef]
- Kim, B.H.; Hong, W.G.; Yu, H.Y.; Han, Y.K.; Lee, S.M.; Chang, S.J.; Moon, H.R.; Jun, Y.; Kim, J.H. Thermally modulated multilayered graphene oxide for hydrogen storage. Phys. Chem. Chem. Phys. 2012, 14, 1480–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.H.; Hong, W.G.; Moon, H.R.; Lee, S.M.; Kim, J.M.; Kang, S.; Jun, Y.; Kim, H.J. Investigation on the existence of optimum interlayer distance for H2 uptake using pillared-graphene oxide. Int. J. Hydrog. Energy 2012, 37, 14217–14222. [Google Scholar] [CrossRef]
- Wang, J.; Kaskel, S. KOH activation of carbon-based materials for energy storage. J. Mater. Chem. 2012, 22, 23710–23725. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Q.; Hu, J. High hydrogen storage capacity of porous carbons prepared by using activated carbon. J. Am. Chem. Soc. 2009, 131, 7016–7022. [Google Scholar] [CrossRef]
- Otowa, T.; Nojima, Y.; Miyazaki, T. Development of KOH activated high surface area carbon and its application to drinking water purification. Carbon 1997, 35, 1315–1319. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T.C.; Rao, M.B. Activated carbon for gas separation and storage. Carbon 1996, 34, 1–12. [Google Scholar] [CrossRef]
- Lillo-Ródenas, M.A.; Juan-Juan, J.; Cazorla-Amorós, D.; Linares-Solano, A. About reactions occurring during chemical activation with hydroxides. Carbon 2004, 42, 1371–1375. [Google Scholar] [CrossRef]
- Kim, J.M.; Hong, W.G.; Lee, S.M.; Chang, S.J.; Jun, Y.; Kim, B.H.; Kim, H.J. Energy storage of thermally reduced graphene oxide. Int. J. Hydrog. Energy 2014, 39, 3799–3804. [Google Scholar] [CrossRef]
- Shin, D.S.; Hong, W.G.; Jang, H.S.; Kim, H.J.; Kim, B.H. Optimum interlayer distance for hydrogen storage in pillared-graphene oxide determined by H2 pressure-dependent electrical conductance. Int. J. Hydrog. Energy 2018, 43, 16136–16140. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Bhat, I.U.H.; Jawaid, M.; Zaidon, A.; Hermawan, D.; Hadi, Y.S. Bamboo fibre reinforced biocomposites: A review. Mater. Des. 2012, 42, 353–368. [Google Scholar] [CrossRef]
- Genovese, M.; Jiang, J.; Lian, K.; Holm, N. High capacitive performance of exfoliated biochar nanosheets from biomass waste corn cob. J. Mater. Chem. A 2015, 3, 2903–2913. [Google Scholar] [CrossRef]
- Jang, H.S.; Mun, J.; Hong, W.G.; Lee, S.M.; Jeon, J.W.; Lee, C.Y.; Kim, H.J.; Kim, B.H. The performance of green carbon as a backbone for hydrogen storage materials. Int. J. Hydrog. Energy 2020, 45, 10516–10522. [Google Scholar] [CrossRef]
- Bhuiyan, M.T.R.; Hirai, N.; Sobue, N. Changes of crystallinity in wood cellulose by heat treatment under dried and moist conditions. J. Wood Sci. 2000, 46, 431–436. [Google Scholar] [CrossRef]
- Cho, S.Y.; Yun, Y.S.; Lee, S.; Jang, D.; Park, K.Y.; Kim, J.K.; Kim, B.H.; Kang, K.; Kaplan, D.L.; Jin, H.J. Carbonization of a stable β-sheet-rich silk protein into a pseudographitic pyroprotein. Nat. Commun. 2015, 6, 7145. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, J.S.; Zheng, T.; Dahn, J.R. Mechanism of lithium insertion in hard carbons prepared by pyrolysis of epoxy resins. Carbon 1996, 34, 193–200. [Google Scholar] [CrossRef]
- Agarwal, U.P. Raman imaging to investigate ultrastructure and composition of plant cell walls: Distribution of lignin and cellulose in black spruce wood (Picea mariana). Planta 2006, 224, 1141–1153. [Google Scholar] [CrossRef]
- Zhan, D.; Ni, Z.; Chen, W.; Sun, L.; Luo, Z.; Lai, L.; Yu, T.; Wee, A.T.S.; Shen, Z. Electronic structure of graphite oxide and thermally reduced graphite oxide. Carbon 2011, 49, 1362–1366. [Google Scholar] [CrossRef]
- González, Z.; Botas, C.; Álvarez, P.; Roldán, S.; Blanco, C.; Santamaría, R.; Granda, M.; Menéndez, R. Thermally reduced graphite oxide as positive electrode in vanadium redox flow batteries. Carbon 2012, 50, 828–834. [Google Scholar] [CrossRef] [Green Version]
- Wollbrink, A.; Volgmann, K.; Koch, J.; Kanthasamy, K.; Tegenkamp, C.; Li, Y.; Richter, H.; Kämnitz, S.; Steinbach, F.; Feldhoff, A.; et al. Amorphous, turbostratic and crystalline carbon membranes with hydrogen selectivity. Carbon 2016, 106, 93–105. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys 1970, 53, 1126–1130. [Google Scholar] [CrossRef] [Green Version]
- Al-Jishi, R.; Dresselhaus, G. Lattice-dynamical model for graphite. Phys. Rev. B 1982, 26, 4514. [Google Scholar] [CrossRef]
- Wang, Y.; Alsmeyer, D.C.; McCreery, R.L. Raman spectroscopy of carbon materials: Structural basis of observed spectra. Chem. Mater. 1990, 2, 557–563. [Google Scholar] [CrossRef]
- Sadezky, A.; Muckenhuber, H.; Grothe, H.; Niessner, R.; Pöschl, U. Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Cuesta, A.; Dhamelincourt, P.; Laureyns, J.; Martinez-Alonso, A.; Tascón, J.D. Raman microprobe studies on carbon materials. Carbon 1994, 32, 1523–1532. [Google Scholar] [CrossRef]
- Jawhari, T.; Roid, A.; Casado, J. Raman spectroscopic characterization of some commercially available carbon black materials. Carbon 1995, 33, 1561–1565. [Google Scholar] [CrossRef]
- Dippel, B.; Heintzenberg, J. Soot characterization in atmospheric particles from different sources by NIR FT Raman spectroscopy. J. Aerosol Sci. 1999, 30, S907–S908. [Google Scholar] [CrossRef]
- Boehm, H.P. Surface oxides on carbon and their analysis: A critical assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Zhou, J.H.; Sui, Z.J.; Zhu, J.; Li, P.; Chen, D.; Dai, Y.C.; Yuan, W.K. Characterization of surface oxygen complexes on carbon nanofibers by TPD, XPS and FT-IR. Carbon 2007, 45, 785–796. [Google Scholar] [CrossRef]
- Zielke, U.; Hüttinger, K.J.; Hoffman, W.P. Surface-oxidized carbon fibers: I. Surface structure and chemistry. Carbon 1996, 34, 983–998. [Google Scholar] [CrossRef]
- Boehm, H.P. Some aspects of the surface chemistry of carbon blacks and other carbons. Carbon 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Zhao, W.; Luo, L.; Wang, H.; Fan, M. Synthesis of bamboo-based activated carbons with super-high specific surface area for hydrogen storage. BioResources 2017, 12, 1246–1262. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Deng, S.; Hu, B.; Chen, Z.; Wang, B.; Huang, J.; Yu, G. Granular bamboo-derived activated carbon for high CO2 adsorption: The dominant role of narrow micropores. ChemSusChem 2012, 5, 2354–2360. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Portet, C.; Osswald, S.; Simmons, J.M.; Yildirim, T.; Laudisio, G.; Fischer, J.E. Importance of pore size in high-pressure hydrogen storage by porous carbons. Int. J. Hydrog. Energy 2009, 34, 6314–6319. [Google Scholar] [CrossRef]
- Rzepka, M.; Lamp, P.; De la Casa-Lillo, M.A. Physisorption of hydrogen on microporous carbon and carbon nanotubes. J. Phys. Chem. B 1998, 102, 10894–10898. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Pantarotto, D.; Lacerda, L.; Pastorin, G.; Klumpp, C.; Prato, M.; Bianco, A.; Kostarelos, K. Tissue biodistribution and blood clearance rates of intravenously administered carbon nanotube radiotracers. Proc. Natl. Acad. Sci. USA 2006, 103, 3357–3362. [Google Scholar] [CrossRef] [Green Version]
- Aga, R.S.; Fu, C.L.; Krčmar, M.; Morris, J.R. Theoretical investigation of the effect of graphite interlayer spacing on hydrogen absorption. Phys. Rev. B 2007, 76, 165404. [Google Scholar] [CrossRef]
- Cabria, I.; López, M.J.; Alonso, J.A. Hydrogen storage capacities of nanoporous carbon calculated by density functional and Møller-Plesset methods. Phys. Rev. B 2008, 78, 075415. [Google Scholar] [CrossRef]
- Jelea, A.; Marinelli, F.; Ferro, Y.; Allouche, A.; Brosset, C. Quantum study of hydrogen–oxygen–graphite interactions. Carbon 2004, 42, 3189–3198. [Google Scholar] [CrossRef]
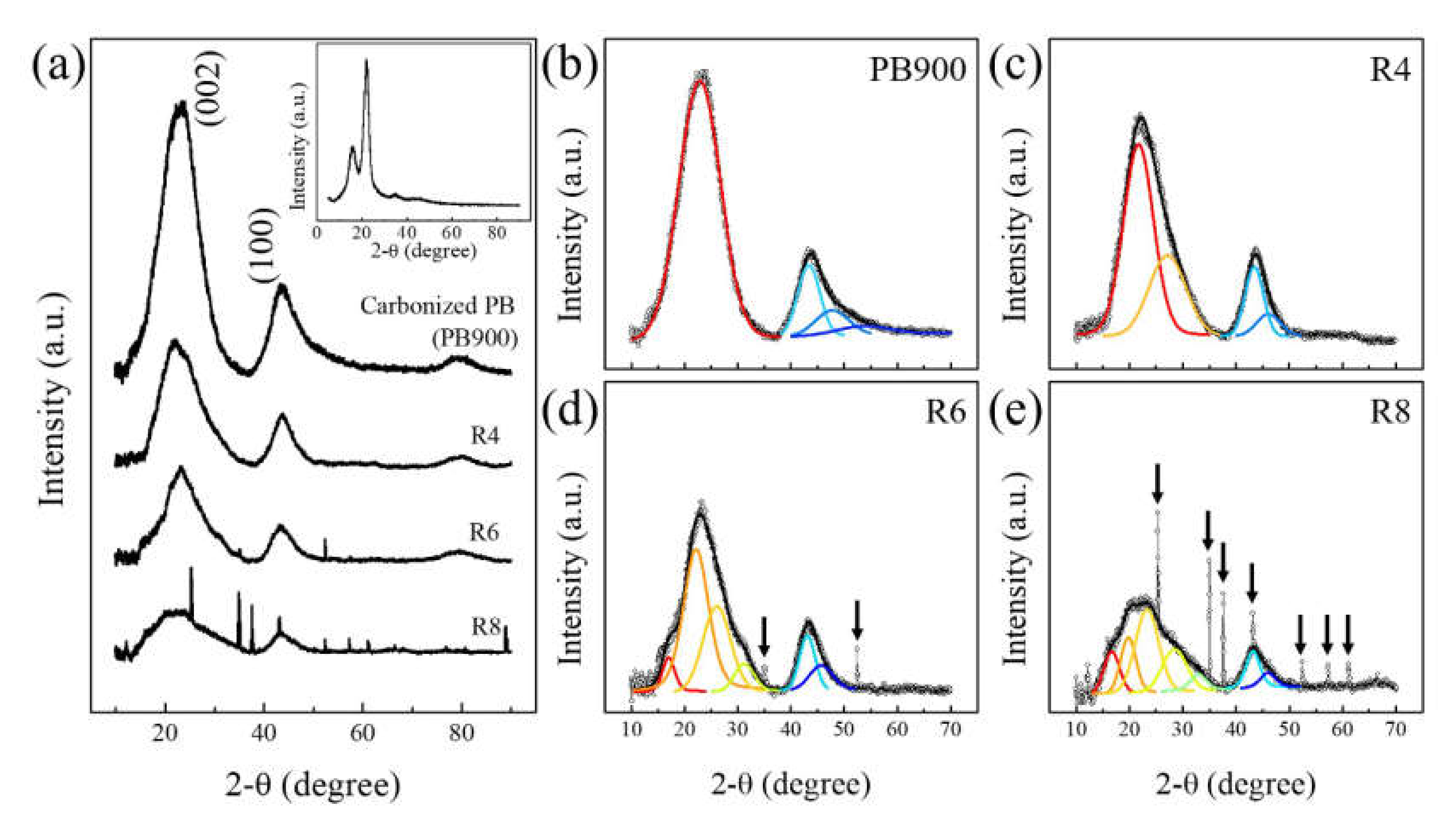





| Sample | Peak Position (2Ɵ, °) | FWHM (2Ɵ, °) | d-Spacing (Å) |
|---|---|---|---|
| PB900 | 22.86 | 9.25 | 3.89 |
| R4 | 21.57 | 6.47 | 4.12 |
| 26.51 | 8.72 | 3.36 | |
| R6 | 17.38 | 3.50 | 5.10 |
| 22.34 | 5.28 | 3.98 | |
| 26.27 | 6.07 | 3.39 | |
| 31.25 | 4.67 | 2.86 | |
| R8 | 16.01 | 5.01 | 5.53 |
| 20.03 | 5.24 | 4.43 | |
| 23.84 | 4.91 | 3.73 | |
| 27.01 | 5.95 | 3.29 | |
| 31.52 | 4.96 | 2.84 |
| Band | Parameters | PB900 | R4 | R6 | R8 | |
|---|---|---|---|---|---|---|
| G | Raman shift (cm−1) | 1589 | 1591 | 1587 | 1587 | |
| FWHM (cm−1) | 71.53 | 75.23 | 72.04 | 66.45 | ||
| Integrated Area | 51.43 | 49.45 | 51.15 | 44.92 | ||
| D | D (D1) | Raman shift (cm−1) | 1348 | 1344 | 1340 | 1338 |
| FWHM (cm−1) | 152.2 | 147.9 | 148.1 | 142.0 | ||
| Integrated Area | 125.2 | 122.5 | 126.9 | 124.3 | ||
| ID/IG | 2.43 | 2.48 | 2.48 | 2.77 | ||
| D2 | Raman shift (cm−1) | 1662 | 1672 | 1669 | 1663 | |
| FWHM (cm−1) | 94.21 | 65.79 | 86.07 | 60.59 | ||
| Integrated Area | 10.82 | 7.94 | 7.91 | 5.36 | ||
| ID2/IG | 0.21 | 0.16 | 0.15 | 0.12 | ||
| D3 | Raman shift (cm−1) | 1513 | 1509 | 1511 | 1511 | |
| FWHM (cm−1) | 129.9 | 141.8 | 150.1 | 151.1 | ||
| Integrated Area | 59.54 | 59.35 | 72.27 | 66.67 | ||
| ID3/IG | 1.16 | 1.20 | 1.41 | 1.48 | ||
| D4 | Raman shift (cm−1) | 1211 | 1227 | 1209 | 1215 | |
| FWHM (cm−1) | 298.0 | 292.9 | 292.4 | 287.2 | ||
| Integrated Area | 75.03 | 71.95 | 70.75 | 60.45 | ||
| ID4/IG | 1.46 | 1.46 | 1.38 | 1.35 | ||
| Element (Chemical Species Indication) | Parameters | PB900 | R4 | R6 | R8 | |
|---|---|---|---|---|---|---|
| C | Sp3 (C−C) | Position (eV) | 285.4 | 285.3 | 285.2 | 285.2 |
| FWHM (eV) | 1.59 | 1.77 | 1.57 | 1.90 | ||
| Integrated Area | 1207.1 | 1308.4 | 915.9 | 759.1 | ||
| Concentration (%) | 22.42 (30.06) | 18.90 (29.03) | 16.45 (28.72) | 19.59 (30.33) | ||
| Sp2 (C=C) | Position (eV) | 284.5 | 284.4 | 284.4 | 284.4 | |
| FWHM (eV) | 1.03 | 1.03 | 1.05 | 1.05 | ||
| Integrated Area | 2808.6 | 3198.4 | 2273.5 | 1744.0 | ||
| Concentration (%) | 52.16 (69.94) | 46.21 (70.97) | 40.83 (71.28) | 45.02 (69.67) | ||
| O | C−O | Position (eV) | 532.9 | 532.9 | 532.8 | 532.9 |
| FWHM (eV) | 2.07 | 2.56 | 2.12 | 2.36 | ||
| Integrated Area | 856.5 | 1004.2 | 188.8 | 385.9 | ||
| Concentration (%) | 15.91 (62.59) | 14.51 (41.58) | 3.39 (7.94) | 9.96 (28.15) | ||
| C=O | Position (eV) | 531.1 | 531.1 | 531.1 | 531.2 | |
| FWHM (eV) | 2.36 | 2.58 | 2.66 | 2.67 | ||
| Integrated Area | 511.9 | 1411.2 | 1577.8 | 784.7 | ||
| Concentration (%) | 9.51 (37.41) | 20.39 (58.42) | 28.34 (66.32) | 20.25 (57.24) | ||
| O–C=O | Position (eV) | - | - | 530.0 | 530.0 | |
| FWHM (eV) | - | - | 1.68 | 1.84 | ||
| Integrated Area | - | - | 612.3 | 200.3 | ||
| Concentration (%) | - | - | 11.00 (25.74) | 5.17 (14.61) | ||
| Element (Primary Region) | PB900 | R4 | R6 | R8 |
|---|---|---|---|---|
| C (C1s) | 74.58 | 65.11 | 57.28 | 64.61 |
| O (O1s) | 25.42 | 34.89 | 42.72 | 35.39 |
| Sample | SBET (m2/g) | Vt-Mic (cm3/g) | Vmeso (cm3/g) | Vtot (cm3/g) | Davg* (nm) | Avg. WHK (nm) | H2 Uptake (wt%) at 77 K | CO2 Uptake (mmol/g) at 298 K |
|---|---|---|---|---|---|---|---|---|
| PB900 | 680 | 0.26 | 0.03 | 0.29 | 1.71 | 0.55 | 1.35 | 2.20 |
| R4 | 590 | 0.21 | 0.01 | 0.22 | 1.60 | 0.54 | 1.48 | 2.98 |
| R6 | 842 | 0.43 | 0.05 | 0.48 | 2.26 | 0.56 | 1.86 | 3.44 |
| R8 | 1150 | 0.43 | 0.03 | 0.46 | 1.30 | 0.58 | 1.82 | 3.34 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, H.-S.; Lee, C.Y.; Jeon, J.W.; Jung, W.T.; Hong, W.G.; Lee, S.M.; Kim, H.; Mun, J.; Kim, B.H. Effect of Oxygen for Enhancing the Gas Storage Performance of Activated Green Carbon. Energies 2020, 13, 3893. https://doi.org/10.3390/en13153893
Jang H-S, Lee CY, Jeon JW, Jung WT, Hong WG, Lee SM, Kim H, Mun J, Kim BH. Effect of Oxygen for Enhancing the Gas Storage Performance of Activated Green Carbon. Energies. 2020; 13(15):3893. https://doi.org/10.3390/en13153893
Chicago/Turabian StyleJang, Hyun-Seok, Chang Yeon Lee, Jun Woo Jeon, Won Taek Jung, Won G. Hong, Sang Moon Lee, Haejin Kim, Junyoung Mun, and Byung Hoon Kim. 2020. "Effect of Oxygen for Enhancing the Gas Storage Performance of Activated Green Carbon" Energies 13, no. 15: 3893. https://doi.org/10.3390/en13153893





