Improving the Energy Balance of Hydrocarbon Production Using an Inclined Solid–Liquid Separator with a Wedge-Wire Screen and Easy Hydrocarbon Recovery from Botryococcus braunii
Abstract
1. Introduction
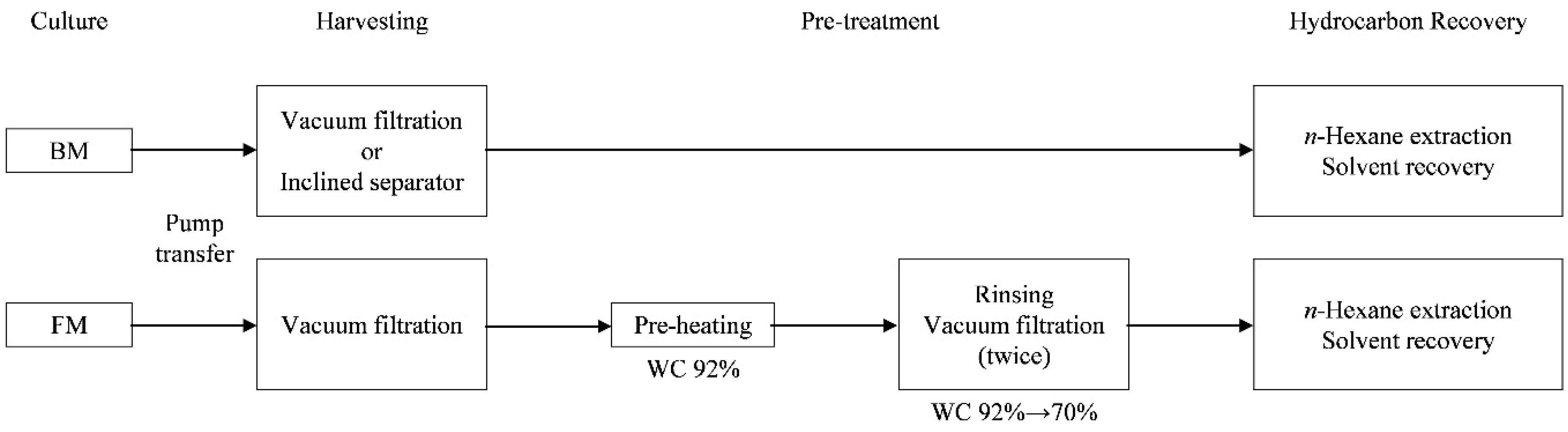
2. Materials and Methods
2.1. Microalgae Cultivation
2.2. Filtration Using an Inclined Solid–Liquid Separator with a Wedge-Wire Screen
2.3. Hydrocarbon Recovery from Concentrated Algal Slurries
2.4. Energy Profit Ratio Calculation
3. Results and Discussions
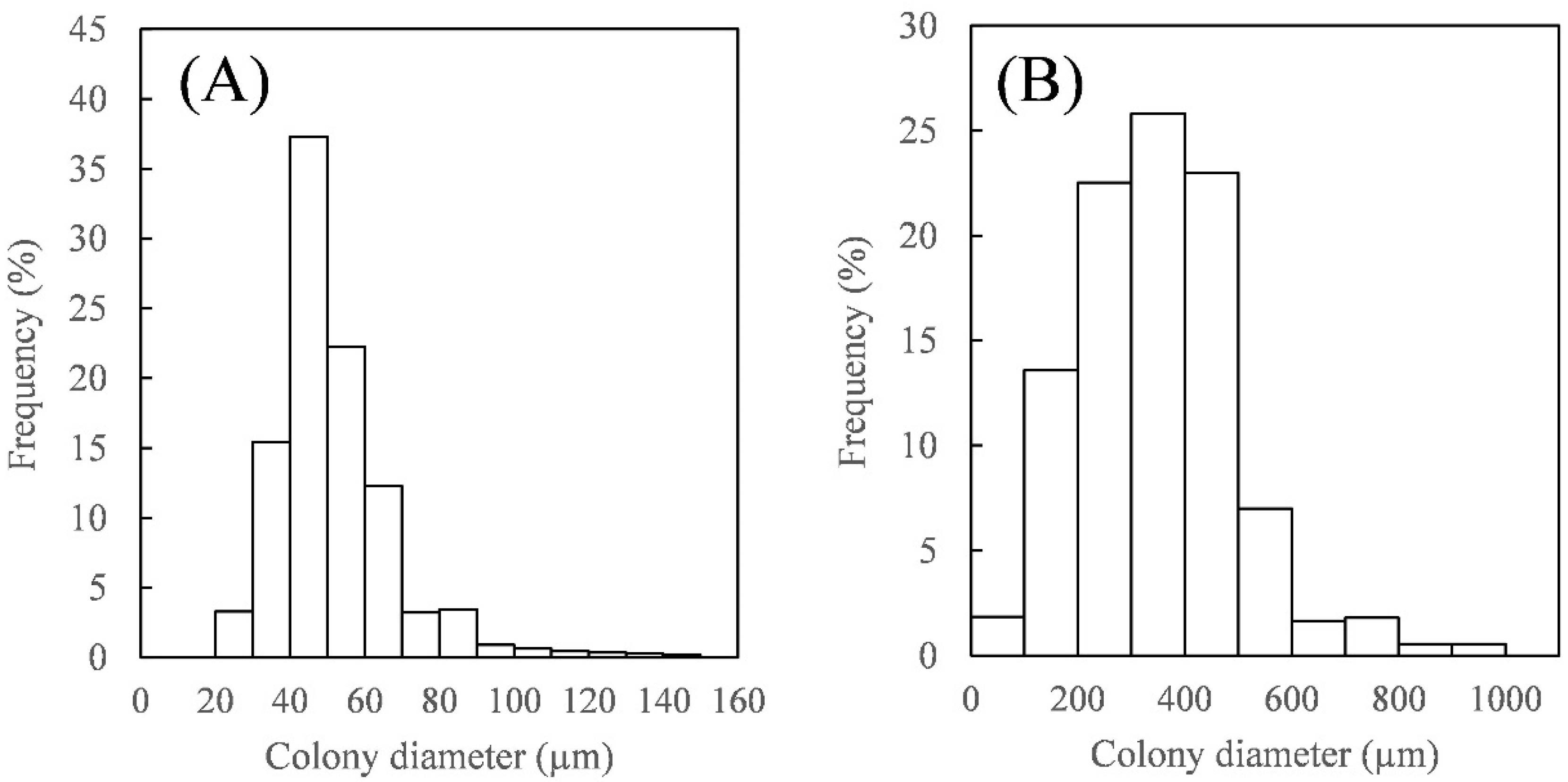
3.1. Harvesting Using the Inclined Solid–Liquid Separator with Wedge-Wire Screens
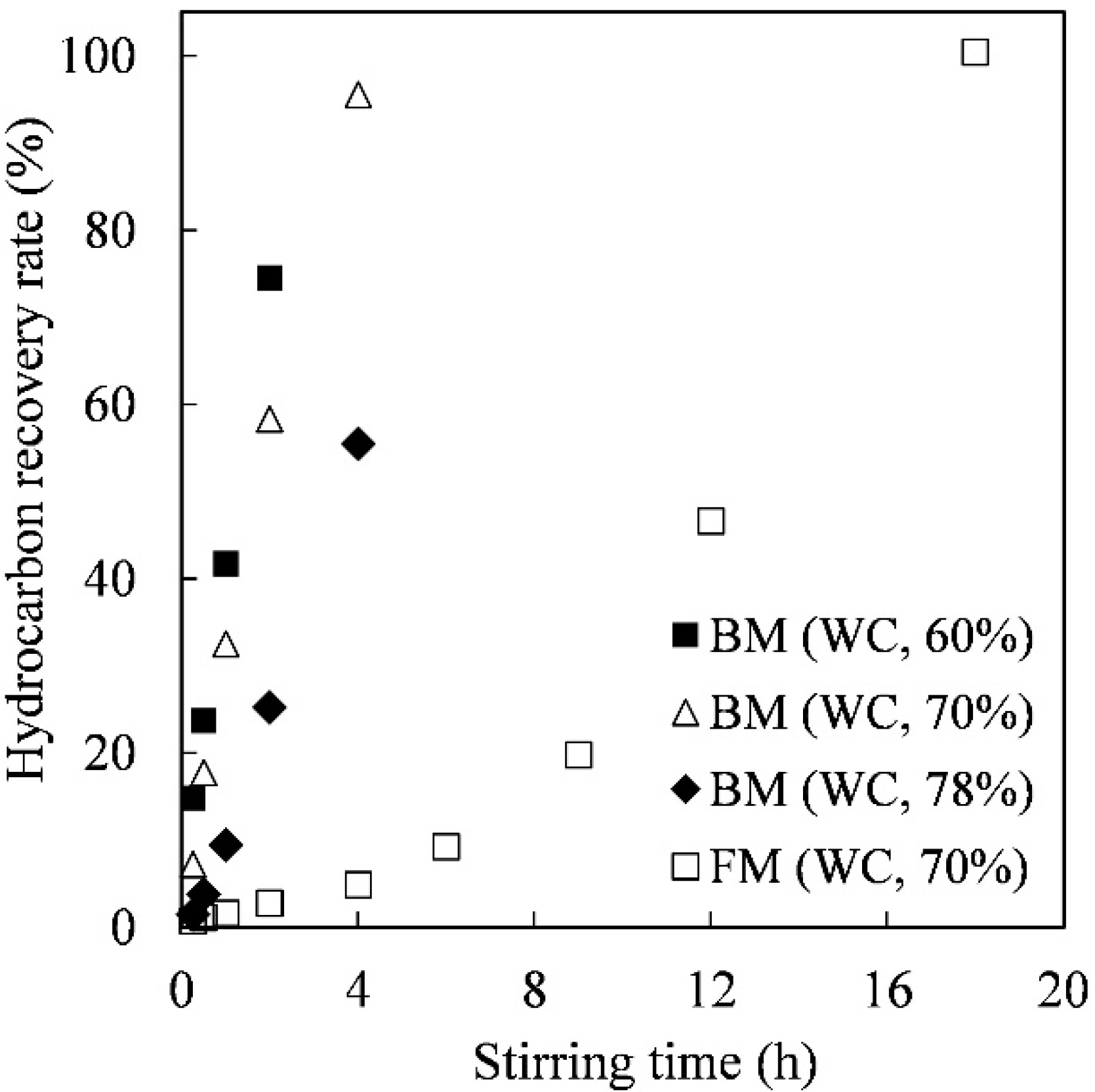
3.2. Hydrocarbon Recovery Rate from the Concentrated Algal Slurry
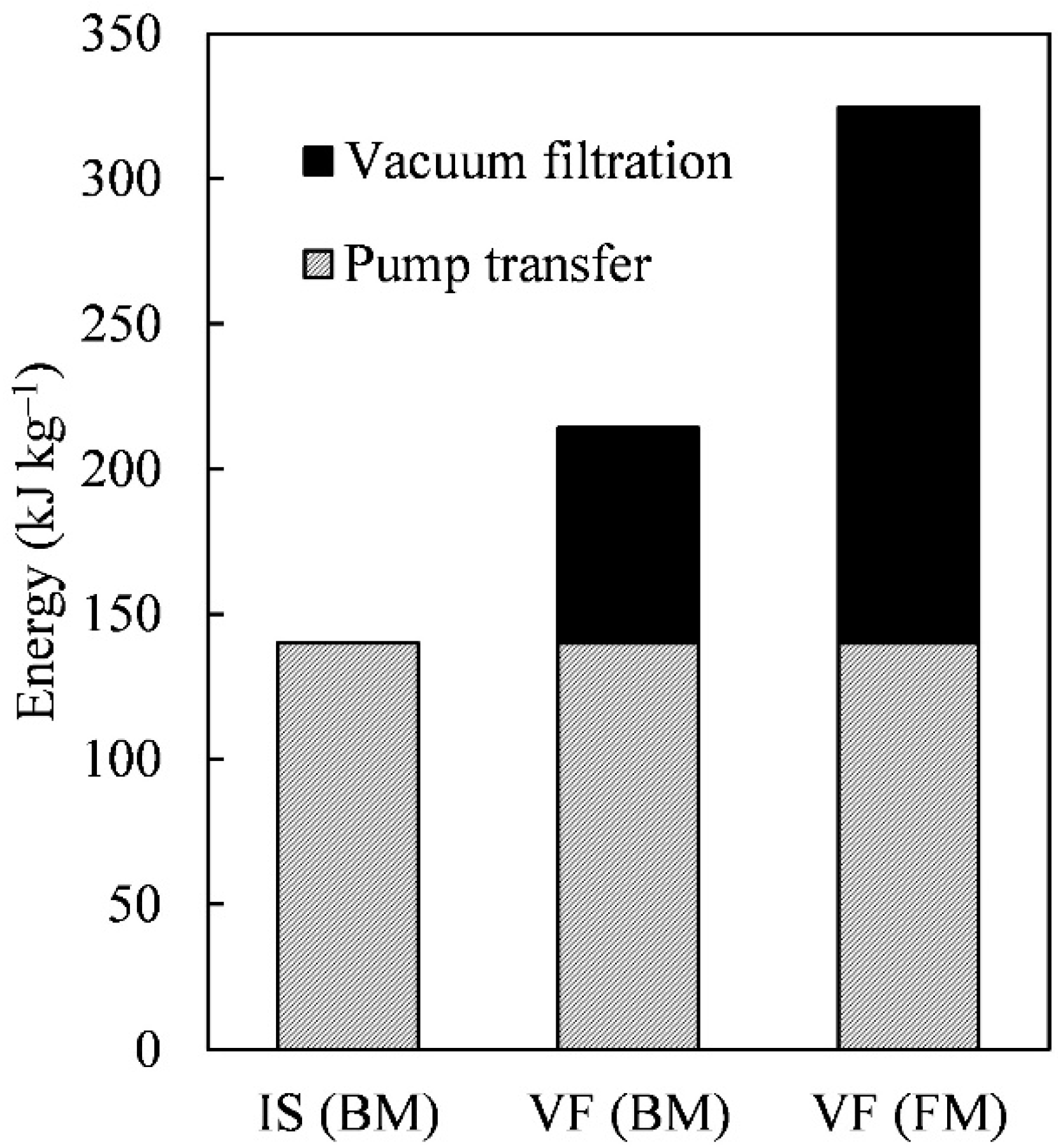
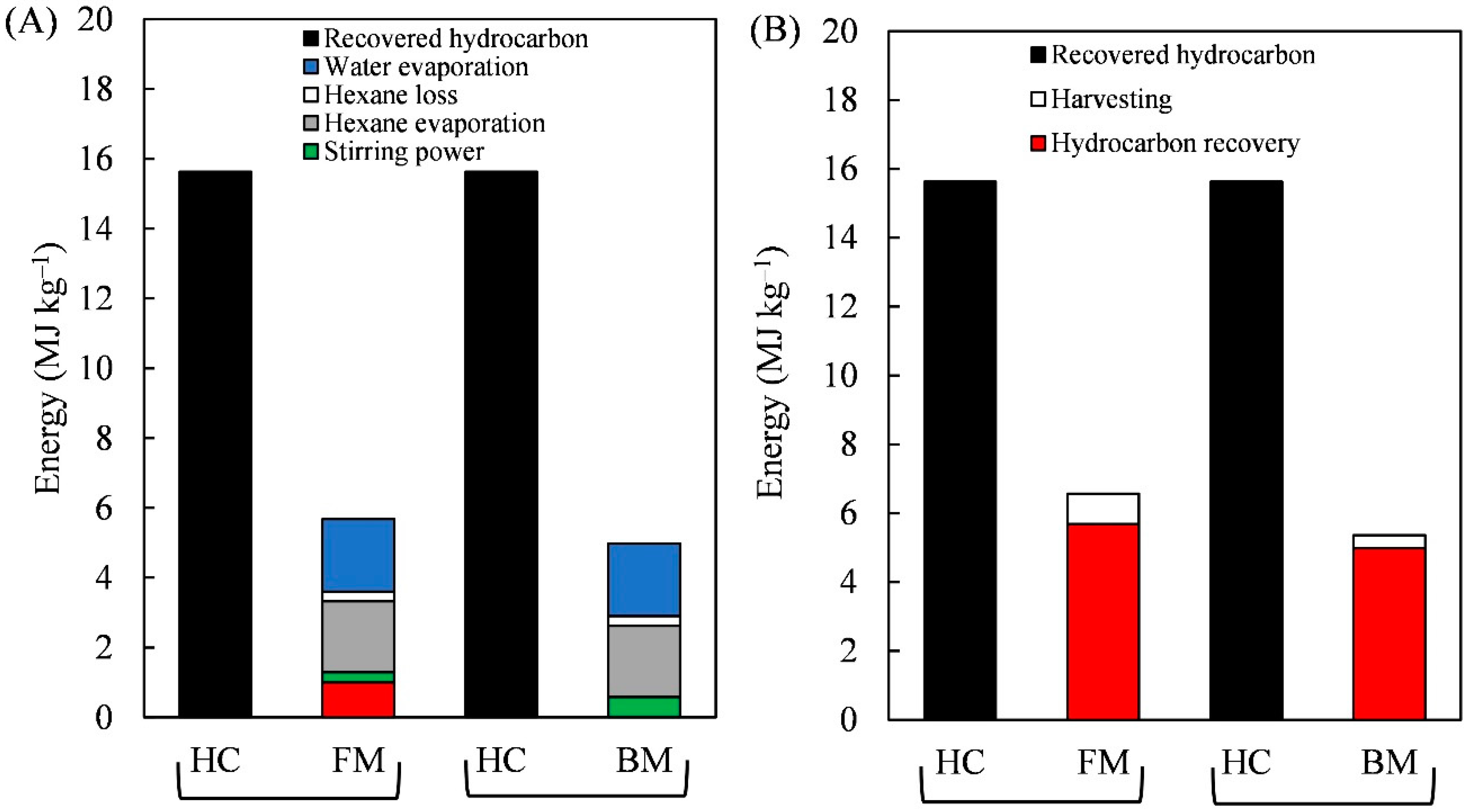
3.3. The Energy Profit Ratio from Harvesting to Hydrocarbon Recovery
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brown, A.C.; Knights, B.A.; Conway, E. Hydrocarbon content and its relationship to physiological state in the green alga Botryococcus braunii. Phytochemistry 1969, 8, 543–547. [Google Scholar] [CrossRef]
- Qin, J.G. Hydrocarbons from algae. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Heidelberg/Berlin, Germany, 2010; pp. 2817–2826. [Google Scholar]
- Sharma, K.K.; Schuhmann, H.; Schenk, P.M. High Lipid Induction in Microalgae for Biodiesel Production. Energies 2012, 5, 1532–1553. [Google Scholar] [CrossRef]
- Metzger, P.; Metzger, P.; Largeau, C.; Largeau, C. Botryococcus braunii: A rich source for hydrocarbons and related ether lipids. Appl. Microbiol. Biotechnol. 2005, 66, 486–496. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Bartlett, J.R.; Kannangara, G.S.K.; Milev, A.S.; Volk, H.; Wilson, M.A. Catalytic upgrading of biorefinery oil from micro-algae. Fuel 2010, 89, 265–274. [Google Scholar] [CrossRef]
- Okada, S.; Murakami, M.; Yamaguchi, K. Hydrocarbon production by the yayoi, a new strain of the green microalga Botryococcus braunii. Appl. Biochem. Biotechnol. 1997, 67, 79–86. [Google Scholar] [CrossRef]
- Largeau, C.; Casadevall, E.; Berkaloff, C.; Dhamelincourt, P. Sites of accumulation and composition of hydrocarbons in Botryococcus braunii. Phytochemistry 1980, 19, 1043–1051. [Google Scholar] [CrossRef]
- Suzuki, R.; Ito, N.; Uno, Y.; Nishii, I.; Kagiwada, S.; Okada, S.; Noguchi, T. Transformation of Lipid Bodies Related to Hydrocarbon Accumulation in a Green Alga, Botryococcus braunii (Race B). PLoS ONE 2013, 8, e81626. [Google Scholar] [CrossRef]
- Lee, A.K.; Lewis, D.M.; Ashman, P.J. Disruption of microalgal cells for the extraction of lipids for biofuels: Processes and specific energy requirements. Biomass Bioenrgy 2012, 46, 89–101. [Google Scholar] [CrossRef]
- Zhang, S.; Hou, Y.; Liu, Z.; Ji, X.; Wu, D.; Wang, W.; Zhang, D.; Wang, W.; Chen, S.; Chen, F. Electro-fenton based technique to enhance cell harvest and lipid extraction from microalgae. Energies 2020, 13, 3813. [Google Scholar] [CrossRef]
- Kanda, H.; Li, P.; Yoshimura, T.; Okada, S. Wet extraction of hydrocarbons from Botryococcus braunii by dimethyl ether as compared with dry extraction by hexane. Fuel 2013, 105, 535–539. [Google Scholar] [CrossRef]
- Hidalgo, P.; Ciudad, G.; Navia, R. Evaluation of different solvent mixtures in esterifiable lipids extraction from microalgae Botryococcus braunii for biodiesel production. Bioresour. Technol. 2016, 201, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.L.; Nobre, B.P.; Cardoso, M.T.; Pereira, A.P.; Palavra, A.F. Supercritical carbon dioxide extraction of compounds with pharmaceutical importance from microalgae. Inorg. Chim. Acta 2003, 356, 328–334. [Google Scholar] [CrossRef]
- Dote, Y.; Sawayama, S.; Inoue, S.; Minowa, T.; Yokoyama, S. Recovery of liquid fuel from hydrocarbon-rich microalgae by thermochemical liquefaction. Fuel 1994, 73, 1855–1857. [Google Scholar] [CrossRef]
- Frenz, J.; Largeau, C.; Casadevall, E.; Kollerup, F.; Daugulis, A.J. Hydrocarbon recovery and biocompatibility of solvents for extraction from cultures of Botryococcus braunii. Biotechnol. Bioeng. 1989, 34, 755–762. [Google Scholar] [CrossRef]
- Weiss, T.L.; Roth, R.; Goodson, C.; Vitha, S.; Black, I.; Azadi, P.; Rusch, J.; Holzenburg, A.; Devarenne, T.P.; Goodenough, U. Colony organization in the green alga Botryococcus braunii (Race B) is specified by a complex extracellular matrix. Eukaryot. Cell 2012, 11, 1424–1440. [Google Scholar]
- Uno, Y.; Nishii, I.; Kagiwada, S.; Noguchi, T. Colony sheath formation is accompanied by shell formation and release in the green alga Botryococcus braunii (race B). Algal Res. 2015, 8, 214–223. [Google Scholar] [CrossRef]
- Furuhashi, K.; Noguchi, T.; Okada, S.; Hasegawa, F.; Kaizu, Y.; Imou, K. The surface structure of Botryococcus braunii colony prevents the entry of extraction solvents into the colony interior. Algal Res. 2016, 16, 160–166. [Google Scholar] [CrossRef]
- Saga, K.; Hasegawa, F.; Miyagi, S.; Atobe, S.; Okada, S.; Imou, K.; Osaka, N.; Yamagishi, T. Comparative evaluation of wet and dry processes for recovering hydrocarbon from Botryococcus Braunii. Appl. Energy 2015, 141, 90–95. [Google Scholar] [CrossRef]
- Lee, J.; Yoo, C.; Jun, S.; Ahn, C.; Oh, H. Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef]
- Kita, K.; Okada, S.; Sekino, H.; Imou, K.; Yokoyama, S.; Amano, T. Thermal pre-treatment of wet microalgae harvest for efficient hydrocarbon recovery. Appl. Energy 2010, 87, 2420–2423. [Google Scholar] [CrossRef]
- Furuhashi, K.; Hasegawa, F.; Saga, K.; Kudou, S.; Okada, S.; Kaizu, Y.; Imou, K. Effects of culture medium salinity on the hydrocarbon extractability, growth and morphology of Botryococcus braunii. Biomass Bioenergy 2016, 91, 83–90. [Google Scholar] [CrossRef]
- Yoo, G.; Park, W.; Kim, C.W.; Choi, Y.; Yang, J. Direct lipid extraction from wet Chlamydomonas reinhardtii biomass using osmotic shock. Bioresour. Technol. 2012, 123, 717–722. [Google Scholar] [CrossRef] [PubMed]
- Saga, K.; Magota, A.; Atobe, S.; Okada, S.; Imou, K.; Osaka, N.; Matsui, T. Hydrocarbon Recovery from Concentrated Algae Slurry via Thermal Pretreatment. J. Jpn. Inst. Energy 2013, 92, 1212–1217. [Google Scholar] [CrossRef][Green Version]
- Zhang, K.; Kojima, E. Effect of light intensity on colony size of microalga Botryococcus braunii in bubble column photobioreactors. J. Ferment. Bioeng. 1998, 86, 573–576. [Google Scholar] [CrossRef]
- Tanoi, T.; Kawachi, M.; Watanabe, M.M. Iron and glucose effects on the morphology of Botryococcus braunii with assumption on the colony formation variability. J. Appl. Phycol. 2014, 26, 1–8. [Google Scholar] [CrossRef]
- Ishizaki, R.; Noguchi, R.; Putra, A.S.; Ichikawa, S.; Ahamed, T.; Watanabe, M.M. Reduction in energy requirement and CO2 emission for microalgae oil production using wastewater. Energies 2020, 13, 1641. [Google Scholar] [CrossRef]
- Atobe, S.; Saga, K.; Hasegawa, F.; Magota, A.; Furuhashi, K.; Okada, S.; Suzuki, T.; Imou, K. The effect of the water-soluble polymer released from Botryococcus braunii Showa strain on solvent extraction of hydrocarbon. J. Appl. Phycol. 2015, 27, 755–761. [Google Scholar] [CrossRef]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]
- Moheimani, N.R.; Matsuura, H.; Watanabe, M.M.; Borowitzka, M.A. Non-destructive hydrocarbon extraction from Botryococcus braunii BOT-22 (race B). J. Appl. Phycol. 2014, 26, 1453–1463. [Google Scholar] [CrossRef]
- Inoue, S.; Dote, Y.; Sawayama, S.; Minowa, T.; Ogi, T.; Yokoyama, S. Analysis of oil derived from liquefaction of Botryococcus Braunii. Biomass Bioenergy 1994, 6, 269–274. [Google Scholar] [CrossRef]
- Aramaki, T.; Watanabe, M.M.; Nakajima, M.; Ichikawa, S. Bench-scale dehydration of a native microalgae culture by centrifugation, flocculation and filtration in Minamisoma city, Fukushima, Japan. Bioresour. Technol. Rep. 2020, 10, 100414. [Google Scholar] [CrossRef]
- Wiedemann, T.; Stahl, W. Experimental investigation of the shrinkage and cracking behaviour of fine participate filter cakes. Chem. Eng. Process. 1996, 35, 35–42. [Google Scholar] [CrossRef]
- Slade, R.; Bauen, A. Micro-algae cultivation for biofuels: Cost, energy balance, environmental impacts and future prospects. Biomass Bioenergy 2013, 53, 29–38. [Google Scholar] [CrossRef]
- Das, S.; Das, S.; Das, I.; Ghangrekar, M.M. Application of bioelectrochemical systems for carbon dioxide sequestration and concomitant valuable recovery: A review. Mater. Sci. Energy Technol. 2019, 2, 687–696. [Google Scholar] [CrossRef]
- Das, S.; Das, S.; Ghangrekar, M.M. Quorum-sensing mediated signals: A promising multi-functional modulators for separately enhancing algal yield and power generation in microbial fuel cell. Bioresour. Technol. 2019, 294, 122138. [Google Scholar] [CrossRef]


| Item | Units | Value | Ref. | |
|---|---|---|---|---|
| Rotary vacuum belt filter | Drum diameter | m | 4.2 | TSK |
| Drum length | m | 7.6 | ||
| Rotational speed | rpm | 1.0 | ||
| Dipping rate | - | 0.33 | ||
| Motor output | kW | 10 | ||
| Gauge pressure | kPa | 40 | ||
| Pump head | m | 10 | Set in this study | |
| Algal throughput by one belt filter unit | FM | kg s−1 | 0.23 | Leaf test |
| BM | kg s−1 | 0.58 | Leaf test | |
| Elemental composition of B. braunii | C | % | 71.4 | Elemental analyzer |
| H | % | 10.3 | ||
| N | % | 1.21 | ||
| O | % | 13.6 | ||
| Elemental composition of the hydrocarbons | C | % | 87.4 | Elemental analyzer |
| H | % | 12.1 | ||
| Higher heating value | B. braunii | MJ kg−1 | 36.6 | Dulong’s formula |
| Hydrocarbons | MJ kg−1 | 47.0 | ||
| n-hexane | MJ kg−1 | 46.2 | ||
| Hydrocarbon content | FM | kg kg−1 | 0.35 | [22] |
| BM | kg kg−1 | 0.35 | [22] | |
| Hydrocarbon recovery rate | Pre-heating | % | 95.0 | [24] |
| BM | 95.0 | From this study | ||
| Pinch temperature for pre-heating | °C | 20 | [19] | |
| Specific heat | B. braunii | kJ kg−1 K−1 | 1.9 | [19] |
| Water | kJ kg−1 K−1 | 4.18 | ||
| Latent heat | n-hexane | MJ kg−1 | 0.34 | [19] |
| Water | MJ kg−1 | 2.25 | ||
| n-hexane recovery rate | 99.9 | [19] | ||
| Evaporated water loss/residual hexane | kg kg−1 | 154 | [19] | |
| Stirring power on extraction from slurry | W kg−1 | 15 | Torque meter | |
| Receiving and efficiency | Japan | % | 36.9 |
| Medium | Slit Size (µm) | Aperture Ratio (%) | Separation Rate (%) | Water Content (%) |
|---|---|---|---|---|
| FM | 10 | 2.0 | 96.3 | 99.9 |
| 20 | 3.8 | 93.0 | 99.7 | |
| 30 | 5.7 | 91.3 | 99.3 | |
| 50 | 4.8 | 51.3 | 99.1 | |
| 75 | 7.0 | ND | ND | |
| BM | 50 | 4.8 | 96.0 | 96.6 |
| 100 | 9.1 | 92.6 | 83.0 | |
| 150 | 13.0 | 86.7 | 78.9 | |
| 200 | 16.7 | 77.8 | 77.4 | |
| 300 | 16.7 | 66.2 | 78.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuhashi, K.; Hasegawa, F.; Yamauchi, M.; Kaizu, Y.; Imou, K. Improving the Energy Balance of Hydrocarbon Production Using an Inclined Solid–Liquid Separator with a Wedge-Wire Screen and Easy Hydrocarbon Recovery from Botryococcus braunii. Energies 2020, 13, 4139. https://doi.org/10.3390/en13164139
Furuhashi K, Hasegawa F, Yamauchi M, Kaizu Y, Imou K. Improving the Energy Balance of Hydrocarbon Production Using an Inclined Solid–Liquid Separator with a Wedge-Wire Screen and Easy Hydrocarbon Recovery from Botryococcus braunii. Energies. 2020; 13(16):4139. https://doi.org/10.3390/en13164139
Chicago/Turabian StyleFuruhashi, Kenichi, Fumio Hasegawa, Manabu Yamauchi, Yutaka Kaizu, and Kenji Imou. 2020. "Improving the Energy Balance of Hydrocarbon Production Using an Inclined Solid–Liquid Separator with a Wedge-Wire Screen and Easy Hydrocarbon Recovery from Botryococcus braunii" Energies 13, no. 16: 4139. https://doi.org/10.3390/en13164139
APA StyleFuruhashi, K., Hasegawa, F., Yamauchi, M., Kaizu, Y., & Imou, K. (2020). Improving the Energy Balance of Hydrocarbon Production Using an Inclined Solid–Liquid Separator with a Wedge-Wire Screen and Easy Hydrocarbon Recovery from Botryococcus braunii. Energies, 13(16), 4139. https://doi.org/10.3390/en13164139




