Cryo-Mechanical Treatment and Hydrometallurgical Process for Recycling Li-MnO2 Primary Batteries with the Direct Production of LiMnPO4 Nanoparticles
Abstract
:1. Introduction
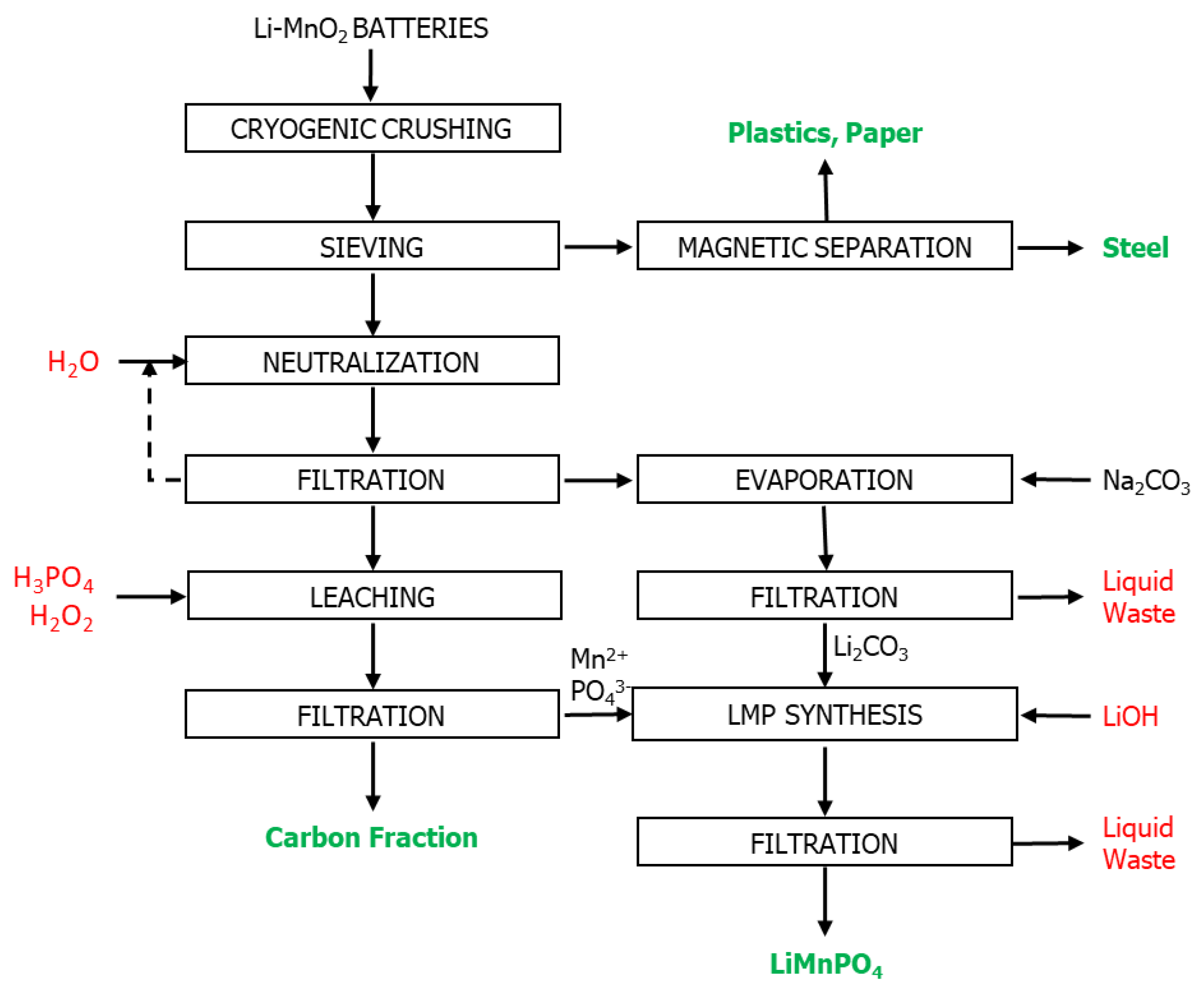
2. Materials and Methods
2.1. Mechanical Pre-Treatments
2.2. Lithium and Manganese Extraction and Recovery
2.3. Synthesis of LiMnPO4
2.4. Characterizations
3. Results
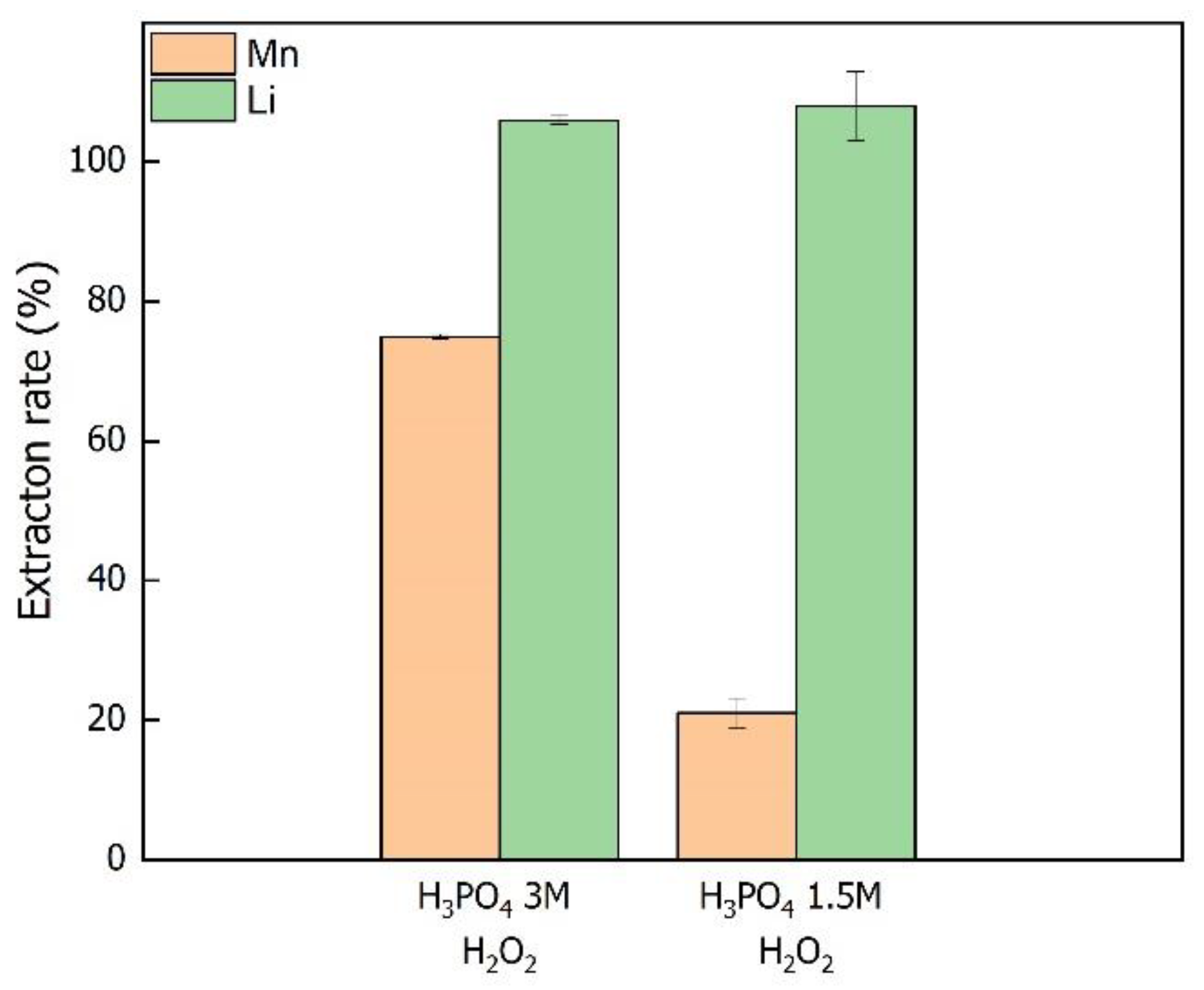
3.1. Recovery of Lithium and Production of the Mn2+-PO43− Precursor Solution
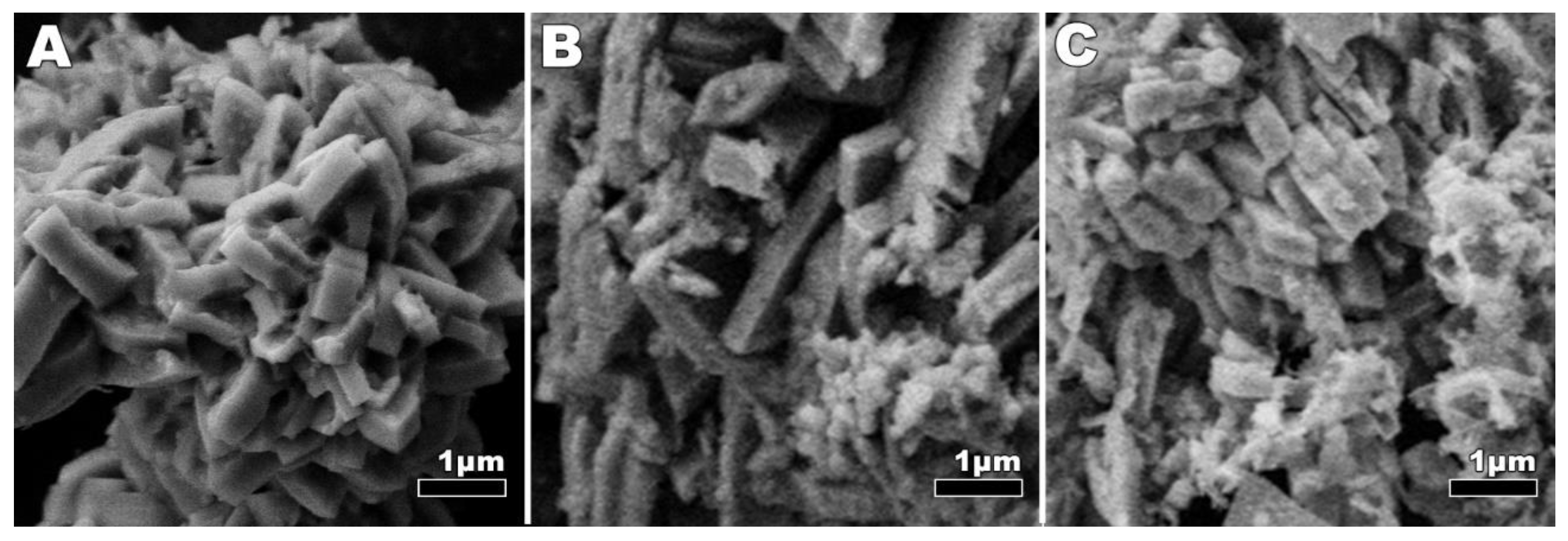
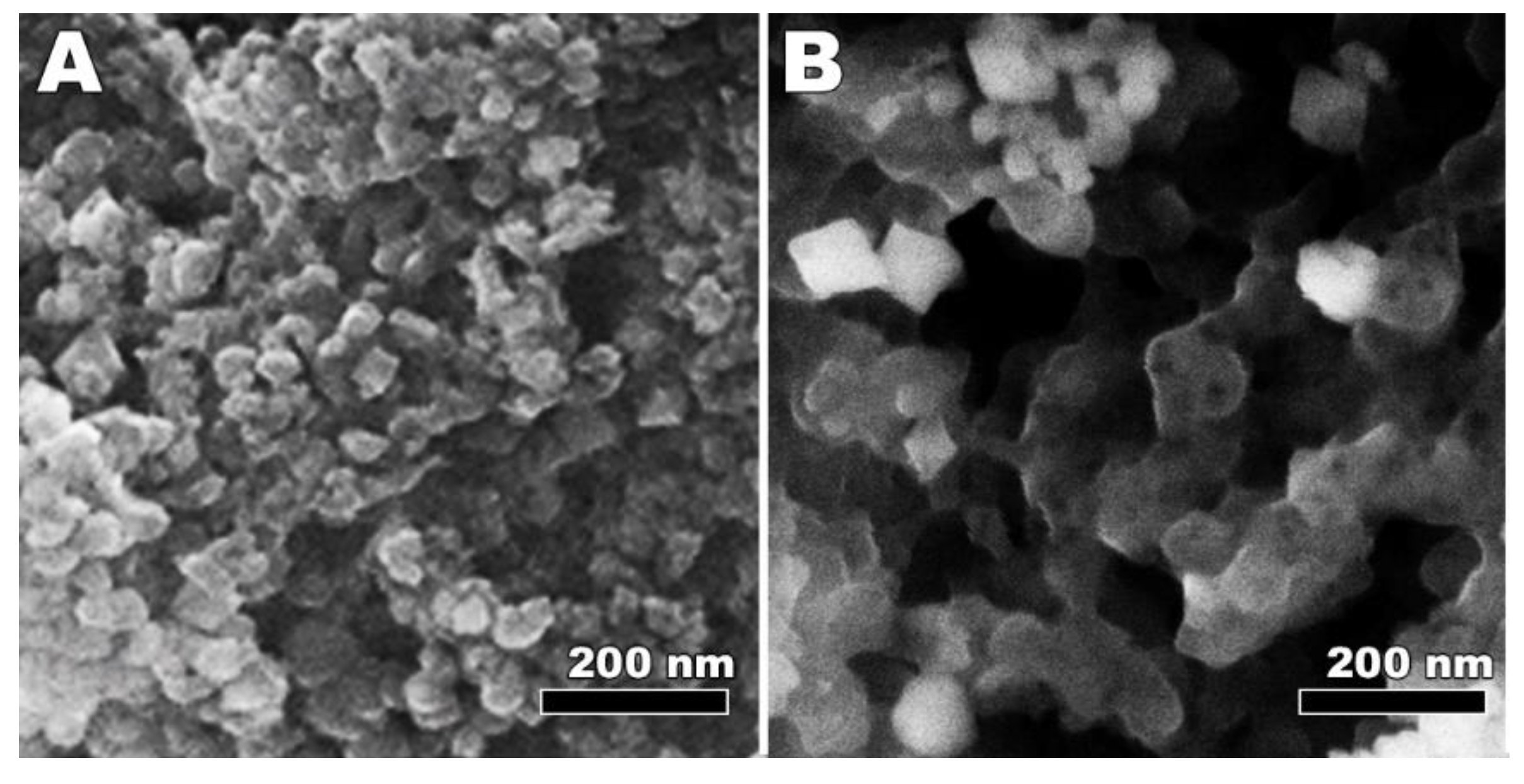
3.2. Synthesis and Characterization of LiMnPO4
3.3. Mass Balance of the Proposed Process
- The results of the electrodic powder washing showed about half of the total lithium contained in the batteries was present as an insoluble species. The insoluble lithium was considered to be LiMnO2.
- The carbon fraction was estimated by subtracting the LiMnO2 and MnO2 amounts remaining in the fine fraction that resulted after water leaching. Here it was assumed that after water washing, the residual insoluble species were carbon, LiMnO2, and MnO2. The LiMnO2 and MnO2 amounts were estimated from the Li and Mn analysis in the washed electrodic powder.
- The remainder of the residue was considered to be made up of solvents and organic salts based on the datasheet of the most common coin-cell batteries on the market.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Directive, B. Directive 2006/66/EC of the European Parliament and of the Council of 6 September 2006 on batteries and accumulators and waste batteries and accumulators and repealing Directive 91/157/EEC. Off. J. Eur. Union 2006, 266, 1–14. [Google Scholar]
- Stahl, H.; Baron, Y.; Hay, D.; Hermann, A.; Mehlhart, G.; Baroni, L.; Rademaekers, K.; Williams, R.; Paha, S. Study in Support of Evaluation of the Directive 2006/66/EC on Batteries and Accumulators and Waste Batteries and Accumulators; Final Report; European Commission: Rotterdam, The Netherlands, 2018. [Google Scholar]
- Espinosa, D.; Bernardes, A.M.; Tenório, J. An overview on the current processes for the recycling of batteries. J. Power Sources 2004, 135, 311–319. [Google Scholar] [CrossRef]
- European Commission. Study on the Calculation of Recycling Efficiencies and Implementation of Export Article (Art. 15) of the Batteries Directive 2006/66/EC; Final Report; European Commission: Brussels, Belgium, 2009. [Google Scholar]
- Liu, C.; Lin, J.; Cao, H.; Zhang, Y.; Sun, Z.H. Recycling of spent lithium-ion batteries in view of lithium recovery: A critical review. J. Clean. Prod. 2019, 228, 801–813. [Google Scholar] [CrossRef]
- Sattar, R.; Ilyas, S.; Bhatti, H.N.; Ghaffar, A. Resource recovery of critically-rare metals by hydrometallurgical recycling of spent lithium ion batteries. Sep. Purif. Technol. 2019, 209, 725–733. [Google Scholar] [CrossRef]
- Huang, T.; Liu, L.; Zhang, S. Recovery of cobalt, lithium, and manganese from the cathode active materials of spent lithium-ion batteries in a bio-electro-hydrometallurgical process. Hydrometallurgy 2019, 188, 101–111. [Google Scholar] [CrossRef]
- Kondás, J.; Jandová, J.; Nemeckova, M. Processing of spent Li/MnO2 batteries to obtain Li2CO3. Hydrometallurgy 2006, 84, 247–249. [Google Scholar] [CrossRef]
- Paulino, J.F.; Busnardo, N.G.; Afonso, J.C. Recovery of valuable elements from spent Li-batteries. J. Hazard. Mater. 2008, 150, 843–849. [Google Scholar] [CrossRef]
- Atia, T.A.; Elia, G.; Hahn, R.; Altimari, P.; Pagnanelli, F. Closed-loop hydrometallurgical treatment of end-of-life lithium ion batteries: Towards zero-waste process and metal recycling in advanced batteries. J. Energy Chem. 2019, 35, 220–227. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.; Huo, G.; Tang, W. Removal of Fe(III) from Ni-Co-Fe chloride solutions using solvent extraction with TBP. Hydrometallurgy 2020, 192, 105265. [Google Scholar] [CrossRef]
- Schiavi, P.G.; Farina, L.; Zanoni, R.; Altimari, P.; Cojocariu, I.; Rubino, A.; Navarra, M.A.; Panero, S.; Pagnanelli, F. Electrochemical synthesis of nanowire anodes from spent lithium ion batteries. Electrochim. Acta 2019, 319, 481–489. [Google Scholar] [CrossRef]
- Gratz, E.; Sa, Q.; Apelian, D.; Wang, Y. A closed loop process for recycling spent lithium ion batteries. J. Power Sources 2014, 262, 255–262. [Google Scholar] [CrossRef]
- Hu, X.; Sun, X.; Yang, M.; Ji, H.; Li, X.; Cai, S.; Guo, R.; Hou, F.; Zheng, C.; Hu, W. Sandwich nanostructured LiMnPO 4/C as enhanced cathode materials for lithium-ion batteries. J. Mater. Sci. 2017, 52, 3597–3612. [Google Scholar] [CrossRef]
- Drezen, T.; Kwon, N.-H.; Bowen, P.; Teerlinck, I.; Isono, M.; Exnar, I. Effect of particle size on LiMnPO4 cathodes. J. Power Sources 2007, 174, 949–953. [Google Scholar] [CrossRef]
- Wang, R.; Cui, W.; Chu, F.; Wu, F. Lithium metal anodes: Present and future. J. Energy Chem. 2020, 48, 145–159. [Google Scholar] [CrossRef]
- Shen, X.; Li, Y.; Qian, T.; Liu, J.; Zhou, J.; Yan, C.; Goodenough, J.B. Lithium anode stable in air for low-cost fabrication of a dendrite-free lithium battery. Nat. Commun. 2019, 10, 900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, H.; Yang, G.; Ni, H.; Roy, S.; Pinto, J.L.; Jiang, X. General synthesis and morphology control of LiMnPO4 nanocrystals via microwave-hydrothermal route. Electrochim. Acta 2011, 56, 3093–3100. [Google Scholar] [CrossRef]
- Wang, W.; Chen, W.; Liu, H. Hydrometallurgical preparation of lithium carbonate from lithium-rich electrolyte. Hydrometallurgy 2019, 185, 88–92. [Google Scholar] [CrossRef]
- Salomon, M. Solubility problems relating to lithium battery electrolytes. Pure Appl. Chem. 1998, 70, 1905–1912. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.-H.; Lu, M.-C.; Chen, J.-N.; Lee, C.-T. Catalytic decomposition of hydrogen peroxide and 4-chlorophenol in the presence of modified activated carbons. Chemosphere 2003, 51, 935–943. [Google Scholar] [CrossRef] [Green Version]
- Petlicki, J.; Palusova, D.; Van De Ven, T.G.M. Physicochemical Aspects of Catalytic Decomposition of Hydrogen Peroxide by Manganese Compounds. Ind. Eng. Chem. Res. 2005, 44, 2002–2010. [Google Scholar] [CrossRef]
- Pagnanelli, F.; Furlani, G.; Valentini, P.; Veglio, F.; Toro, L. Leaching of low-grade manganese ores by using nitric acid and glucose: Optimization of the operating conditions. Hydrometallurgy 2004, 75, 157–167. [Google Scholar] [CrossRef]
- Fang, H.; Li, L.; Yang, Y.; Yan, G.; Li, G. Carbonate anions controlled morphological evolution of LiMnPO4 crystals. Chem. Commun. 2008, 9, 1118–1120. [Google Scholar] [CrossRef] [PubMed]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiavi, P.G.; dos Santos Martins Padoan, F.C.; Altimari, P.; Pagnanelli, F. Cryo-Mechanical Treatment and Hydrometallurgical Process for Recycling Li-MnO2 Primary Batteries with the Direct Production of LiMnPO4 Nanoparticles. Energies 2020, 13, 4004. https://doi.org/10.3390/en13154004
Schiavi PG, dos Santos Martins Padoan FC, Altimari P, Pagnanelli F. Cryo-Mechanical Treatment and Hydrometallurgical Process for Recycling Li-MnO2 Primary Batteries with the Direct Production of LiMnPO4 Nanoparticles. Energies. 2020; 13(15):4004. https://doi.org/10.3390/en13154004
Chicago/Turabian StyleSchiavi, Pier Giorgio, Flavia Carla dos Santos Martins Padoan, Pietro Altimari, and Francesca Pagnanelli. 2020. "Cryo-Mechanical Treatment and Hydrometallurgical Process for Recycling Li-MnO2 Primary Batteries with the Direct Production of LiMnPO4 Nanoparticles" Energies 13, no. 15: 4004. https://doi.org/10.3390/en13154004





