Efficiency at Maximum Power of the Low-Dissipation Hybrid Electrochemical–Otto Cycle
Abstract
1. Introduction
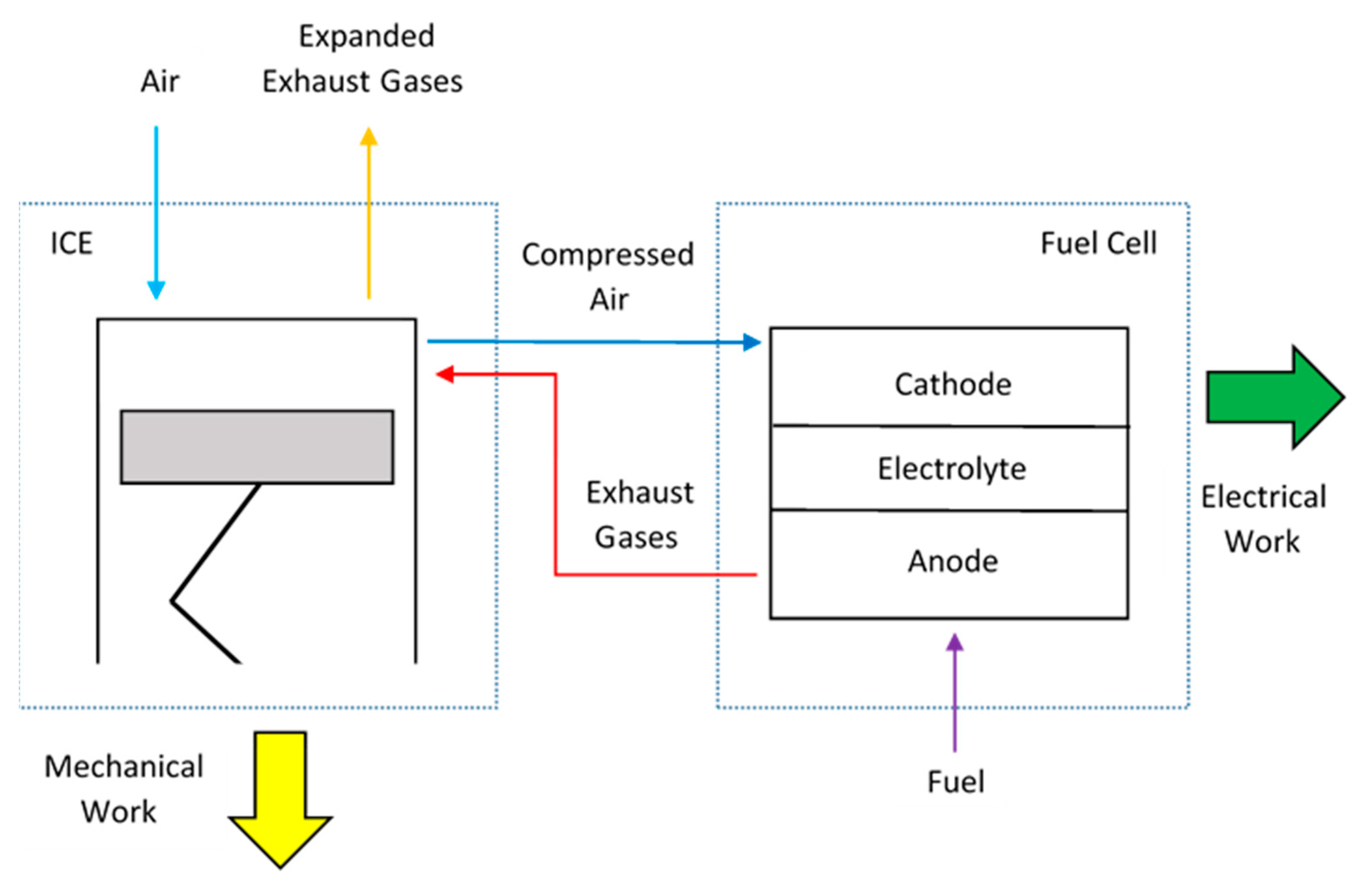
2. Low-Dissipation Model of the Hybrid Cycle
3. Low-Dissipation Model for Partial Conversion
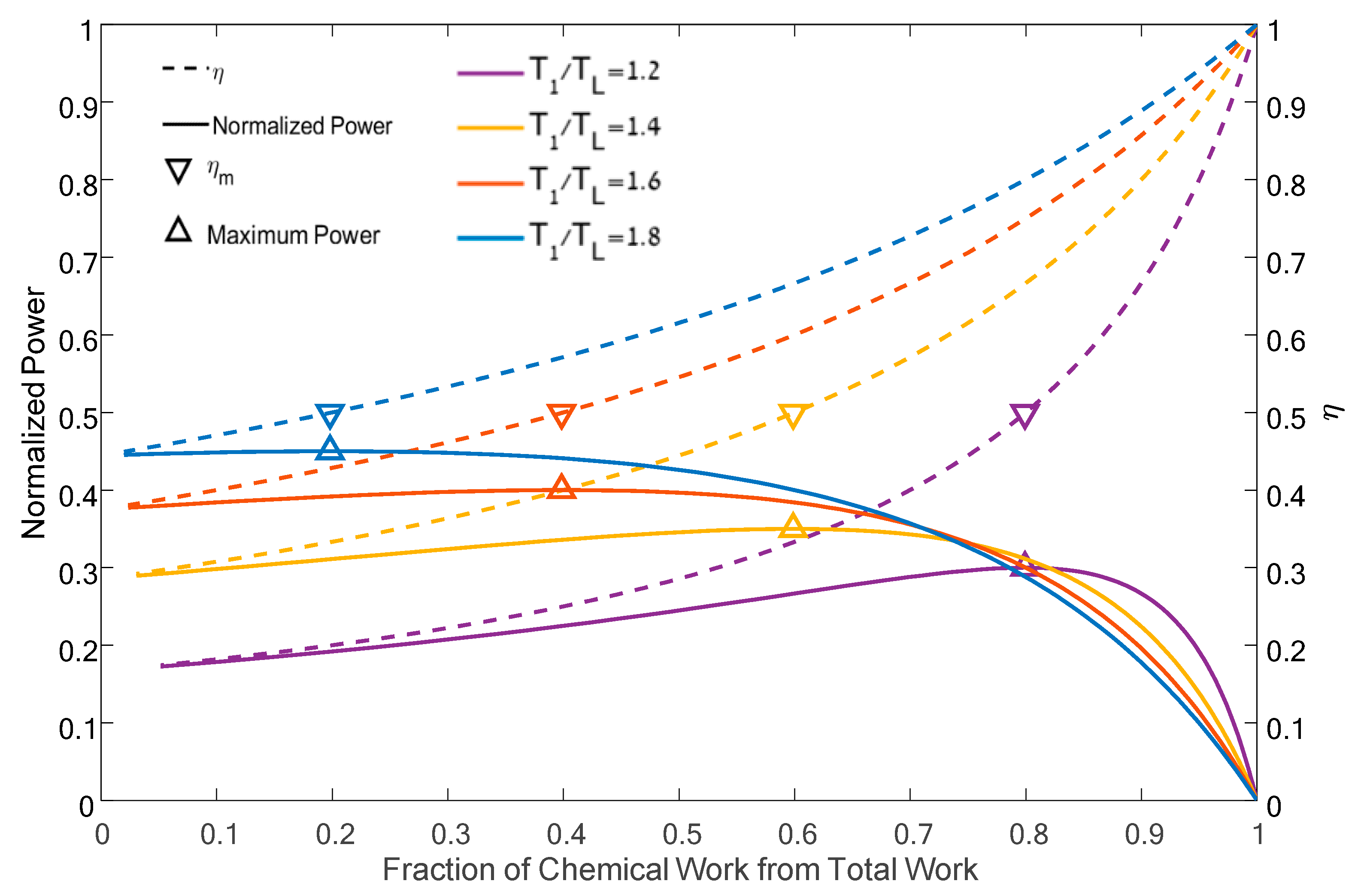
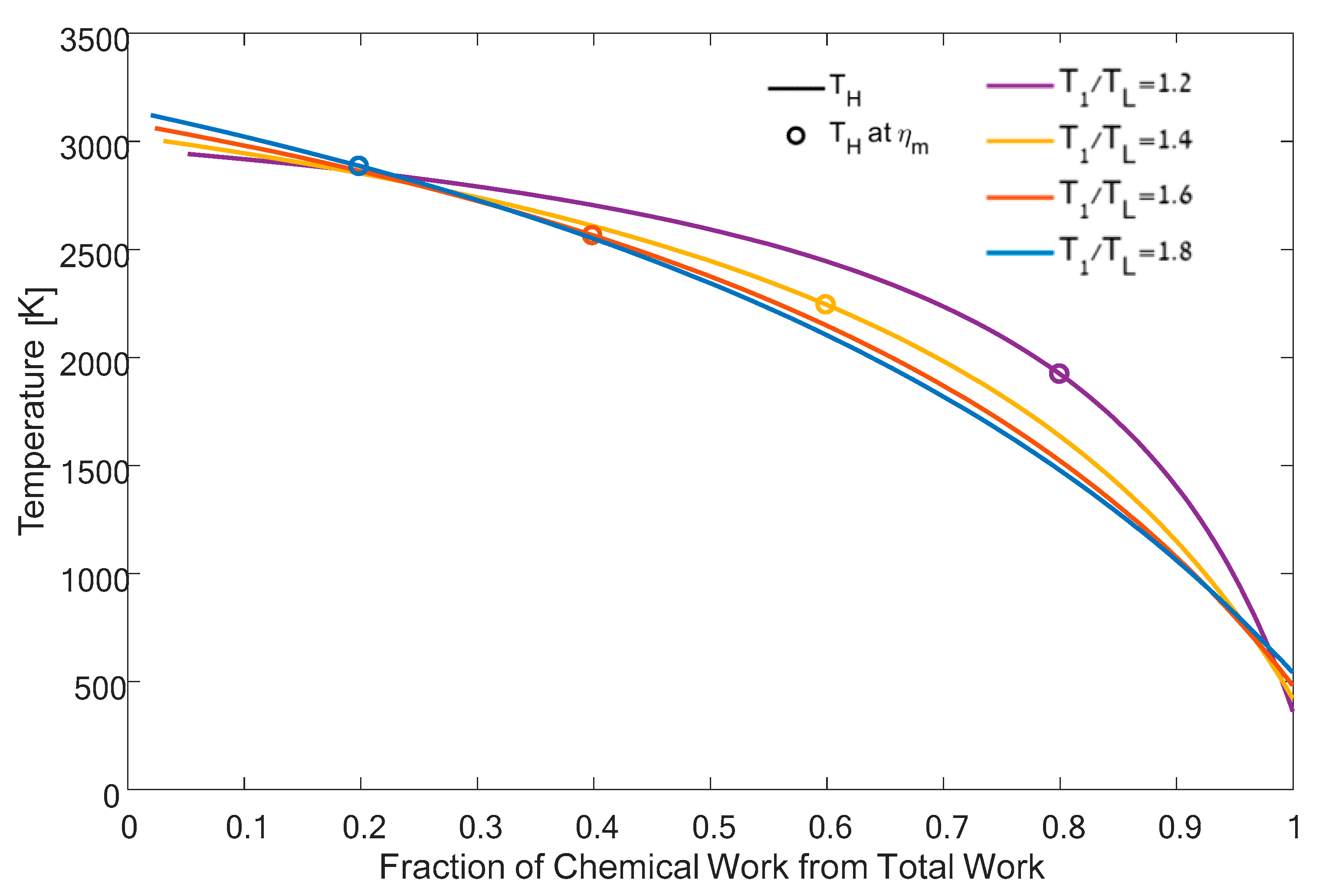
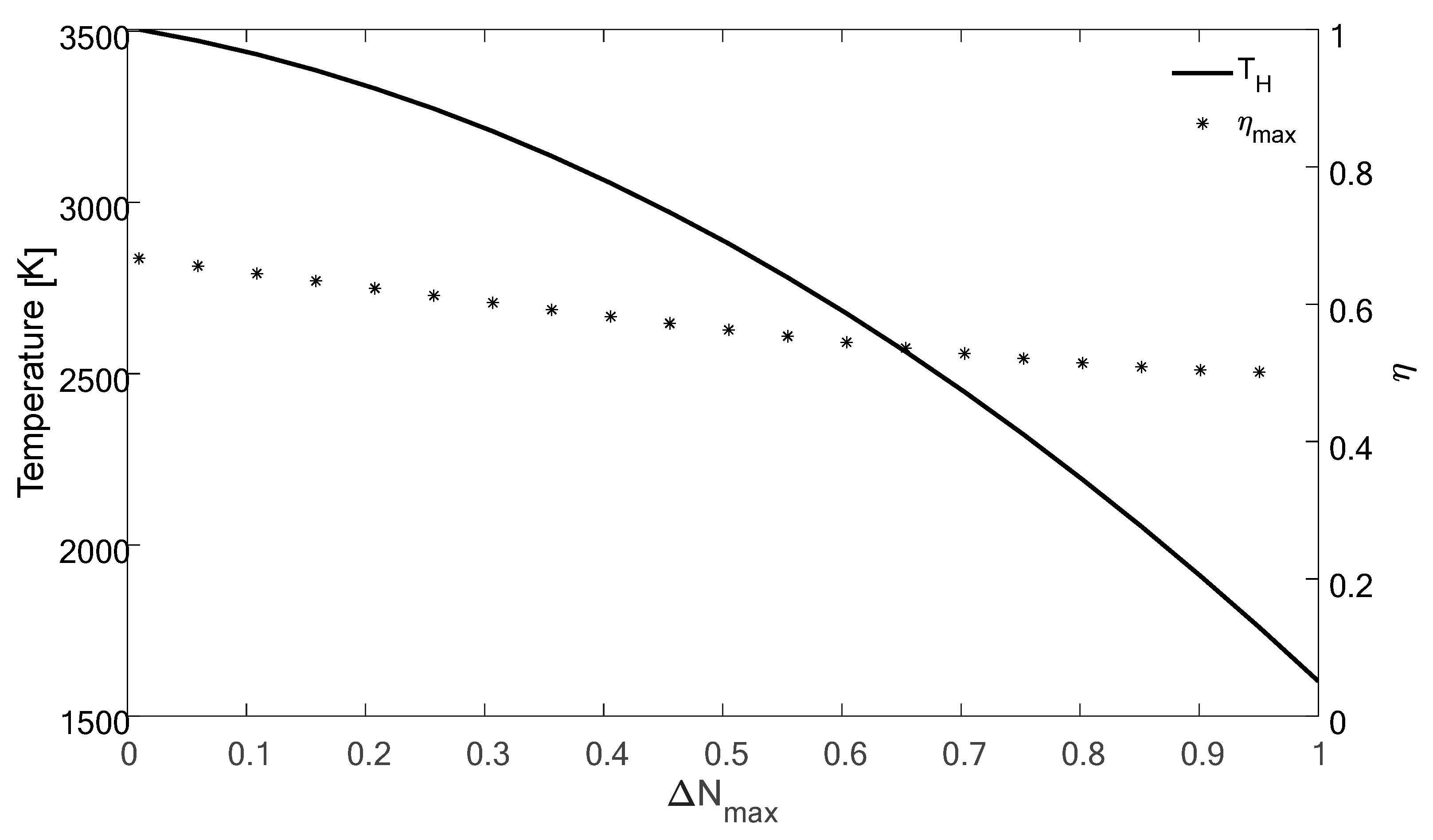
4. Results and Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Novikov, I.I. Efficiency of an atomic power generating installation. Sov. J. At. Energy 1957, 3, 1269–1272. [Google Scholar] [CrossRef]
- Curzon, F.L.; Ahlborn, B. Efficiency of a Carnot engine at maximum power output. Am. J. Phys. 1975, 43, 22–24. [Google Scholar] [CrossRef]
- Hoffmann, K.H.; Burzler, J.M.; Schubert, S. Endoreversible thermodynamics. CiteSeer 1997, 22, 311. [Google Scholar]
- Esposito, M.; Kawai, R.; Lindenberg, K.; Van den Broeck, C. Efficiency at maximum power of low-dissipation Carnot engines. Phys. Rev. Lett. 2010, 105, 150603. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Wang, Y.; Chen, J. Universal efficiency bounds of weak-dissipative thermodynamic cycles at the maximum power output. Phys. Rev. E 2013, 87, 012133. [Google Scholar] [CrossRef] [PubMed]
- Petrescu, S.; Maris, V.; Costea, M.; Boriaru, N.; Stanciu, C.; Dura, I. Comparison between Fuel Cells and Heat Engines. I. A Similar Approach in the Framework of Thermodynamics with Finite Speed. Rev. Chim. 2013, 64, 739–746. [Google Scholar]
- Pavelka, M.; Klika, V.; Vágner, P.; Maršík, F. Generalization of exergy analysis. Appl. Energy 2015, 137, 158–172. [Google Scholar] [CrossRef]
- Vágner, P.; Pavelka, M.; Maršík, F. Pitfalls of exergy analysis. J. Non-Equilib. Thermodyn. 2017, 42, 201–216. [Google Scholar] [CrossRef]
- Zabihian, F.; Fung, A. A review on modeling of hysbrid solid oxide fuel cell systems. Int. J. Eng. 2009, 3, 85–119. [Google Scholar]
- Winkler, W.; Nehter, P.; Williams, M.C.; Tucker, D.; Gemmen, R. General fuel cell hybrid synergies and hybrid system testing status. J. Power Sources 2006, 159, 656–666. [Google Scholar] [CrossRef]
- Dunbar, W.R.; Lior, N.; Gaggioli, R.A. Exergetic advantages of topping rankine power cycles with fuel cell units. Am. Soc. Mech. Eng. Adv. Energy Syst. Div. (AES) 1990, 21, 63–68. [Google Scholar]
- Harvey, S.P.; Richter, H.J. Improved gas turbine power plant efficiency by use of recycled exhaust gases and fuel cell technology. Proc. ASME Winter Annu. Meet. 1993, 30, 199–207. [Google Scholar]
- Gorla, R.S. Probabilistic analysis of a solid-oxide fuel-cell based hybrid gas-turbine system. Appl. Energy 2004, 78, 63–74. [Google Scholar] [CrossRef][Green Version]
- Kim, J.; Kim, Y.; Choi, W.; Ahn, K.Y.; Song, H.H. Analysis on the operating performance of 5-kW class solid oxide fuel cell-internal combustion engine hybrid system using spark-assisted ignition. Appl. Energy 2020, 260, 114231. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, Y.D.; Ahn, K.Y. Performance analysis of an SOFC/HCCI engine hybrid system: System simulation and thermo-economic comparison. Int. J. Hydrog. Energy 2014, 39, 1799–1810. [Google Scholar] [CrossRef]
- Lee, Y.D.; Ahn, K.Y.; Morosuk, T.; Tsatsaronis, G. Exergetic and exergoeconomic evaluation of an SOFC-Engine hybrid power generation system. Energy 2018, 145, 810–822. [Google Scholar] [CrossRef]
- Pettersson, L.J.; Westerholm, R. State of the art of multi-fuel reformers for fuel cell vehicles: Problem identification and research needs. Int. J. Hydrog. Energy 2001, 26, 243–264. [Google Scholar] [CrossRef]
- Tartakovsky, L.; Sheintuch, M. Fuel reforming in internal combustion engines. Prog. Energy Combust. Sci. 2018, 67, 88–114. [Google Scholar] [CrossRef]
- Eyal, A.; Tartakovsky, L. Second-law analysis of the reforming-controlled compression ignition. Appl. Energy 2020, 263, 114622. [Google Scholar] [CrossRef]
- Poran, A.; Thawko, A.; Eyal, A.; Tartakovsky, L. Direct injection internal combustion engine with high-pressure thermochemical recuperation–Experimental study of the first prototype. Int. J. Hydrog. Energy 2018, 43, 11969–11980. [Google Scholar] [CrossRef]
- Chuahy, F.D.; Kokjohn, S.L. Solid oxide fuel cell and advanced combustion engine combined cycle: A pathway to 70% electrical efficiency. Appl. Energy 2019, 235, 391–408. [Google Scholar] [CrossRef]
- Andresen, B. Comment on A fallacious argument in the finite time thermodynamic concept of endoreversibility [J. Appl. Phys. 83, 4561 (1998)]. J. Appl. Phys. 2001, 90, 6557–6559. [Google Scholar] [CrossRef]
- Chen, J.; Yan, Z.; Lin, G.; Andresen, B. On the Curzon–Ahlborn efficiency and its connection with the efficiencies of real heat engines. Energy Convers. Manag. 2001, 42, 173–181. [Google Scholar] [CrossRef]
- Ocampo-García, A.; Barranco-Jiménez, M.A.; Angulo-Brown, F. Thermodynamic and thermoeconomic optimization of coupled thermal and chemical engines by means of an equivalent array of uncoupled endoreversible engines. Eur. Phys. J. Plus 2018, 133, 342. [Google Scholar] [CrossRef]
- Heywood, J.B. Combustion Engine Fundamentals; McGraw-Hill: New York, NY, USA, 1988. [Google Scholar]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diskin, D.; Tartakovsky, L. Efficiency at Maximum Power of the Low-Dissipation Hybrid Electrochemical–Otto Cycle. Energies 2020, 13, 3961. https://doi.org/10.3390/en13153961
Diskin D, Tartakovsky L. Efficiency at Maximum Power of the Low-Dissipation Hybrid Electrochemical–Otto Cycle. Energies. 2020; 13(15):3961. https://doi.org/10.3390/en13153961
Chicago/Turabian StyleDiskin, David, and Leonid Tartakovsky. 2020. "Efficiency at Maximum Power of the Low-Dissipation Hybrid Electrochemical–Otto Cycle" Energies 13, no. 15: 3961. https://doi.org/10.3390/en13153961
APA StyleDiskin, D., & Tartakovsky, L. (2020). Efficiency at Maximum Power of the Low-Dissipation Hybrid Electrochemical–Otto Cycle. Energies, 13(15), 3961. https://doi.org/10.3390/en13153961





