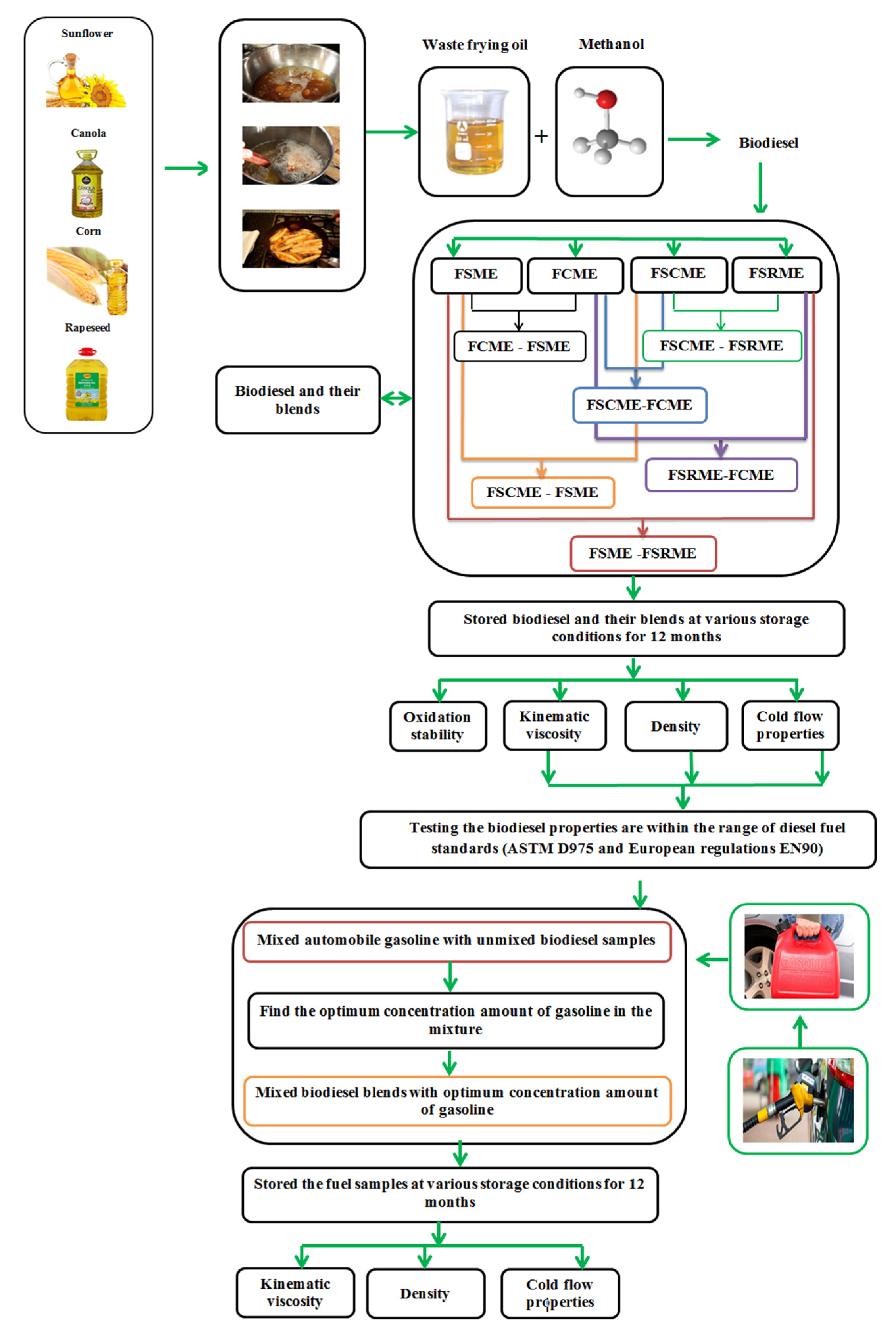
Biodiesel Production from Four Residential Waste Frying Oils: Proposing Blends for Improving the Physicochemical Properties of Methyl Biodiesel
Abstract
:1. Introduction
- Study the physicochemical properties, including kinematic viscosity, density, cold flow properties and oxidation stability, of biodiesels and their blends in different proportions to investigate the possibility of producing a mixture that improves the oxidation stability and the other physicochemical properties. In this study, four methyl biodiesel samples are produced from residential waste frying oils. The first sample (FSME) was prepared from residential waste frying sunflower oil, the second sample (FCME) was obtained from residential waste frying canola oil, and the third sample (FSCME) was prepared from the mixture of residential waste frying sunflower and corn oil. The last sample (FSRME) was obtained from a mixture of residential waste frying sunflower and rapeseed oil;
- In order to use biodiesel in diesel engines according to American Standard Test Method (ASTM) D975 and European regulations EN 590 for diesel fuel, the unmixed biodiesels were blended with automobile gasoline in different proportions, varying from 5% to 95% by volume, and the physicochemical characteristics of the samples were also evaluated in order to determine the optimum concentration amount of gasoline. Moreover, biodiesel blends were mixed with the optimum concentration amount of gasoline so as to reduce the kinematic viscosity, density and cold flow properties of the biodiesel;
- We investigate the effects of long-term storage periods on the properties of all fuel samples, including kinematic viscosity, density, cold flow properties and oxidation stability, with different storage temperatures (5 °C, room temperature, RT, (23 ± 1 °C) and 40 °C). These storage temperatures were selected based on expected average weather temperatures in the winter, spring, summer and autumn seasons.
2. Materials and Methods
2.1. Preparing Biodiesel Sample
- FSME was prepared from frying cooking sunflower oil;
- FCME was obtained from frying cooking canola oil;
- FSCME was produced from a mixture of frying cooking sunflower and corn oils;
- FSRME was prepared from a mixture of frying cooking sunflower and rapeseed oils.
2.2. Biodiesel Characterization
2.3. Fuel Samples
2.4. Preparation of Blends
2.5. Storage Conditions
- For the storage condition of 5 °C, the samples were stored in the refrigerator in which the temperature was fixed at 5 °C.
- For room temperature, the fuel samples were stored in a glass container where the average daily temperature was about 23 °C.
- For the storage condition of 40 °C, the samples were in a temperature-controlled laboratory oven in which the thermostat was used to control the temperature inside the oven and keep it constant.
2.6. Analytical Methods
3. Results and Discussion
3.1. Preliminary Physical Properties of Pure Biodiesel and Blends at 0-Month
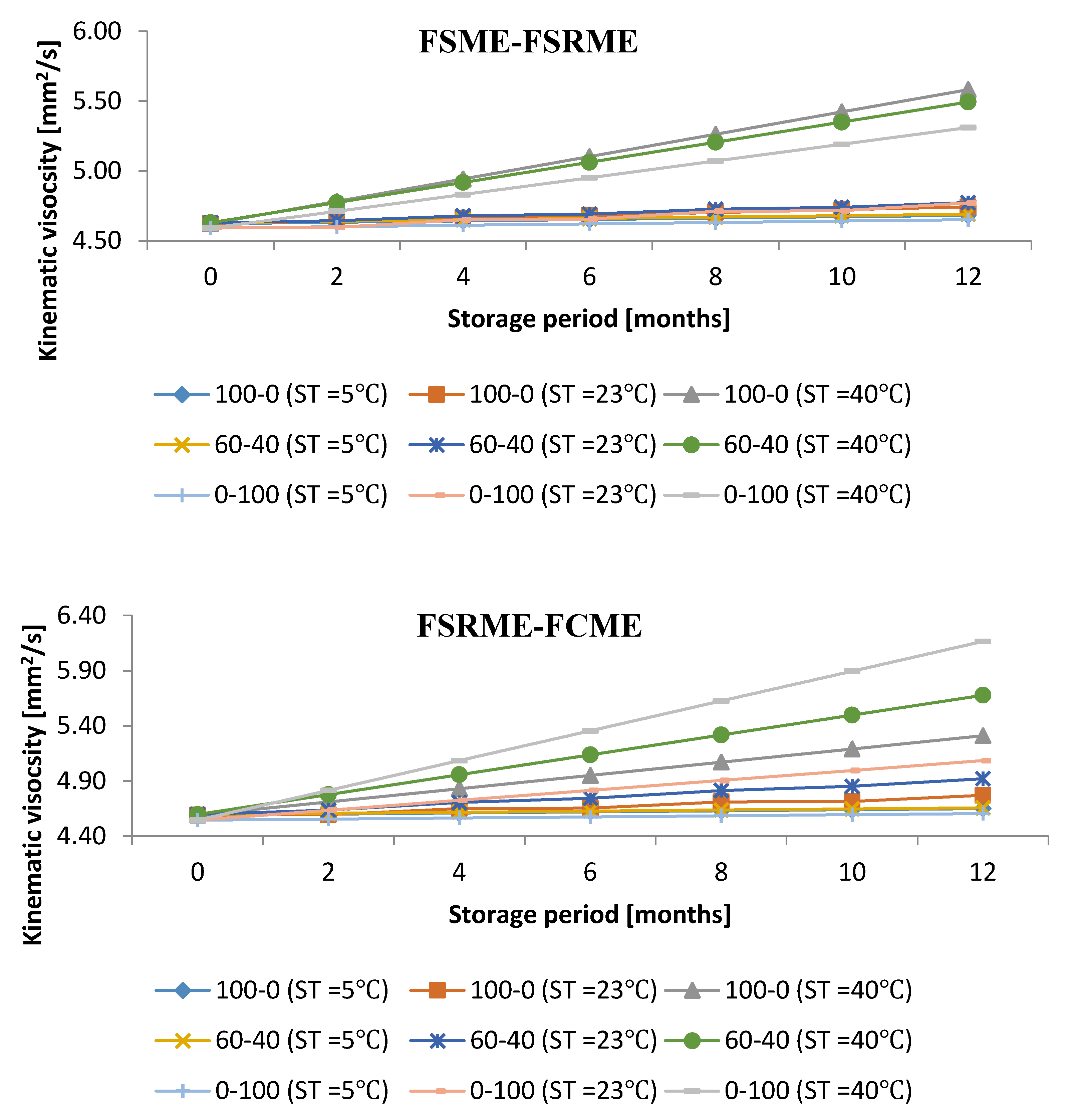
- The kinematic viscosity increased by 0.04 mm2/s to a maximum at 40 vol% FSME in FCME–FSME blends
- The kinematic viscosity increased by 0.04 mm2/s to a maximum at 60 vol% FSME in FSCME–FSME blends
- The kinematic viscosity increased by 0.06 mm2/s to a maximum at 20 vol% FSME in FSME–FSRME blends
- The kinematic viscosity increased by 0.09 mm2/s to a maximum at 20 vol% FCME in FSRME–FCME blends
3.2. Influence of Storage Period, with Various Storage Conditions, on Oxidation Stability
3.3. Impact of Storage Period under Various Storage Conditions on Kinematic Viscosity
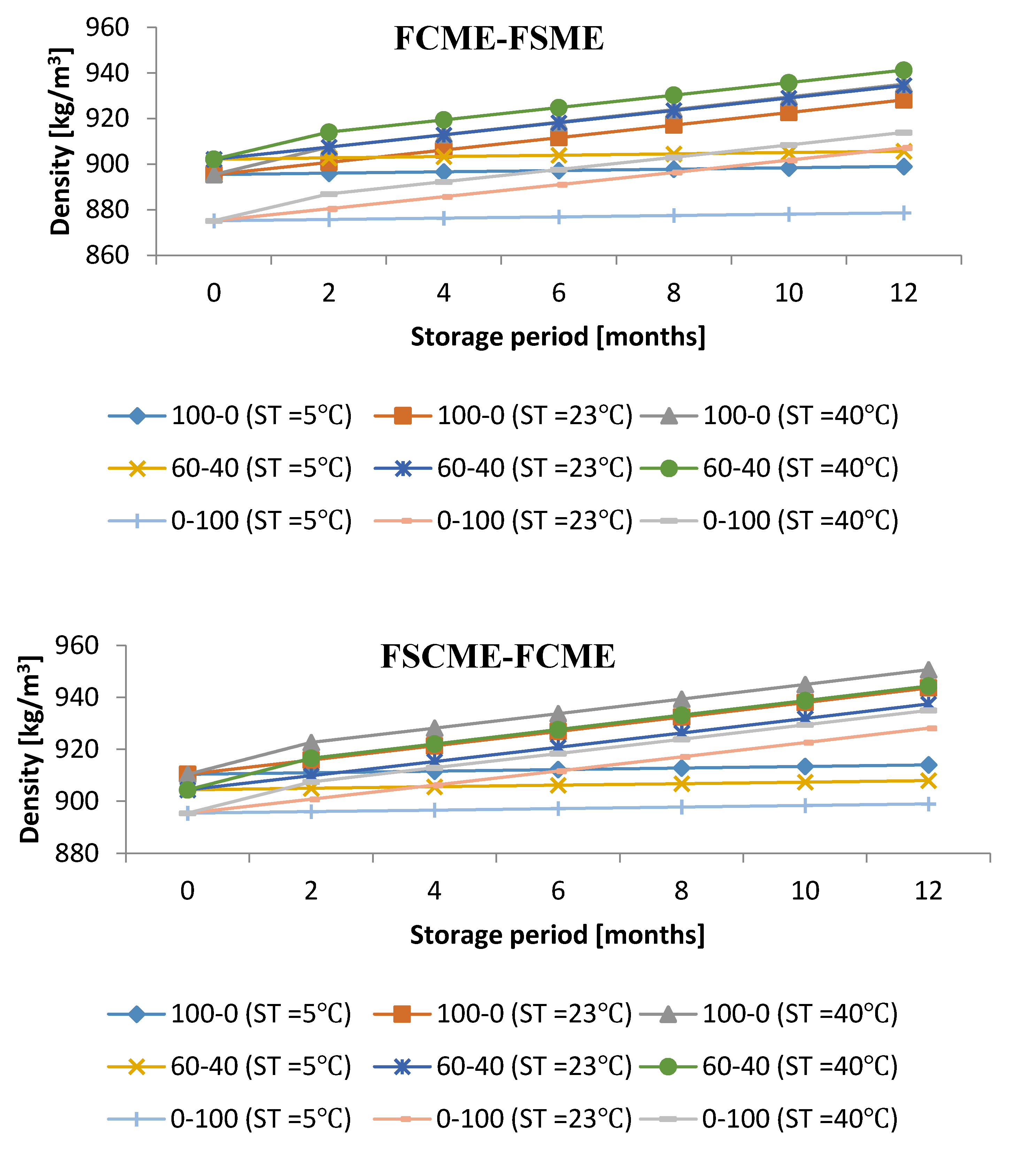
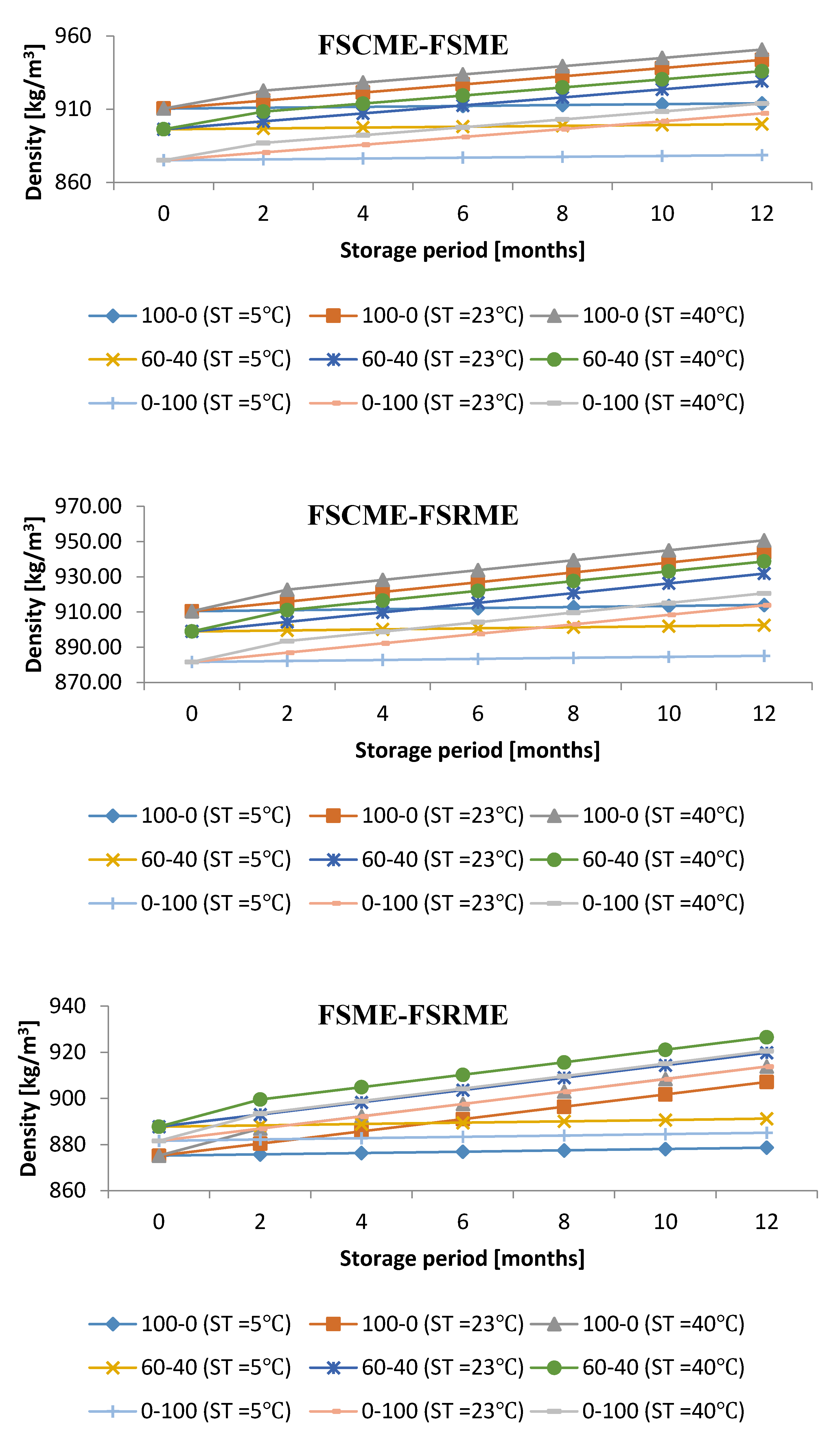
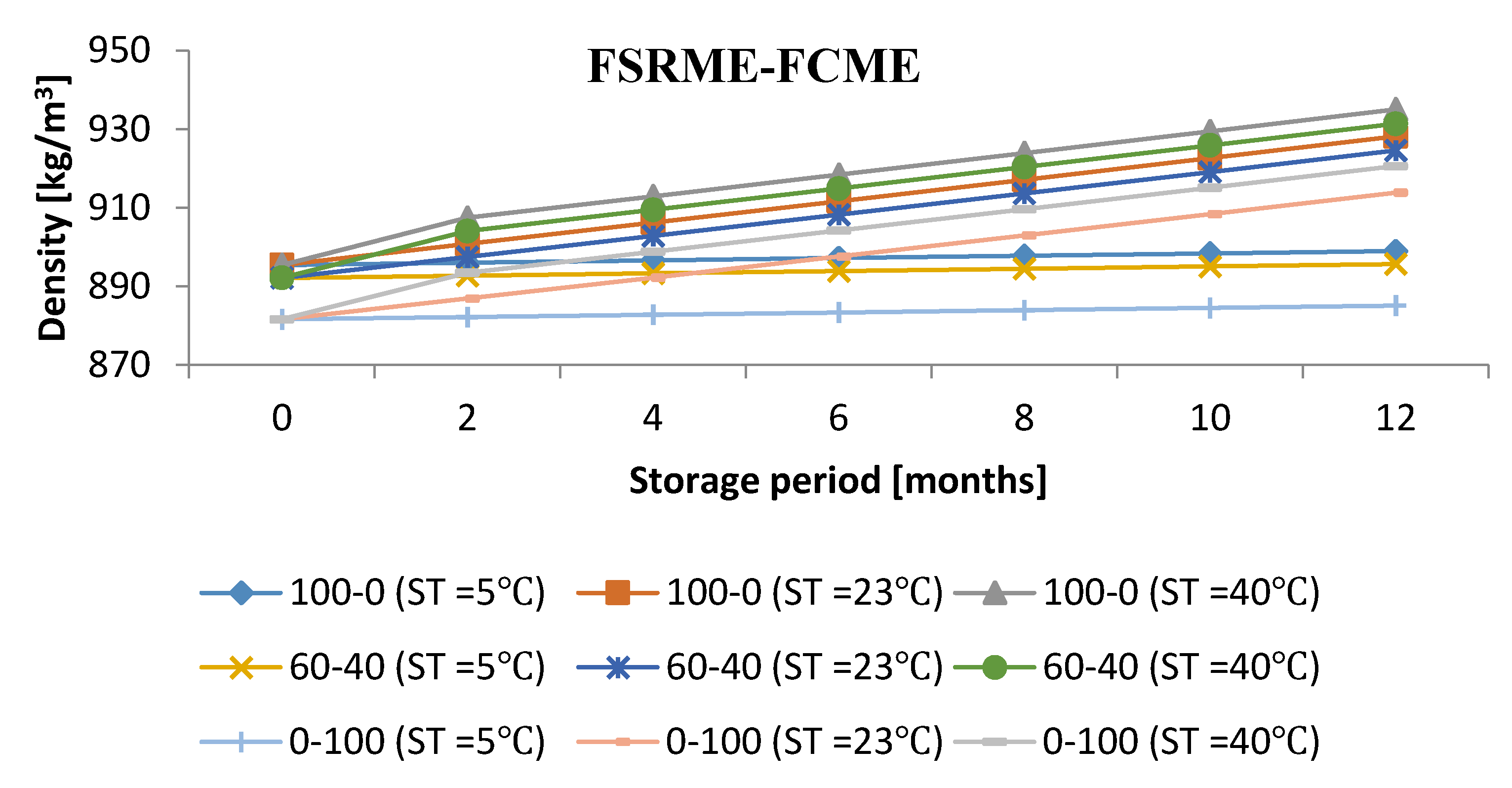
3.4. Influence of Storage Period with Various Storage Conditions on Density
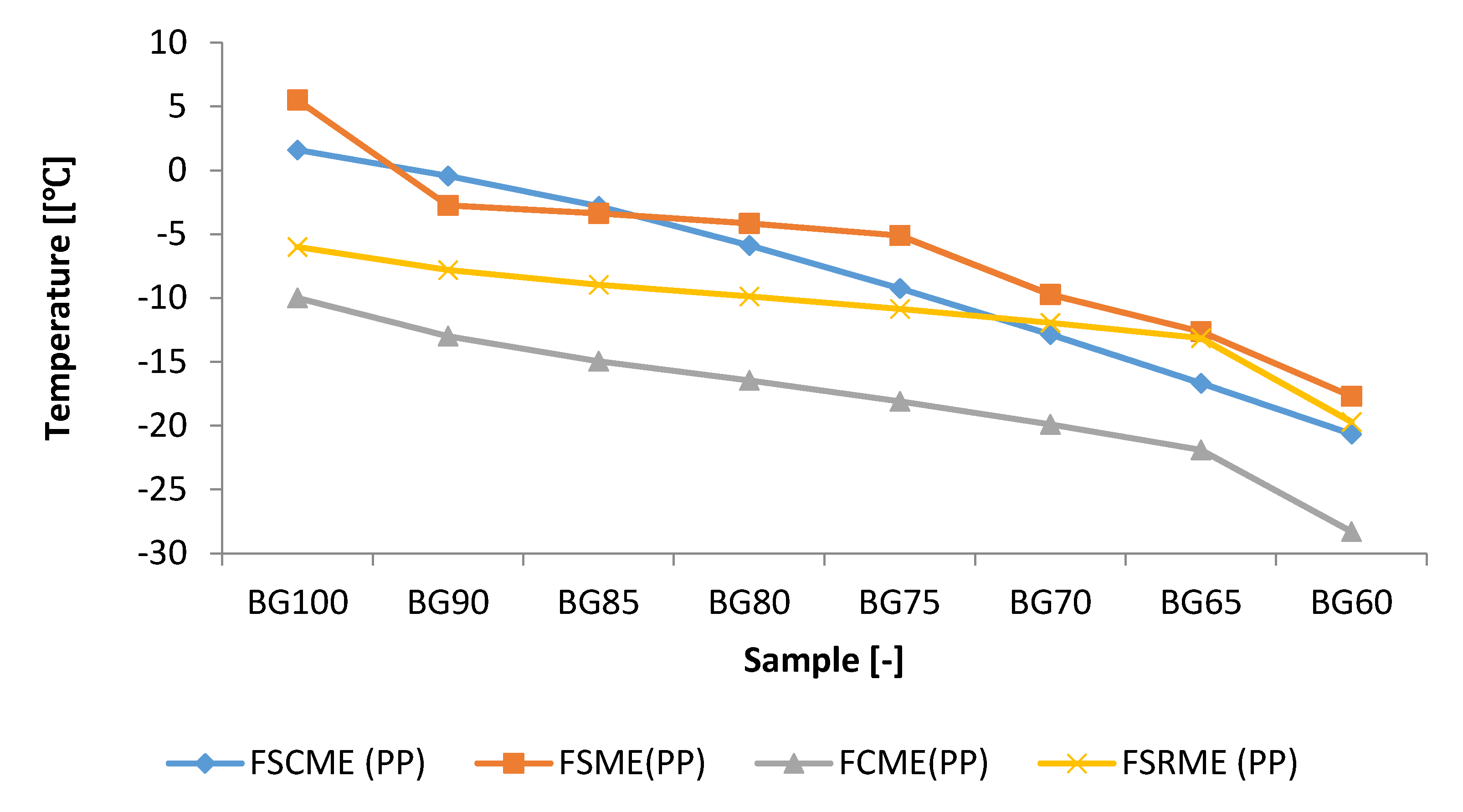
3.5. Impact of Storage Period with Various Storage Conditions on Cold Flow Properties
3.6. Physical Properties of Unmixed Biodiesel–Gasoline Blends
3.6.1. Influence of Storage Period with Various Storage Condition on Kinematic Viscosity
3.6.2. Influence of Storage Period with Various Storage Condition on Cold Flow Properties
3.7. Physical Properties of Triple Blends
- The kinematic viscosities of all blends are above the limit specification of ASTM D975, while some samples with various concentrations of FSCME fulfill the European regulations EN 590.
- Based on the oxidation stability specification listed in EN 14214:2014, the minimum specified limit of 8 h is required to ensure adequate biodiesel stability during a typical 6-month fuel consumption timeframe [32,37]. The results indicated that out of 26 samples, 10 samples had an oxidation stability above 10 h for 0-month. These samples are mixed with various percentages of FSCME. It can be noted that storage conditions strongly influence the stability of biodiesel [31,32]. According to Plessis et al. [42], biodiesel remains stable if stored at 20 °C in closed containers. The results showed that when the sample is stored at 5 °C, all these samples remain stable. It was also observed that when the concentration amount of FSCME decreased, the samples after 2 or 4 months of storage at RT were no longer above the minimum oxidative stability specification.
- The cold flow results showed that these samples have poor cold flow properties. It was found that CP and PP values were within the ranges of −0.2–8.80 °C and −7.68–3.94 °C.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Filho, S.C.; Miranda, A.C.; Silva, T.A.; Calarge, F.A.; Souza, R.R.; Santana, J.C.; Tambourgi, E.B. Environmental and techno-economic considerations on biodiesel production from waste frying oil in São Paulo city. J. Clean. Prod. 2018, 183, 1034–1042. [Google Scholar] [CrossRef]
- Diya’Uddeen, B.H.; Aziz, A.A.; Daud, W.; Chakrabarti, M. Performance evaluation of biodiesel from used domestic waste oils: A review. Process Saf. Environ. Prot. 2012, 90, 164–179. [Google Scholar] [CrossRef]
- Fawaz, E.G.; Salam, D.A. Preliminary economic assessment of the use of waste frying oils for biodiesel production in Beirut, Lebanon. Sci. Total Environ. 2018, 637–638, 1230–1240. [Google Scholar] [CrossRef]
- Freitas, O.N.; Rial, R.C.; Cavalheiro, L.F.; Barbosa, J.M.; Nazário, C.E.; Viana, L.H. Evaluation of the oxidative stability and cold filter plugging point of soybean methyl biodiesel/bovine tallow methyl biodiesel blends. Ind. Crops Prod. 2019, 140, 111667. [Google Scholar] [CrossRef]
- Rezania, S.; Oryani, B.; Park, J.; Hashemi, B.; Yadav, K.K.; Kwon, E.E.; Cho, J. Review on transesterification of non-edible sources for biodiesel production with a focus on economic aspects, fuel properties and by-product applications. Energy Convers. Manag. 2019, 201, 112155. [Google Scholar] [CrossRef]
- Fonseca, J.M.; Teleken, J.G.; Almeida, V.D.; Silva, C.D. Biodiesel from waste frying oils: Methods of production and purification. Energy Convers. Manag. 2019, 184, 205–218. [Google Scholar] [CrossRef]
- Sia, C.B.; Kansedo, J.; Tan, Y.H.; Lee, K.T. Evaluation on biodiesel cold flow properties, oxidative stability and enhancement strategies: A review. Biocatal. Agric. Biotechnol. 2020, 24, 101514. [Google Scholar] [CrossRef]
- Giakoumis, E.G.; Sarakatsanis, C.K. Estimation of biodiesel cetane number, density, kinematic viscosity and heating values from its fatty acid weight composition. Fuel 2018, 222, 574–585. [Google Scholar] [CrossRef]
- Kassem, Y.; Çamur, H. Prediction of biodiesel density for extended ranges of temperature and pressure using adaptive neuro-fuzzy inference system (ANFIS) and radial basis function (RBF). Procedia Comput. Sci. 2017, 120, 311–316. [Google Scholar] [CrossRef]
- Kassem, Y.; Çamur, H.; Esenel, E. Adaptive neuro-fuzzy inference system (ANFIS) and response surface methodology (RSM) prediction of biodiesel dynamic viscosity at 313 K. Procedia Comput. Sci. 2017, 120, 521–528. [Google Scholar] [CrossRef]
- Verma, P.; Sharma, M.; Dwivedi, G. Evaluation and enhancement of cold flow properties of palm oil and its biodiesel. Energy Rep. 2016, 2, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. Safflower Biodiesel: Improvement of its Oxidative Stability by Using BHA and TBHQ. Energies 2019, 12, 1940. [Google Scholar] [CrossRef] [Green Version]
- Varatharajan, K.; Pushparani, D. Screening of antioxidant additives for biodiesel fuels. Renew. Sustain. Energy Rev. 2018, 82, 2017–2028. [Google Scholar] [CrossRef]
- Magalhães, A.M.; Pereira, E.; Meirelles, A.J.; Sampaio, K.A.; Maximo, G.J. Proposing blends for improving the cold flow properties of ethylic biodiesel. Fuel 2019, 253, 50–59. [Google Scholar] [CrossRef]
- Lv, P.; Cheng, Y.; Yang, L.; Yuan, Z.; Li, H.; Luo, W. Improving the low temperature flow properties of palm oil biodiesel: Addition of cold flow improver. Fuel Proc. Technol. 2013, 110, 61–64. [Google Scholar] [CrossRef]
- Kumar, M.; Sharma, M. Investigating and Improving the Cold Flow Properties of Waste Cooking Biodiesel Using Winterization and Blending. Mater. Today Proc. 2018, 5, 23051–23056. [Google Scholar] [CrossRef]
- Dwivedi, G.; Sharma, M.P. Investigation of Cold Flow Properties of Waste Cooking Biodiesel. J. Clean Energy Technol. 2015, 4, 205–208. [Google Scholar] [CrossRef] [Green Version]
- Amaral, B.E.; Rezende, D.B.; Pasa, V.M. Aging and stability evaluation of diesel/ biodiesel blends stored in amber polyethylene bottles under different humidity conditions. Fuel 2020, 279, 118289. [Google Scholar] [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; Guiberteau, A.; Márquez, S. The Effect of Antioxidants on Corn and Sunflower Biodiesel Properties under Extreme Oxidation Conditions. J. Am. Oil Chem. Soc. 2020, 97, 201–212. [Google Scholar] [CrossRef]
- Moser, B.R. Influence of extended storage on fuel properties of methyl esters prepared from canola, palm, soybean and sunflower oils. Renew. Energy 2011, 36, 1221–1226. [Google Scholar] [CrossRef]
- Kanca, A.; Temur, H. The effects of long-term storage on the cold flow properties and viscosity of Canola-based biodiesel. Energy Sour. Part A Recovery Util. Environ. Eff. 2016, 38, 2205–2210. [Google Scholar] [CrossRef]
- Jayaraman, P.P.; Pugazhvadivu, M. Studies on long-term storage stability of biodiesel (B100) and its blend (B20) using box-behnken response surface method. Int. J. Ambient Energy 2017, 39, 270–277. [Google Scholar] [CrossRef]
- Kassem, Y.; Çamur, H. A Laboratory Study of the Effects of Wide Range Temperature on the Properties of Biodiesel Produced from Various Waste Vegetable Oils. Waste Biomass Valoriz. 2017, 8, 1995–2007. [Google Scholar] [CrossRef] [Green Version]
- Kassem, Y.; Çamur, H. Effects of storage under different conditions on the fuel properties of biodiesel admixtures derived from waste frying and canola oils. Biomass Convers. Biorefin. 2018, 8, 825–845. [Google Scholar] [CrossRef]
- Kassem, Y.; Aktuğ, B.; Özgenç, E.; Dib, M.S.; Ghisheer, M.M.; Cole, O.A.; Çamur, H. Effects of storage period on kinematic viscosity and density of biodiesel and its blends with ultra-low-sulfur diesel fuel at constant storage temperature. Int. J. Smart Grid Clean Energy 2018, 7, 130–144. [Google Scholar] [CrossRef]
- Saeed, R.H.S.; Kassem, Y.; Çamur, H. Effect of biodiesel mixture derived from waste frying-corn, Frying-Canola-Corn and Canola-corn cooking oils with various Ages on physicochemical properties. Energies 2019, 12, 3729. [Google Scholar] [CrossRef] [Green Version]
- Mejía, J.; Salgado, N.; Orrego, C. Effect of blends of Diesel and Palm-Castor biodiesels on viscosity, cloud point and flash point. Ind. Crops Prod. 2013, 43, 791–797. [Google Scholar] [CrossRef]
- Doğan, T.H. The testing of the effects of cooking conditions on the quality of biodiesel produced from waste cooking oils. Renew. Energy 2016, 94, 466–473. [Google Scholar] [CrossRef]
- Freire, L.M.; Filho, J.R.; Moura, C.V.; Soledade, L.E.; Stragevitch, L.; Cordeiro  MSouza, A.G. Evaluation of the oxidative stability and flow properties of quaternary mixtures of vegetable oils for biodiesel production. Fuel 2012, 95, 126–130. [Google Scholar] [CrossRef] [Green Version]
- Freire, L.M.; Santos, I.M.; Filho, J.R.; De Magalhães Cordeiro, A.M.T.; Soledade, L.E.B.; Fernandes, V.J.; Souza, A.G. Influence of the synthesis process on the properties of flow and oxidative stability of biodiesel from Jatropha curcas biodiesel. Fuel 2012, 94, 313–316. [Google Scholar] [CrossRef] [Green Version]
- Khalid, A.; Tamaldin, N.; Jaat, M.; Ali, M.; Manshoor, B.; Zaman, I. Impacts of Biodiesel Storage Duration on Fuel Properties and Emissions. Procedia Eng. 2013, 68, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Christensen, E.; Mccormick, R.L. Long-term storage stability of biodiesel and biodiesel blends. Fuel Proc. Technol. 2014, 128, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Meher, L.; Vidyasagar, D.; Naik, S. Technical aspects of biodiesel production by transesterification—A review. Renew. Sustain. Energy Rev. 2006, 10, 248–268. [Google Scholar] [CrossRef]
- Bouaid, A.; Martinez, M.; Aracil, J. Long storage stability of biodiesel from vegetable and used frying oils. Fuel 2007, 86, 2596–2602. [Google Scholar] [CrossRef]
- Cavalheiro, L.F.; Misutsu, M.Y.; Rial, R.C.; Viana, L.H.; Oliveira, L.C. Characterization of residues and evaluation of the physico chemical properties of soybean biodiesel and biodiesel: Diesel blends in different storage conditions. Renew. Energy 2020, 151, 454–462. [Google Scholar] [CrossRef]
- Zuleta, E.C.; Baena, L.; Rios, L.A.; Calderón, J.A. The oxidative stability of biodiesel and its impact on the deterioration of metallic and polymeric materials: A review. J. Brazil. Chem. Soc. 2012, 23, 2159–2175. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; He, Q.S.; Corscadden, K.; Caldwell, C. Improvement on oxidation and storage stability of biodiesel derived from an emerging feedstock camelina. Fuel Proc. Technol. 2017, 157, 90–98. [Google Scholar] [CrossRef]
- Fajar, B.; Sukarn. Experimental Study of Additives on Viscosity biodiesel at Low Temperature. IOP Conf. Ser. Mater. Sci. Eng. 2015, 88, 012070. [Google Scholar] [CrossRef]
- Jiaqiang, Z.; Zhang, J.; Chen, M.; Pham, X.; Zhao, Q.; Peng, B.; Yin, U. Performance and emission evaluation of a marine diesel engine fueled by water biodiesel-diesel emulsion blends with a fuel additive of a cerium oxide nanoparticle. Energy Convers. Manag. 2018, 169, 194–205. [Google Scholar] [CrossRef]
- Kumar, M.V.; Babu, A.V.; Kumar, P.R. The impacts on combustion, performance and emissions of biodiesel by using additives in direct injection diesel engine. Alex. Eng. J. 2018, 57, 509–516. [Google Scholar] [CrossRef]
- Gürü, M.; Karakaya, U.; Altıparmak, D.; Alıcılar, A. Improvement of Diesel fuel properties by using additives. Energy Convers. Manag. 2002, 43, 1021–1025. [Google Scholar] [CrossRef]
- Plessis, L.M.; Villiers, J.B.; Walt, W.H. Stability studies on methyl and ethyl fatty acid esters of sunflowerseed oil. J. Am. Oil Chem. Soc. 1985, 62, 748–752. [Google Scholar] [CrossRef]



| Composition (wt. %) | FCME | FSME | FSCME | FSRME | Literature Data [23] | |
|---|---|---|---|---|---|---|
| Min | Max | |||||
| C8:0 | 0.00 | 0.05 | 0.15 | 0.00 | - | - |
| C10:0 | 0.00 | 0.33 | 0.16 | 0.00 | - | - |
| C12:0 | 0.08 | 1.18 | 2.06 | 0.00 | 0.0 | 49.2 |
| C14:0 | 0.00 | 0.10 | 1.05 | 0.00 | 0.0 | 25.9 |
| C16:0 | 5.63 | 37.29 | 13.62 | 21.47 | 0.9 | 44.1 |
| C16:1 | 0.00 | 0.00 | 0.45 | 0.09 | - | - |
| C18:0 | 1.57 | 4.04 | 4.65 | 2.75 | 0.3 | 23.5 |
| C18:1 | 63.97 | 40.42 | 50.75 | 46.18 | 1.8 | 92.5 |
| C18:2 | 20.34 | 16.84 | 20.28 | 25.17 | 0.0 | 77.3 |
| C18:3 | 6.99 | 0.18 | 5.20 | 3.99 | 0.0 | 72.3 |
| C20:0 | 0.46 | 0.00 | 0.44 | 0.51 | 0.0 | 7.5 |
| C20:1 | 1.46 | 0.00 | 0.87 | 0.44 | 0.0 | 66.5 |
| C22:0 | 0.00 | 0.00 | 0.00 | 0.14 | - | - |
| C24:0 | 0.00 | 0.00 | 0.00 | 0.13 | - | - |
| MUFAMEs | 65.43 | 40.42 | 52.07 | 46.71 | - | - |
| PUFAMEs | 27.33 | 17.02 | 25.48 | 29.16 | - | - |
| SFAMEs | 7.74 | 42.61 | 21.82 | 24.87 | - | - |
| DU | 120.09 | 74.46 | 103.03 | 105.03 | - | - |
| LCSF | 1.81 | 5.75 | 4.13 | 4.50 | - | - |
| Property | Unit | Test Method | Limits | FCME | FSME | FSCME | FSRME |
|---|---|---|---|---|---|---|---|
| Kinematic viscosity at 40 °C | mm2/s | ASTM D445 | 1.9–6.0 | 4.55 | 4.62 | 4.33 | 4.59 |
| Density at 15 °C | kg/m3 | ASTM D854 | 867 min. | 895.44 | 875.19 | 910.42 | 881.63 |
| Cloud Point | °C | ASTM D2500 | Report | −2.0 | 10.0 | 7.0 | −1.2 |
| Cold Filter Plugging Point | °C | ASTM D6371 | Report | −6.0 | 7.5 | 4.5 | −3.0 |
| Pour Point | °C | ASTMD97 | Report | −10.0 | 5.5 | 1.6 | −6.0 |
| Acid value | mg KOH/g | ASTM D664 | 0.5 max. | 0.35 | 0.37 | 0.3 | 0.4 |
| Oxidation Stability (at 110 °C) | h | EN 14112:2003 | 3.0 min. | 7.5 | 7.85 | 14.0 | 8.27 |
| h | EN 14112:2014 | 8.0 min. |
| Property | Unit | Test Method | Limits | Results |
|---|---|---|---|---|
| Density at 15 °C | kg/m3 | ASTMD 4052 | 720–775 | 740.94 |
| Viscosity at 40 °C | mm2/s | EN ISO 3104 | - | 0.59 |
| Oxidation stability | minute | >360 | - | |
| Cloud point | °C | ISO 3015 | - | −57 |
| Pour point | °C | ASTM D6749 | - | −57 |
| Octane number, Mon | ASTM D2700 | 85.0–... | 85.1 | |
| Octane number, Ron | ASTM D 2699 | 95.0–... | 95 | |
| Evaporated at 70 °C | VOL PCT | ASTM D86 | 22.0–50.0 | 41.4 |
| Evaporated at 100 °C | VOL PCT | ASTM D86 | 46.0–71.0 | 58.6 |
| Evaporated at 150 °C | VOL PCT | ASTM D86 | 75.0–... | 81.9 |
| Distillation residue | VOL PCT | ASTM D86 | ...–2 | 1 |
| Blend | Designation | VF [%] | KV [mm2/s] | D [kg/m3] | Blend | Designation | VF [%] | KV [mm2/s] | D [kg/m3] |
|---|---|---|---|---|---|---|---|---|---|
| FCME–FSME | FCME | 100–0 | 4.55 | 895.44 | FSCME–FCME | FSCME | 100–0 | 4.33 | 910.42 |
| 20-FSME | 80–20 | 4.58 | 879.77 | 20-FCME | 80–20 | 4.37 | 907.42 | ||
| 40-FSME | 60–40 | 4.66 | 902.18 | 40-FCME | 60–40 | 4.42 | 904.43 | ||
| 60-FSME | 40–60 | 4.64 | 892.34 | 60-FCME | 40–60 | 4.59 | 912.93 | ||
| 80-FSME | 20–80 | 4.63 | 882.50 | 80-FCME | 20–80 | 4.57 | 903.44 | ||
| FSME | 0–100 | 4.62 | 875.19 | FCME | 0–100 | 4.55 | 895.44 | ||
| EFSCME–FSRME | FSCME | 100–0 | 4.33 | 910.42 | FSME–FSRME | FSME | 100–0 | 4.62 | 875.19 |
| 20-FSRME | 80–20 | 4.36 | 904.64 | 20-FSRME | 80–20 | 4.65 | 892.28 | ||
| 40-FSRME | 60–40 | 4.49 | 898.96 | 40-FSRME | 60–40 | 4.63 | 887.76 | ||
| 60-FSRME | 40–60 | 4.51 | 893.16 | 60-FSRME | 40–60 | 4.61 | 885.85 | ||
| 80-FSRME | 20–80 | 4.57 | 887.42 | 80-FSRME | 20–80 | 4.60 | 883.32 | ||
| FSRME | 0–100 | 4.59 | 881.63 | FSRME | 0–100 | 4.59 | 881.63 | ||
| FSRME–FCME | FSRME | 100–0 | 4.59 | 881.63 | FSCME–FSME | FSCME | 100–0 | 4.33 | 910.42 |
| 20-FCME | 80–20 | 4.63 | 900.95 | 20-FSME | 80–20 | 4.37 | 903.37 | ||
| 40-FCME | 60–40 | 4.60 | 892.15 | 40-FSME | 60–40 | 4.43 | 896.33 | ||
| 60-FCME | 40–60 | 4.58 | 889.94 | 60-FSME | 40–60 | 4.48 | 889.28 | ||
| 80-FCME | 20–80 | 4.56 | 886.10 | 80-FSME | 20–80 | 4.55 | 882.24 | ||
| FCME | 0–100 | 4.55 | 895.44 | FSME | 0–100 | 4.62 | 875.19 |
| Blend | VF [%] | IP [h] | Blend | VF [%] | IP [h] |
|---|---|---|---|---|---|
| FCME–FSME | 100–0 | 7.50 | FSCME–FCME | 100–0 | 14.00 |
| 80–20 | 7.95 | 80–20 | 12.70 | ||
| 60–40 | 8.19 | 60–40 | 11.40 | ||
| 40–60 | 7.90 | 40–60 | 13.33 | ||
| 20–80 | 7.87 | 20–80 | 10.30 | ||
| 0–100 | 7.85 | 0–100 | 7.50 | ||
| FSCME–FSRME | 100–0 | 14.00 | FSME–FSRME | 100–0 | 7.85 |
| 80–20 | 12.85 | 80–20 | 8.59 | ||
| 60–40 | 11.71 | 60–40 | 8.47 | ||
| 40–60 | 10.56 | 40–60 | 8.40 | ||
| 20–80 | 9.42 | 20–80 | 8.34 | ||
| 0–100 | 8.27 | 0–100 | 8.27 | ||
| FSRME–FCME | 100–0 | 8.27 | FSCME–FSME | 100–0 | 14.00 |
| 80–20 | 8.62 | 80–20 | 12.77 | ||
| 60–40 | 7.96 | 60–40 | 11.54 | ||
| 40–60 | 7.81 | 40–60 | 10.31 | ||
| 20–80 | 7.65 | 20–80 | 9.08 | ||
| 0–100 | 7.50 | 0–100 | 7.85 |
| Blend | VF [%] | CP [°C] | PP [°C] | Blend | VF [%] | CP [°C] | PP [°C] |
|---|---|---|---|---|---|---|---|
| FCME–FSME | 100–0 | −2.00 | −10.00 | FSCME–FCME | 100–0 | 7.00 | 1.60 |
| 80–20 | 0.40 | −6.90 | 80–20 | 5.20 | −0.72 | ||
| 60–40 | 2.80 | −3.80 | 60–40 | 3.40 | −3.04 | ||
| 40–60 | 5.20 | −0.70 | 40–60 | 1.60 | −5.36 | ||
| 20–80 | 7.60 | 2.40 | 20–80 | −0.20 | −7.68 | ||
| 0–100 | 10.00 | 5.50 | 0–100 | −2.00 | −10.00 | ||
| FSCME–FSRME | 100–0 | 7.00 | 1.60 | FSME–FSRME | 100–0 | 10.00 | 5.50 |
| 80–20 | 5.36 | 0.08 | 80–20 | 7.76 | 3.20 | ||
| 60–40 | 3.72 | −1.44 | 60–40 | 5.52 | 0.90 | ||
| 40–60 | 2.08 | −2.96 | 40–60 | 3.28 | −1.40 | ||
| 20–80 | 0.44 | −4.48 | 20–80 | 1.04 | −3.70 | ||
| 0–100 | −1.20 | −6.00 | 0–100 | −1.20 | −6.00 | ||
| FSRME–FCME | 100–0 | −1.20 | −6.00 | FSCME–FSME | 100–0 | 7.00 | 1.60 |
| 80–20 | −1.36 | −6.80 | 80–20 | 7.60 | 2.38 | ||
| 60–40 | −1.52 | −7.60 | 60–40 | 8.20 | 3.16 | ||
| 40–60 | −1.68 | −8.40 | 40–60 | 8.80 | 3.94 | ||
| 20–80 | −1.84 | −9.20 | 20–80 | 9.40 | 4.72 | ||
| 0–100 | −2.00 | −10.00 | 0–100 | 10.00 | 5.50 |
| Volume Fraction [%] | Kinematic Viscosity [mm2/s] | Density [kg/m3] | ||||||
|---|---|---|---|---|---|---|---|---|
| FCME | FSME | FSCME | FSRME | FCME | FSME | FSCME | FSRME | |
| 100 (Pure biodiesel) | 4.55 | 4.62 | 4.33 | 4.59 | 895.44 | 875.19 | 910.42 | 881.63 |
| 95 | 4.35 | 4.42 | 4.14 | 4.39 | 888.28 | 867.75 | 900.86 | 874.13 |
| 90 | 4.00 | 4.07 | 3.81 | 4.04 | 879.84 | 860.38 | 891.40 | 866.70 |
| 85 | 3.67 | 3.73 | 3.49 | 3.70 | 871.48 | 853.06 | 882.04 | 859.34 |
| 80 | 3.30 | 3.36 | 3.14 | 3.33 | 863.20 | 845.81 | 872.78 | 852.03 |
| 75 | 2.87 | 2.92 | 2.73 | 2.90 | 855.00 | 838.62 | 863.62 | 844.79 |
| 70 | 2.59 | 2.63 | 2.47 | 2.62 | 846.88 | 831.49 | 854.55 | 837.61 |
| 65 | 2.33 | 2.37 | 2.22 | 2.35 | 838.83 | 824.43 | 845.58 | 830.49 |
| 60 | 2.10 | 2.13 | 2.00 | 2.12 | 830.87 | 817.42 | 836.70 | 823.43 |
| 55 | 1.89 | 1.92 | 1.80 | 1.91 | 822.97 | 810.47 | 827.91 | 816.43 |
| 50 | 1.70 | 1.73 | 1.62 | 1.72 | 815.15 | 803.58 | 819.22 | 809.49 |
| 45 | 1.53 | 1.55 | 1.46 | 1.54 | 807.41 | 796.75 | 810.62 | 802.61 |
| 40 | 1.38 | 1.40 | 1.31 | 1.39 | 799.74 | 789.98 | 802.11 | 795.79 |
| 35 | 1.24 | 1.26 | 1.18 | 1.25 | 792.14 | 783.26 | 793.68 | 789.02 |
| 30 | 1.11 | 1.13 | 1.06 | 1.13 | 784.62 | 776.61 | 785.35 | 782.32 |
| 25 | 1.00 | 1.02 | 0.96 | 1.01 | 777.16 | 770.00 | 777.10 | 775.67 |
| 20 | 0.90 | 0.92 | 0.86 | 0.91 | 769.78 | 763.46 | 768.94 | 769.07 |
| 15 | 0.81 | 0.83 | 0.77 | 0.82 | 762.47 | 756.97 | 760.87 | 762.54 |
| 10 | 0.73 | 0.74 | 0.70 | 0.74 | 755.22 | 750.54 | 752.88 | 756.06 |
| 5 | 0.66 | 0.67 | 0.63 | 0.66 | 748.05 | 744.16 | 744.98 | 749.63 |
| 0 (Pure fuel additive) | 0.59 | 0.59 | 0.59 | 0.59 | 740.94 | 740.94 | 740.94 | 740.94 |
| Blend | VF [%] | Storage Period [month] | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | ||
| EFSCME–FSRME (80–20)–Gasoline | 100–0 | 4.36 | 4.37 | 4.40 | 4.42 | 4.45 | 4.46 | 4.49 |
| 85–15 | 3.52 | 3.59 | 3.66 | 3.73 | 3.81 | 3.88 | 3.96 | |
| 80–20 | 2.55 | 2.60 | 2.66 | 2.71 | 2.76 | 2.82 | 2.87 | |
| EFSCME–FSRME (60–40)–Gasoline | 100–0 | 4.49 | 4.50 | 4.54 | 4.55 | 4.58 | 4.60 | 4.63 |
| 85–15 | 3.62 | 3.70 | 3.79 | 3.88 | 3.97 | 4.06 | 4.15 | |
| 80–20 | 2.63 | 2.68 | 2.73 | 2.79 | 2.85 | 2.90 | 2.96 | |
| EFSCME–FSRME (40–60)–Gasoline | 100–0 | 4.51 | 4.52 | 4.56 | 4.57 | 4.61 | 4.62 | 4.66 |
| 85–15 | 3.63 | 3.73 | 3.82 | 3.91 | 4.01 | 4.11 | 4.22 | |
| 80–20 | 2.64 | 2.70 | 2.76 | 2.83 | 2.89 | 2.96 | 3.02 | |
| FSCME-FCME (80–20)–Gasoline | 100–0 | 4.37 | 4.41 | 4.44 | 4.47 | 4.51 | 4.54 | 4.58 |
| 85–15 | 3.53 | 3.58 | 3.63 | 3.69 | 3.74 | 3.80 | 3.86 | |
| 80–20 | 2.56 | 2.61 | 2.66 | 2.71 | 2.77 | 2.82 | 2.88 | |
| FSCME-FCME (60–40)–Gasoline | 100–0 | 4.42 | 4.46 | 4.51 | 4.56 | 4.61 | 4.66 | 4.70 |
| 85–15 | 3.56 | 3.62 | 3.67 | 3.72 | 3.78 | 3.84 | 3.89 | |
| 80–20 | 2.59 | 2.64 | 2.69 | 2.74 | 2.79 | 2.85 | 2.90 | |
| FSCME-FCME (40–60)–Gasoline | 100–0 | 4.59 | 4.52 | 4.58 | 4.64 | 4.71 | 4.77 | 4.83 |
| 85–15 | 3.70 | 3.79 | 3.89 | 3.99 | 4.09 | 4.19 | 4.29 | |
| 80–20 | 2.69 | 2.75 | 2.81 | 2.88 | 2.94 | 3.01 | 3.08 | |
| FSCME-FCME (20–80)–Gasoline | 100–0 | 4.57 | 4.65 | 4.73 | 4.80 | 4.88 | 4.95 | 5.03 |
| 85–15 | 3.69 | 3.77 | 3.86 | 3.95 | 4.04 | 4.13 | 4.23 | |
| 80–20 | 2.68 | 2.73 | 2.79 | 2.84 | 2.90 | 2.96 | 3.02 | |
| FSCME–FSME (80–20)–Gasoline | 100–0 | 4.37 | 4.39 | 4.41 | 4.43 | 4.45 | 4.47 | 4.49 |
| 85–15 | 3.52 | 3.61 | 3.70 | 3.79 | 3.89 | 3.99 | 4.09 | |
| 80–20 | 2.56 | 2.62 | 2.68 | 2.74 | 2.80 | 2.87 | 2.93 | |
| FSCME–FSME (60–40)–Gasoline | 100–0 | 4.43 | 4.45 | 4.47 | 4.49 | 4.51 | 4.53 | 4.55 |
| 85–15 | 3.57 | 3.64 | 3.71 | 3.79 | 3.86 | 3.94 | 4.02 | |
| 80–20 | 2.59 | 2.64 | 2.69 | 2.75 | 2.80 | 2.85 | 2.91 | |
| FSCME–FSME (40–60)–Gasoline | 100–0 | 4.48 | 4.50 | 4.52 | 4.54 | 4.56 | 4.58 | 4.60 |
| 85–15 | 3.61 | 3.70 | 3.78 | 3.87 | 3.96 | 4.05 | 4.14 | |
| 80–20 | 2.62 | 2.68 | 2.73 | 2.78 | 2.84 | 2.90 | 2.95 | |
| Blend | VF [%] | Storage Period [Month] | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | ||
| EFSCME–FSRME (80–20)–Gasoline | 100–0 | 904.64 | 910.07 | 915.53 | 921.02 | 926.55 | 932.11 | 937.70 |
| 85–15 | 881.77 | 889.70 | 898.60 | 907.58 | 916.66 | 921.24 | 930.46 | |
| 80–20 | 852.17 | 859.84 | 868.43 | 877.12 | 885.89 | 890.32 | 899.22 | |
| EFSCME–FSRME (60–40)–Gasoline | 100–0 | 898.96 | 904.35 | 909.78 | 915.24 | 920.73 | 926.25 | 931.81 |
| 85–15 | 876.23 | 884.11 | 892.96 | 901.89 | 910.90 | 915.46 | 924.61 | |
| 80–20 | 846.81 | 854.44 | 862.98 | 871.61 | 880.33 | 884.73 | 893.58 | |
| EFSCME–FSRME (40–60)–Gasoline | 100–0 | 893.16 | 898.52 | 903.91 | 909.34 | 914.79 | 920.28 | 925.80 |
| 85–15 | 870.58 | 878.42 | 887.20 | 896.07 | 905.03 | 909.56 | 918.65 | |
| 80–20 | 841.36 | 848.93 | 857.42 | 865.99 | 874.65 | 879.03 | 887.82 | |
| FSCME-FCME (80–20)–Gasoline | 100–0 | 907.42 | 912.87 | 918.35 | 923.86 | 929.40 | 934.98 | 940.59 |
| 85–15 | 884.48 | 892.44 | 901.37 | 910.38 | 919.48 | 924.08 | 933.32 | |
| 80–20 | 854.79 | 862.48 | 871.11 | 879.82 | 888.62 | 893.06 | 901.99 | |
| FSCME-FCME (60–40)–Gasoline | 100–0 | 904.43 | 909.86 | 915.31 | 920.81 | 926.33 | 931.89 | 937.48 |
| 85–15 | 881.56 | 889.50 | 898.39 | 907.37 | 916.45 | 921.03 | 930.24 | |
| 80–20 | 851.97 | 859.64 | 868.23 | 876.91 | 885.68 | 890.11 | 899.01 | |
| FSCME-FCME (40–60)–Gasoline | 100–0 | 912.93 | 918.34 | 923.78 | 929.26 | 934.76 | 940.30 | 945.88 |
| 85–15 | 889.85 | 897.86 | 906.84 | 915.91 | 925.07 | 929.69 | 938.99 | |
| 80–20 | 859.98 | 867.72 | 876.40 | 885.16 | 894.01 | 898.48 | 907.47 | |
| FSCME-FCME (20–80)–Gasoline | 100–0 | 903.44 | 908.83 | 914.25 | 919.71 | 925.20 | 930.72 | 936.27 |
| 85–15 | 880.60 | 888.52 | 897.41 | 906.38 | 915.44 | 920.02 | 929.22 | |
| 80–20 | 851.03 | 858.69 | 867.28 | 875.95 | 884.71 | 889.14 | 898.03 | |
| FSCME–FSME (80–20)–Gasoline | 100–0 | 903.37 | 908.79 | 914.25 | 919.73 | 925.25 | 930.80 | 936.39 |
| 85–15 | 880.53 | 888.46 | 897.34 | 906.32 | 915.38 | 919.96 | 929.16 | |
| 80–20 | 850.97 | 858.63 | 867.22 | 875.89 | 884.65 | 889.07 | 897.97 | |
| FSCME–FSME (60–40)–Gasoline | 100–0 | 896.33 | 901.71 | 907.12 | 912.56 | 918.03 | 923.54 | 929.08 |
| 85–15 | 873.67 | 881.53 | 890.34 | 899.25 | 908.24 | 912.78 | 921.91 | |
| 80–20 | 844.34 | 851.94 | 860.46 | 869.06 | 877.75 | 882.14 | 890.96 | |
| FSCME–FSME (40–60)–Gasoline | 100–0 | 889.28 | 894.62 | 899.99 | 905.39 | 910.82 | 916.28 | 921.78 |
| 85–15 | 866.80 | 874.60 | 883.34 | 892.18 | 901.10 | 905.61 | 914.66 | |
| 80–20 | 837.70 | 845.24 | 853.69 | 862.23 | 870.85 | 875.21 | 883.96 | |
| Blend | VF [%] | Storage Period [Month] | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | ||
| EFSCME–FSRME (80–20)–Gasoline | 100–0 | 5.20 | 5.41 | 5.62 | 5.85 | 6.08 | 6.33 | 6.58 |
| 85–15 | −1.04 | −1.0 | −1.0 | −0.9 | −0.9 | −0.8 | −0.8 | |
| 80–20 | −3.12 | −3.0 | −2.9 | −2.8 | −2.6 | −2.5 | −2.4 | |
| EFSCME–FSRME (60–40)–Gasoline | 100–0 | 3.40 | 3.54 | 3.68 | 3.82 | 3.98 | 4.14 | 4.30 |
| 85–15 | −0.68 | −0.7 | −0.6 | −0.6 | −0.6 | −0.6 | −0.5 | |
| 80–20 | −2.04 | −2.0 | −1.9 | −1.8 | −1.7 | −1.7 | −1.6 | |
| EFSCME–FSRME (40–60)–Gasoline | 100–0 | 1.60 | 1.66 | 1.73 | 1.80 | 1.87 | 1.95 | 2.02 |
| 85–15 | −0.32 | −0.3 | −0.3 | −0.3 | −0.3 | −0.3 | −0.2 | |
| 80–20 | −0.96 | −0.9 | −0.9 | −0.8 | −0.8 | −0.8 | −0.8 | |
| FSCME–FCME (80–20)–Gasoline | 100–0 | 5.20 | 5.41 | 5.62 | 5.85 | 6.08 | 6.33 | 6.58 |
| 85–15 | −1.04 | −1.0 | −1.0 | −0.9 | −0.9 | −0.8 | −0.8 | |
| 80–20 | −3.12 | −3.0 | −2.9 | −2.8 | −2.6 | −2.5 | −2.4 | |
| FSCME–FCME (60–40)–Gasoline | 100–0 | 3.40 | 3.54 | 3.68 | 3.82 | 3.98 | 4.14 | 4.30 |
| 85–15 | −0.68 | −0.7 | −0.6 | −0.6 | −0.6 | −0.6 | −0.5 | |
| 80–20 | −2.04 | −2.0 | −1.9 | −1.8 | −1.7 | −1.7 | −1.6 | |
| FSCME–FCME (40–60)–Gasoline | 100–0 | 1.60 | 1.66 | 1.73 | 1.80 | 1.87 | 1.95 | 2.02 |
| 85–15 | −0.32 | −0.3 | −0.3 | −0.3 | −0.3 | −0.3 | −0.2 | |
| 80–20 | −0.96 | −0.9 | −0.9 | −0.8 | −0.8 | −0.8 | −0.8 | |
| FSCME–FCME (20–80)–Gasoline | 100–0 | −0.20 | −0.21 | −0.22 | −0.22 | −0.23 | −0.24 | −0.25 |
| 85–15 | −0.44 | −0.4 | −0.4 | −0.4 | −0.4 | −0.4 | −0.3 | |
| 80–20 | −0.52 | −0.5 | −0.5 | −0.5 | −0.4 | −0.4 | −0.4 | |
| FSCME–FSME (80–20)–Gasoline | 100–0 | 7.60 | 7.90 | 8.22 | 8.55 | 8.89 | 9.25 | 9.62 |
| 85–15 | −1.52 | −1.5 | −1.4 | −1.3 | −1.3 | −1.2 | −1.2 | |
| 80–20 | −4.56 | −4.4 | −4.2 | −4.0 | −3.9 | −3.7 | −3.6 | |
| FSCME–FSME (60–40)–Gasoline | 100–0 | 8.20 | 8.53 | 8.87 | 9.22 | 9.59 | 9.98 | 10.38 |
| 85–15 | −1.64 | −1.6 | −1.5 | −1.5 | −1.4 | −1.3 | −1.3 | |
| 80–20 | −4.92 | −4.7 | −4.5 | −4.4 | −4.2 | −4.0 | −3.9 | |
| FSCME–FSME (40–60)–Gasoline | 100–0 | 8.80 | 9.15 | 9.52 | 9.90 | 10.29 | 10.71 | 11.13 |
| 85–15 | −1.76 | −1.7 | −1.6 | −1.6 | −1.5 | −1.4 | −1.3 | |
| 80–20 | −5.28 | −5.1 | −4.9 | −4.7 | −4.5 | −4.3 | −4.1 | |
| Blend | VF [%] | Storage Period [month] | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 2 | 4 | 6 | 8 | 10 | 12 | ||
| EFSCME–FSRME (80–20)–Gasoline | 100–0 | 0.08 | 0.08 | 0.09 | 0.09 | 0.09 | 0.10 | 0.10 |
| 85–15 | −1.07 | −1.0 | −0.9 | −0.9 | −0.8 | −0.8 | −0.7 | |
| 80–20 | −1.36 | −1.3 | −1.2 | −1.1 | −1.0 | −1.0 | −0.9 | |
| EFSCME–FSRME (60–40)–Gasoline | 100–0 | −1.44 | −1.38 | −1.33 | −1.27 | −1.22 | −1.17 | −1.13 |
| 85–15 | −3.74 | −3.6 | −3.5 | −3.3 | −3.2 | −3.1 | −2.9 | |
| 80–20 | −4.32 | −4.1 | −4.0 | −3.8 | −3.7 | −3.5 | −3.4 | |
| EFSCME–FSRME (40–60)–Gasoline | 100–0 | −2.96 | −2.84 | −2.73 | −2.62 | −2.51 | −2.41 | −2.32 |
| 85–15 | −7.70 | −7.4 | −7.1 | −6.8 | −6.5 | −6.3 | −5.9 | |
| 80–20 | −8.88 | −8.5 | −8.2 | −7.9 | −7.5 | −7.2 | −7.0 | |
| FSCME–FCME (80–20)–Gasoline | 100–0 | −0.72 | −0.69 | −0.66 | −0.64 | −0.61 | −0.59 | −0.56 |
| 85–15 | −1.87 | −1.8 | −1.7 | −1.7 | −1.6 | −1.5 | −1.4 | |
| 80–20 | −2.16 | −2.1 | −2.0 | −1.9 | −1.8 | −1.8 | −1.7 | |
| FSCME–FCME (60–40)–Gasoline | 100–0 | −3.04 | −2.92 | −2.80 | −2.69 | −2.58 | −2.48 | −2.38 |
| 85–15 | −7.90 | −7.6 | −7.3 | −7.0 | −6.7 | −6.4 | −6.1 | |
| 80–20 | −9.12 | −8.8 | −8.4 | −8.1 | −7.7 | −7.4 | −7.1 | |
| FSCME–FCME (40–60)–Gasoline | 100–0 | −5.36 | −5.15 | −4.94 | −4.74 | −4.55 | −4.37 | −4.20 |
| 85–15 | −13.94 | −13.4 | −12.8 | −12.3 | −11.8 | −11.4 | −10.7 | |
| 80–20 | −16.08 | −15.4 | −14.8 | −14.2 | −13.7 | −13.1 | −12.6 | |
| FSCME–FCME (20–80)–Gasoline | 100–0 | −7.68 | −7.37 | −7.08 | −6.79 | −6.52 | −6.26 | −6.01 |
| 85–15 | −19.97 | −19.2 | −18.4 | −17.7 | −17.0 | −16.3 | −15.3 | |
| 80–20 | −23.04 | −22.1 | −21.2 | −20.4 | −19.6 | −18.8 | −18.0 | |
| FSCME–FSME (80–20)–Gasoline | 100–0 | 2.38 | 2.48 | 2.57 | 2.68 | 2.78 | 2.90 | 3.01 |
| 85–15 | −1.43 | −1.4 | −1.3 | −1.3 | −1.2 | −1.2 | −1.1 | |
| 80–20 | −2.38 | −2.3 | −2.2 | −2.1 | −2.0 | −1.9 | −1.9 | |
| FSCME–FSME (60–40)–Gasoline | 100–0 | 3.16 | 3.29 | 3.42 | 3.55 | 3.70 | 3.84 | 4.00 |
| 85–15 | −1.90 | −1.8 | −1.7 | −1.7 | −1.6 | −1.5 | −1.5 | |
| 80–20 | −3.16 | −3.0 | −2.9 | −2.8 | −2.7 | −2.6 | −2.5 | |
| FSCME–FSME (40–60)–Gasoline | 100–0 | 3.94 | 4.10 | 4.26 | 4.43 | 4.61 | 4.79 | 4.99 |
| 85–15 | −2.36 | −2.3 | −2.2 | −2.1 | −2.0 | −1.9 | −1.8 | |
| 80–20 | −3.94 | −3.8 | −3.6 | −3.5 | −3.3 | −3.2 | −3.1 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kassem, Y.; Çamur, H.; Alassi, E. Biodiesel Production from Four Residential Waste Frying Oils: Proposing Blends for Improving the Physicochemical Properties of Methyl Biodiesel. Energies 2020, 13, 4111. https://doi.org/10.3390/en13164111
Kassem Y, Çamur H, Alassi E. Biodiesel Production from Four Residential Waste Frying Oils: Proposing Blends for Improving the Physicochemical Properties of Methyl Biodiesel. Energies. 2020; 13(16):4111. https://doi.org/10.3390/en13164111
Chicago/Turabian StyleKassem, Youssef, Hüseyin Çamur, and Ebaa Alassi. 2020. "Biodiesel Production from Four Residential Waste Frying Oils: Proposing Blends for Improving the Physicochemical Properties of Methyl Biodiesel" Energies 13, no. 16: 4111. https://doi.org/10.3390/en13164111




