Carbon Dioxide Conversion with High-Performance Photocatalysis into Methanol on NiSe2/WSe2
Abstract
1. Introduction
2. Materials and Methods
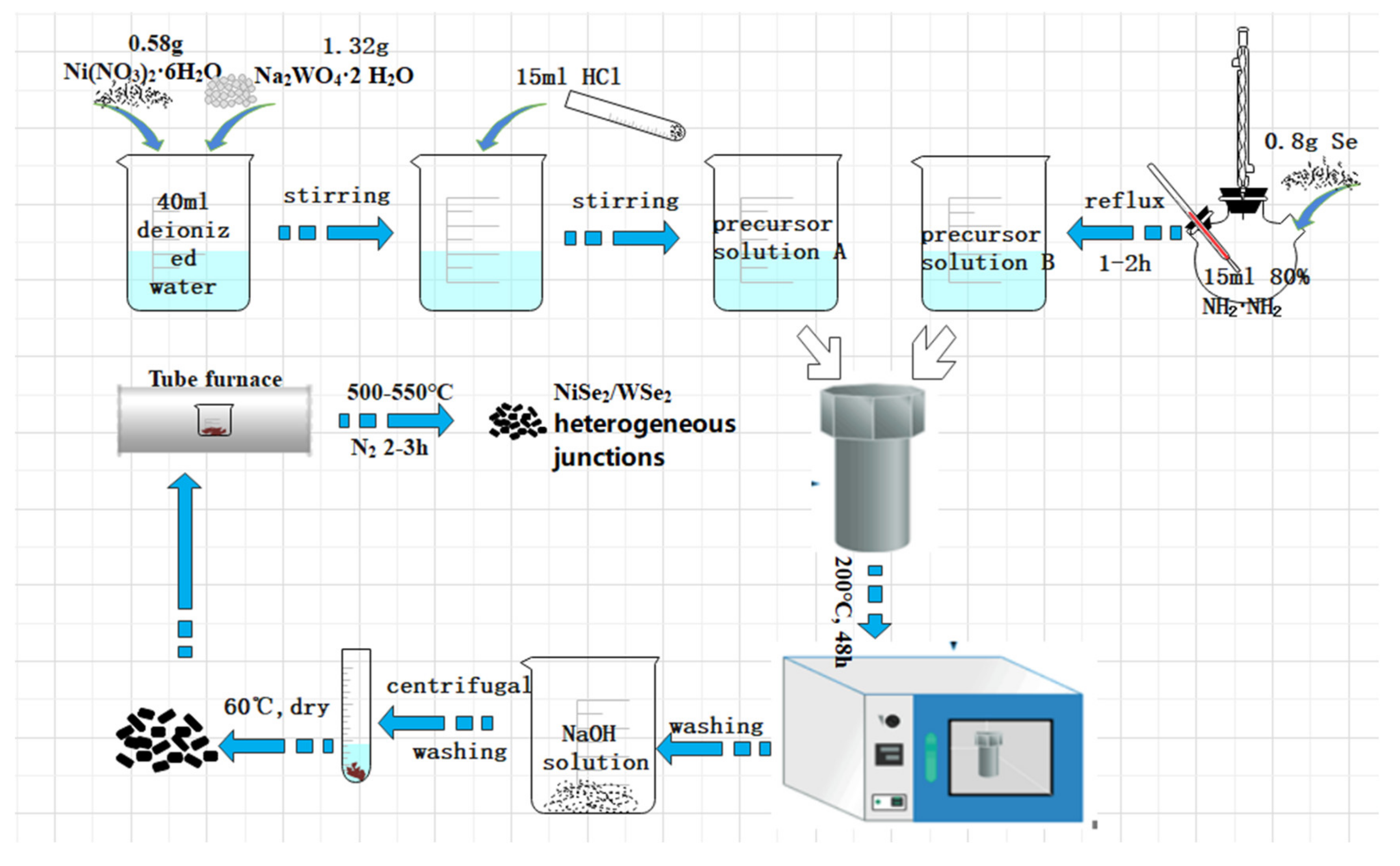
2.1. Preparation of NiSe2/WSe2
2.2. Characterization
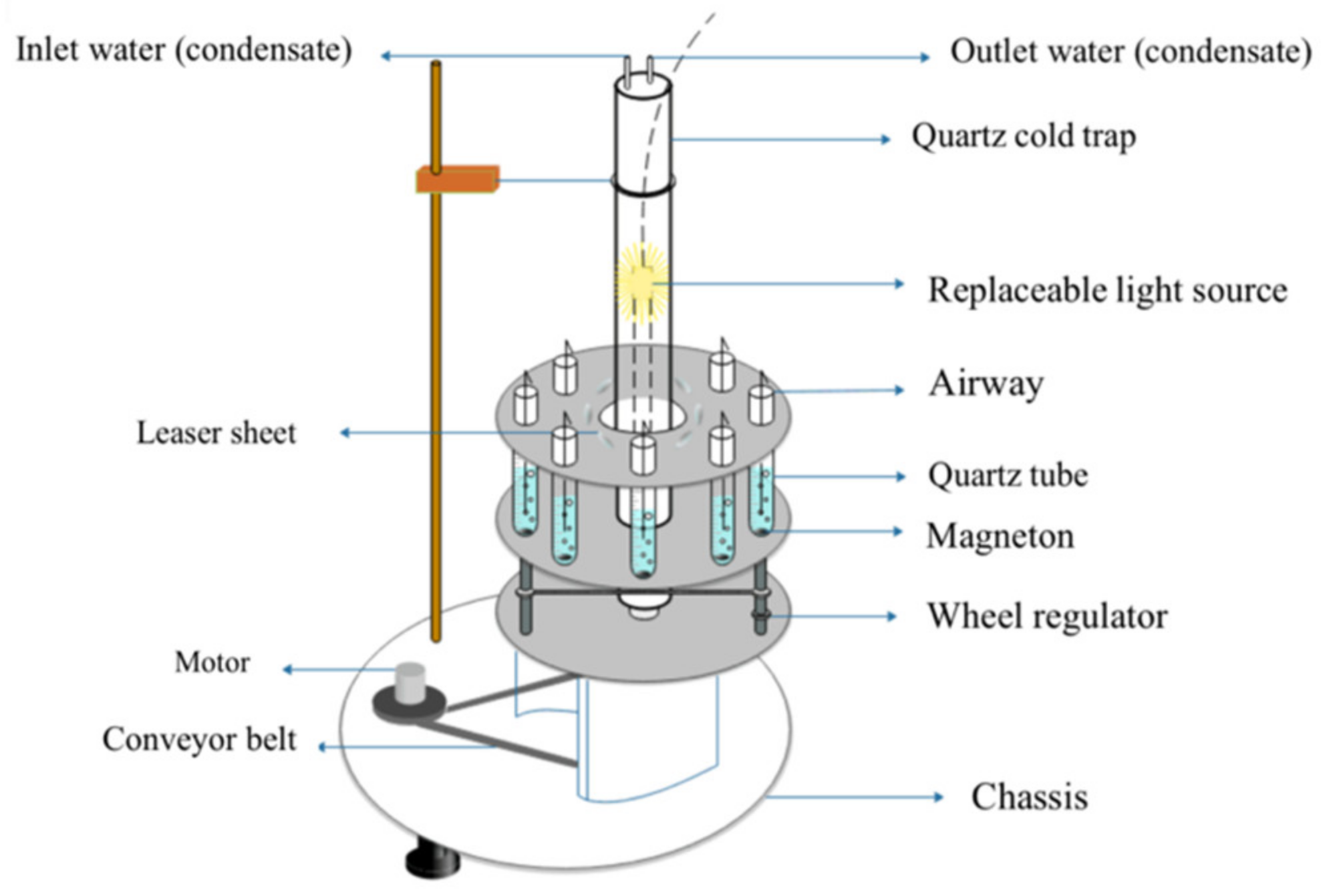
2.3. Photocatalytic Reduction Experiment of CO2
3. Results and Discussion
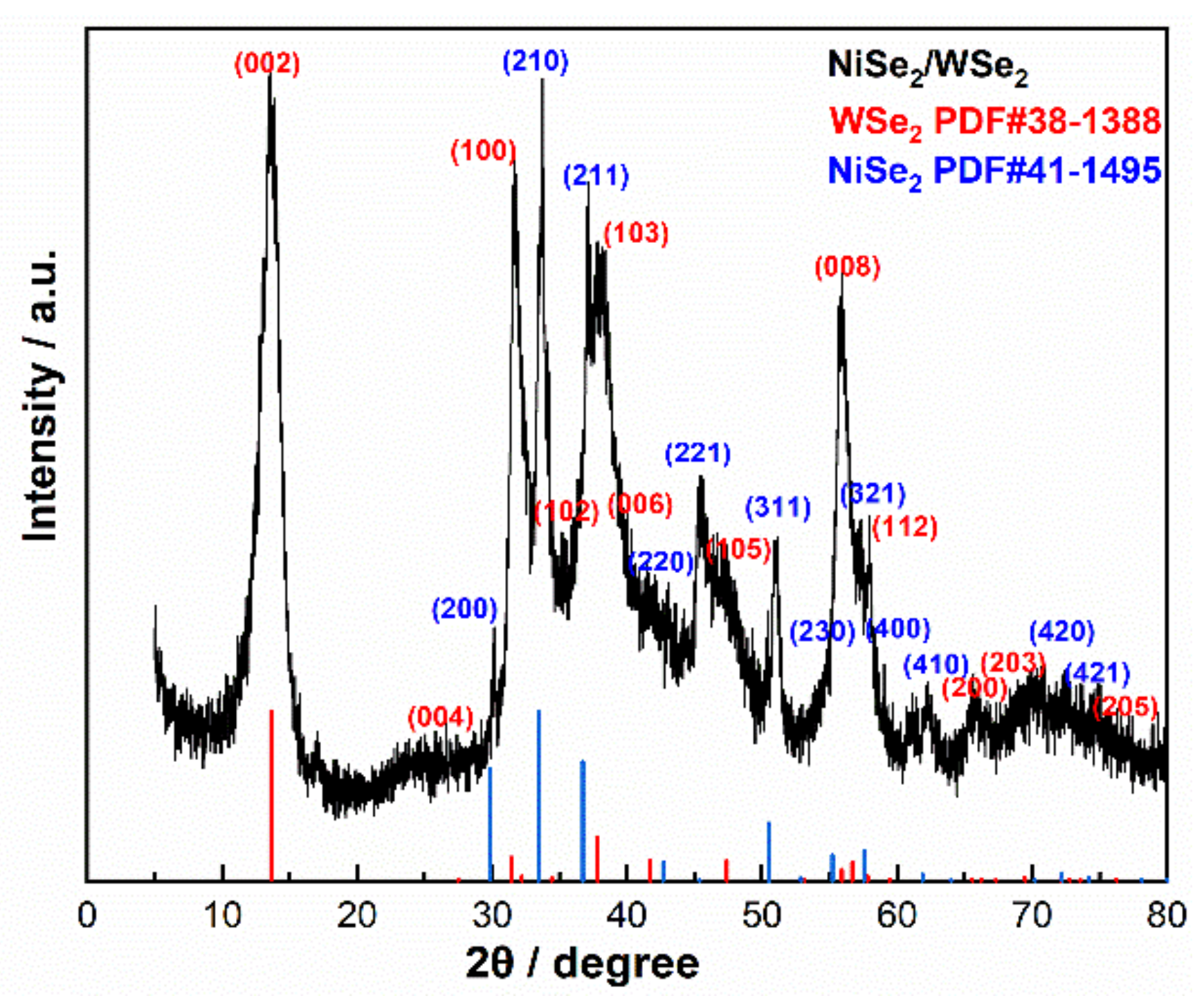
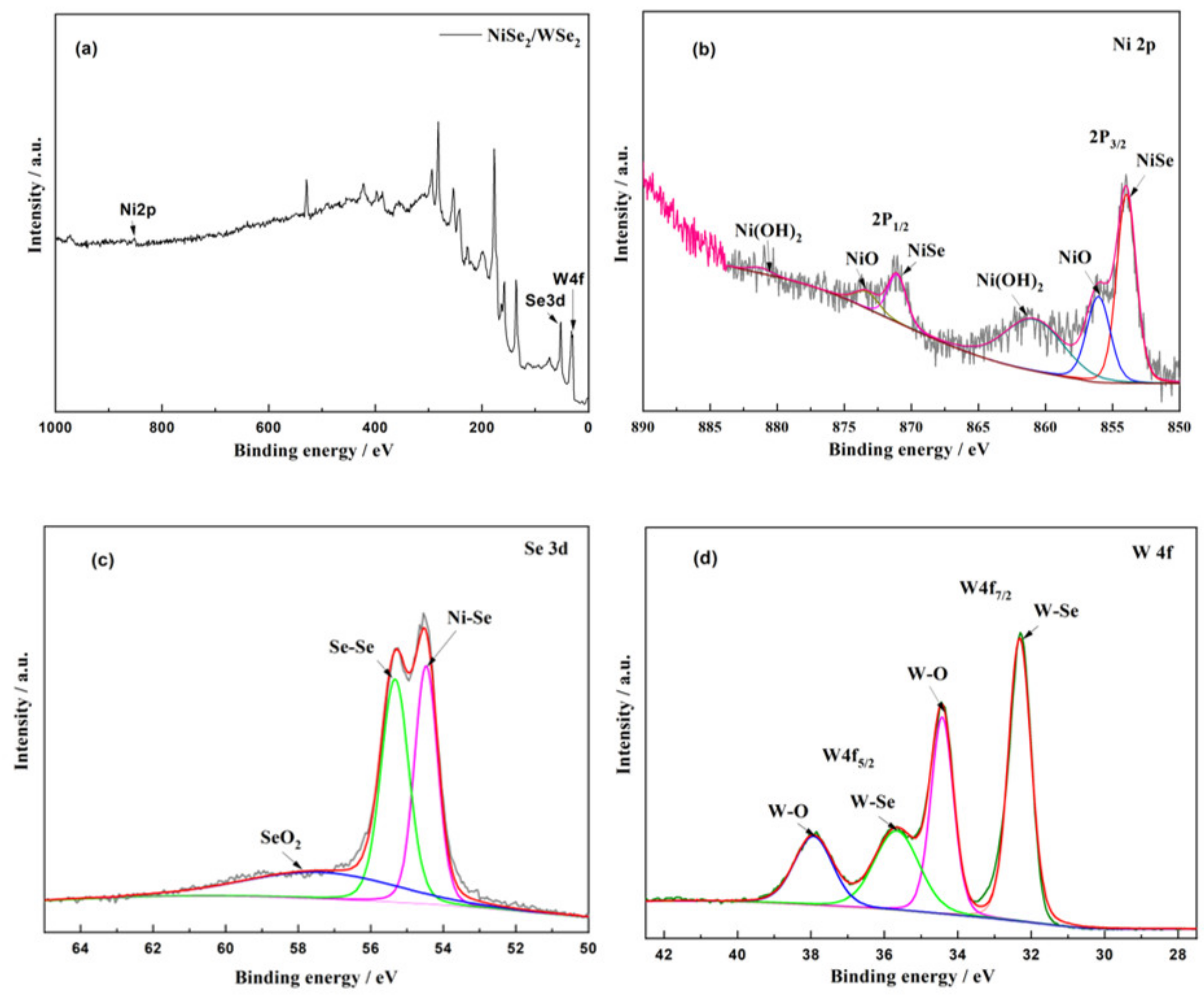
3.1. XRD and XPS Analysis
3.2. SEM Analysis
3.3. BET Analysis
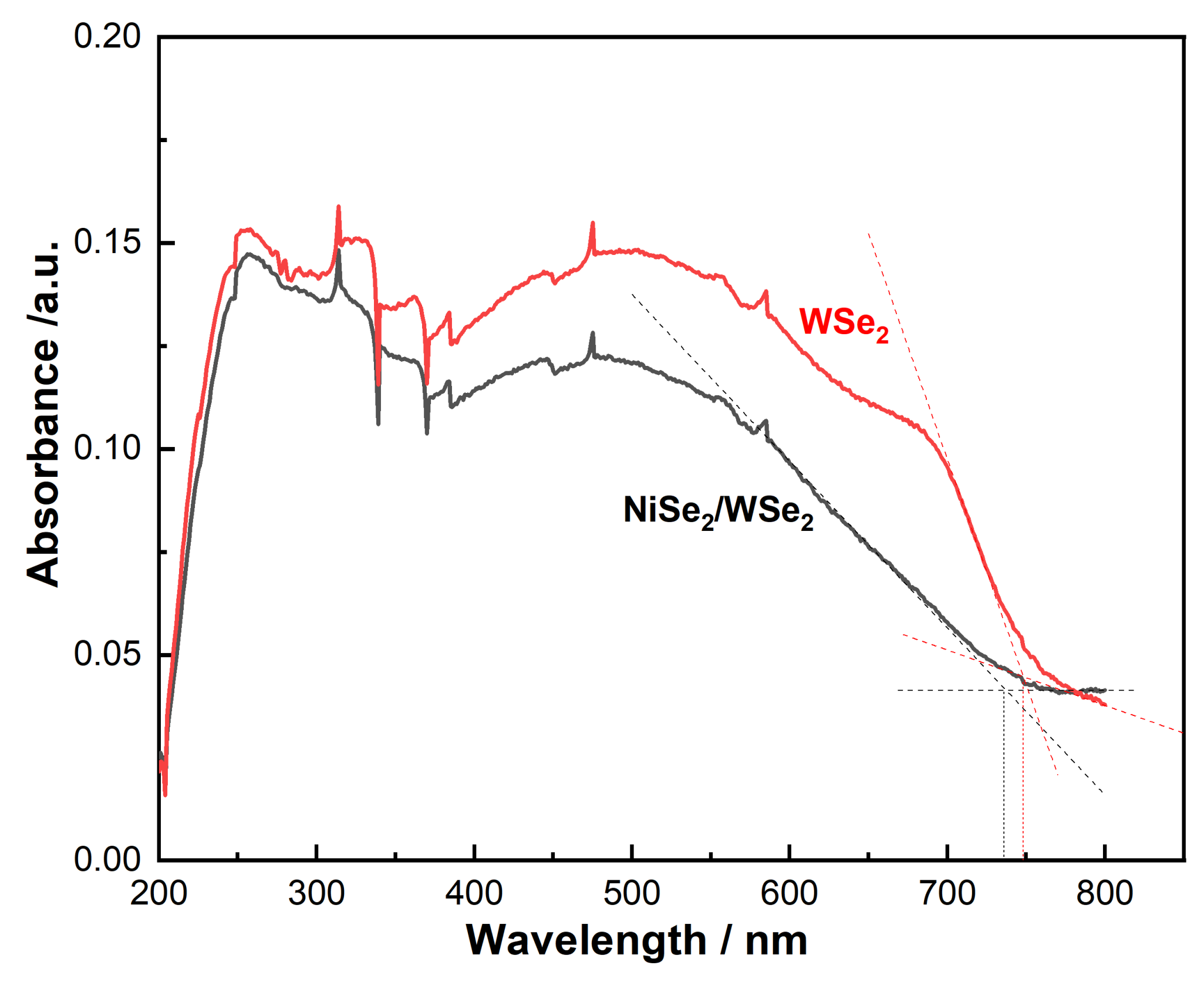
3.4. Analysis of UV-Visible Absorbance Spectrum
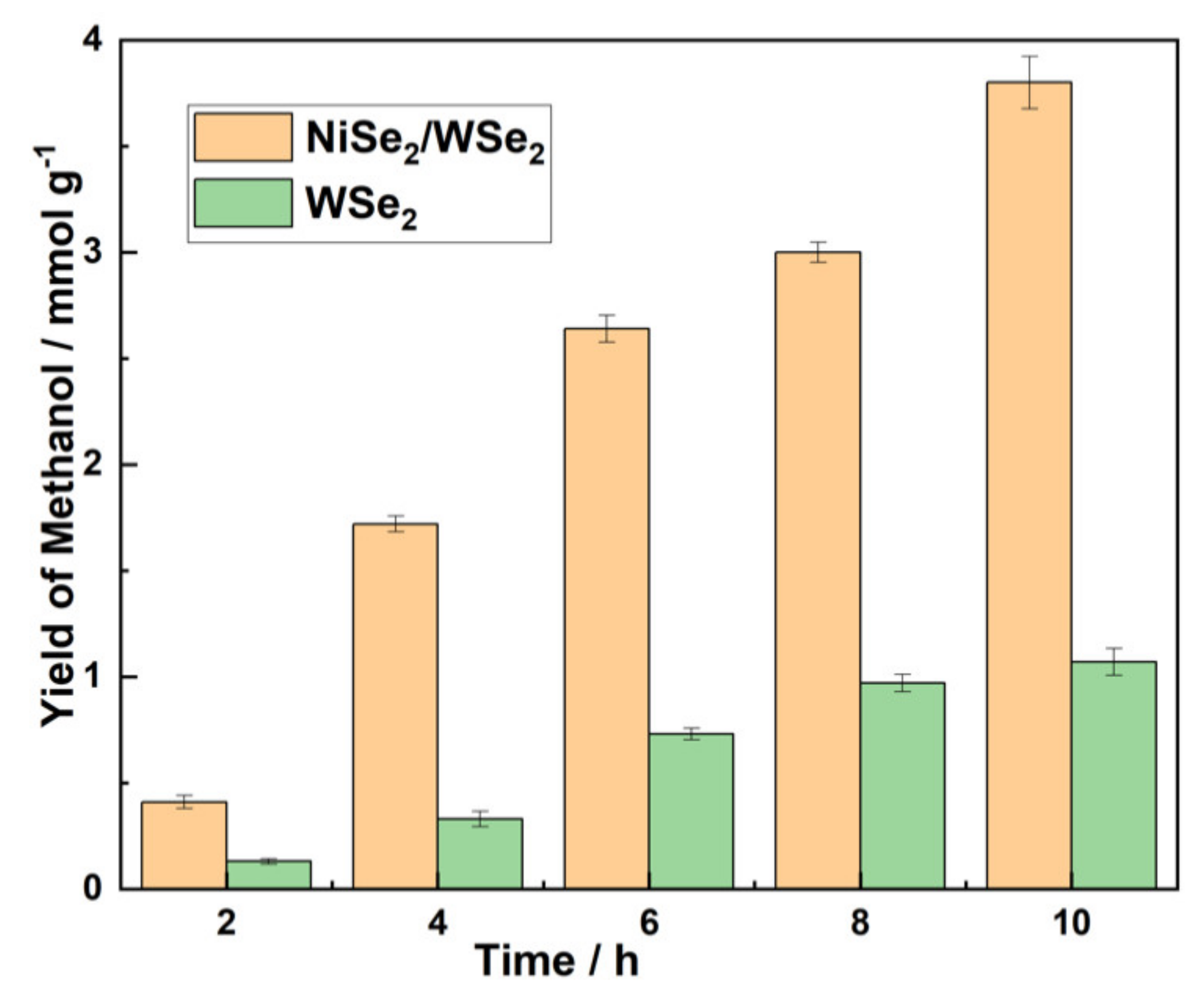
3.5. Photocatalysis Performance
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhao, S.; Jin, R.; Jin, R. Opportunities and Challenges in CO2 Reduction by Gold- and Silver-Based Electrocatalysts: From Bulk Metals to Nanoparticles and Atomically Precise Nanoclusters. ACS Energy Lett. 2018, 3, 452–462. [Google Scholar] [CrossRef]
- Wan, W.; Tackett, B.M.; Chen, J.G. Reactions of water and C1 molecules on carbide and metal-modified carbide surfaces. Chem. Soc. Rev. 2017, 46, 1807–1823. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef] [PubMed]
- Graves, C.; Ebbesen, S.D.; Mogensen, M.; Lackner, K.S. Sustainable hydrocarbon fuels by recycling CO2 and H2O with renewable or nuclear energy. Renew. Sustain. Energy Rev. 2011, 15, 1–23. [Google Scholar] [CrossRef]
- Stolarczyk, J.K.; Bhattacharyya, S.; Polavarapu, L.; Feldmann, J. Challenges and Prospects in Solar Water Splitting and CO2 Reduction with Inorganic and Hybrid Nanostructures. ACS Catal. 2018, 8, 3602–3635. [Google Scholar] [CrossRef]
- Wang, P.; Wang, S.; Wang, H.; Wu, Z.; Wang, L. Recent Progress on Photo-Electrocatalytic Reduction of Carbon Dioxide. Part. Part. Syst. Charact. 2018, 35, 1700371. [Google Scholar] [CrossRef]
- Eklund, P.; Kerdsongpanya, S.; Alling, B. Transition-metal-nitride-based thin films as novel energy harvesting materials. J. Mater. Chem. C 2016, 4, 3905–3914. [Google Scholar] [CrossRef]
- Xiao, Y.; Hwang, J.-Y.; Sun, Y.-K. Transition metal carbide-based materials: Synthesis and applications in electrochemical energy storage. J. Mater. Chem. A 2016, 4, 10379–10393. [Google Scholar] [CrossRef]
- Asadi, M.; Kim, K.; Liu, C.; Addepalli, A.V.; Abbasi, P.; Yasaei, P.; Phillips, P.; Behranginia, A.; Cerrato, J.M.; Haasch, R.; et al. Nanostructured transition metal dichalcogenide electrocatalysts for CO2 reduction in ionic liquid. Science 2016, 353, 467–470. [Google Scholar] [CrossRef]
- Chhowalla, M.; Shin, H.S.; Eda, G.; Li, L.-J.; Loh, K.P.; Zhang, H. The chemistry of two-dimensional layered transition metal dichalcogenide nanosheets. Nat. Chem. 2013, 5, 263–275. [Google Scholar] [CrossRef]
- Tan, C.; Zhang, H. Two-dimensional transition metal dichalcogenide nanosheet-based composites. Chem. Soc. Rev. 2015, 44, 2713–2731. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Dou, K.; Kaun, C.-C.; Kuang, Q.; Yang, S. MoSe2 nanosheets and their graphene hybrids: Synthesis, characterization and hydrogen evolution reaction studies. J. Mater. Chem. A 2014, 2, 360–364. [Google Scholar] [CrossRef]
- Bi, E.; Chen, H.; Yang, X.; Ye, F.; Yin, M.; Han, L. Fullerene-Structured MoSe2 Hollow Spheres Anchored on Highly Nitrogen-Doped Graphene as a Conductive Catalyst for Photovoltaic Applications. Sci. Rep. 2015, 5, 13214. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Chuang, S.; Chang, T.C.; Takei, K.; Takahashi, T.; Javey, A. High-Performance Single Layered WSe2 p-FETs with Chemically Doped Contacts. Nano Lett. 2012, 12, 3788–3792. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Kang, J.; Sarkar, D.; Khatami, Y.; Jena, D.; Banerjee, K. Role of Metal Contacts in Designing High-Performance Monolayer n-Type WSe2 Field Effect Transistors. Nano Lett. 2013, 13, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Teo, W.Z.; Chng, E.L.K.; Sofer, Z. Cytotoxicity of Exfoliated Transition-Metal Dichalcogenides (MoS2, WS2, and WSe2) is Lower Than That of Graphene and its Analogues. Chem. Eur. J. 2014, 20, 9627–9632. [Google Scholar] [CrossRef]
- Wang, H.; Feng, H.; Li, J. Graphene and Graphene-like Layered Transition Metal Dichalcogenides in Energy Conversion and Storage. Small 2014, 10, 2165–2181. [Google Scholar] [CrossRef]
- Yu, B.; Zheng, B.; Wang, X.; Qi, F.; He, J.; Zhang, W.; Chen, Y. Enhanced photocatalytic properties of graphene modified few-layered WSe2 nanosheets. Appl. Surf. Sci. 2017, 400, 420–425. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zheng, B.; Qi, F.; He, J.; Yu, B.; Zhang, W. Significant enhancement of photocatalytic activity of multi-walled carbon nanotubes modified WSe2 composite. Mater. Lett. 2017, 197, 67–70. [Google Scholar] [CrossRef]
- Licht, S.; Myung, N.; Tenne, R.; Hodes, G. Cation Electrolytic Modification of n-WSe2/Aqueous Polyiodide Photoelectrochemistry. J. Electrochem. Soc. 1995, 142, 840. [Google Scholar] [CrossRef]
- Feng, J.; Sun, X.; Wu, C.; Peng, L.; Lin, C.; Hu, S.; Yang, J.; Xie, Y. Metallic Few-Layered VS2 Ultrathin Nanosheets: High Two-Dimensional Conductivity for In-Plane Supercapacitors. J. Am. Chem. Soc. 2011, 133, 17832–17838. [Google Scholar] [CrossRef]
- Li, W.; Yu, B.; Hu, Y.; Wang, X.; Yang, D.; Chen, Y. Core-Shell Structure of NiSe2 Nanoparticles@Nitrogen-Doped Graphene for Hydrogen Evolution Reaction in Both Acidic and Alkaline Media. ACS Sustain. Chem. Eng. 2019, 7, 4351–4359. [Google Scholar] [CrossRef]
- Guo, J.; Shi, Y.; Bai, X.; Wang, X.; Ma, T. Atomically thin MoSe2/graphene and WSe2/graphene nanosheets for the highly efficient oxygen reduction reaction. J. Mater. Chem. A 2015, 3, 24397–24404. [Google Scholar] [CrossRef]
- Prabhu, P.; Vishal, J.; Lee, J.M. Heterostructured Catalysts for Electrocatalytic and Photocatalytic Carbon Dioxide Reduction. Adv. Funct. Mater. 2020, 30, 24. [Google Scholar] [CrossRef]
- Yang, X.; Yang, W.; Xin, X.; Yin, X. Enhancement of photocatalytic activity in reducing CO2 over CdS/g-C3N4 composite catalysts under UV light irradiation Chem. Phys. Lett. 2016, 651, 127. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Peng, T. Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: A direct Z-scheme mechanism. J. Mater. Chem. A 2015, 3, 19936. [Google Scholar] [CrossRef]
- Bafaqeer, A.; Tahir, M. Well-designed 2D/2D ZnV2O6/g-C3N4 nanosheets heterojunction with faster charges separation via pCN as mediator towards enhanced photocatalytic reduction of CO2 to fuels. Appl. Catal. B 2019, 242, 312–326. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Luo, D.; Li, J.; Huang, Y.; Li, H.; Fang, Y.; Xu, Y.-H.; Zhu, L. Adsorption of CO2 on heterostructure CdS(Bi2S3)/TiO2 nanotube photocatalysts and their photocatalytic activities in the reduction of CO2 to methanol under visible light irradiation. Chem. Eng. J. 2012, 180, 151. [Google Scholar] [CrossRef]
- Qin, S.; Xin, F.; Liu, Y.; Yin, X.; Ma, W. Photocatalytic reduction of CO2 in methanol to methyl formate over CuO–TiO2 composite catalysts. Chem. Eng. 2011, 356, 257. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.C.; Stulp, S.; de Brito, J.F.; Flor, J.B.S.; Frem, R.C.G.; Zanoni, M.V.B. MOFs based on ZIF-8 deposited on TiO2 nanotubes increase the surface adsorption of CO2 and its photoelectrocatalytic reduction to alcohols in aqueous media. Appl. Catal. B Environ. 2018, 225, 563–573. [Google Scholar] [CrossRef]
- Cao, K.S. Preparation and Tribological Properties of WSe2 and WSe2/C Nanostructured Materials. Master‘s. Thesis, Jiangsu University, Jiangsu, China, 2011. [Google Scholar]
- Cho, S.; Park, S.-K.; Jeon, K.M.; Piao, Y.; Kang, Y.C. Mesoporous reduced graphene oxide/WSe2 composite particles for efficient sodium-ion batteries and hydrogen evolution reactions. Appl. Sur. Sci. 2018, 459, 309–317. [Google Scholar] [CrossRef]
- Ge, P.; Li, S.; Xu, L.; Zou, K.; Gao, X.; Cao, X.; Zou, G.; Hou, H.; Ji, X. Hierarchical Hollow-Microsphere Metal–Selenide@Carbon Composites with Rational Surface Engineering for Advanced Sodium Storage. Adv. Energy Mater. 2019, 9, 1803035. [Google Scholar] [CrossRef]
- Gu, C.; Hu, S.; Zheng, X.; Gao, M.-R.; Zheng, Y.-R.; Shi, L.; Gao, Q.; Zheng, X.; Chu, W.; Yao, H.-B.; et al. Synthesis of Sub-2 nm Iron-Doped NiSe2 Nanowires and Their Surface-Confined Oxidation for Oxygen Evolution Catalysis. Angew. Chem. Int. 2018, 57, 4084–4088. [Google Scholar] [CrossRef]

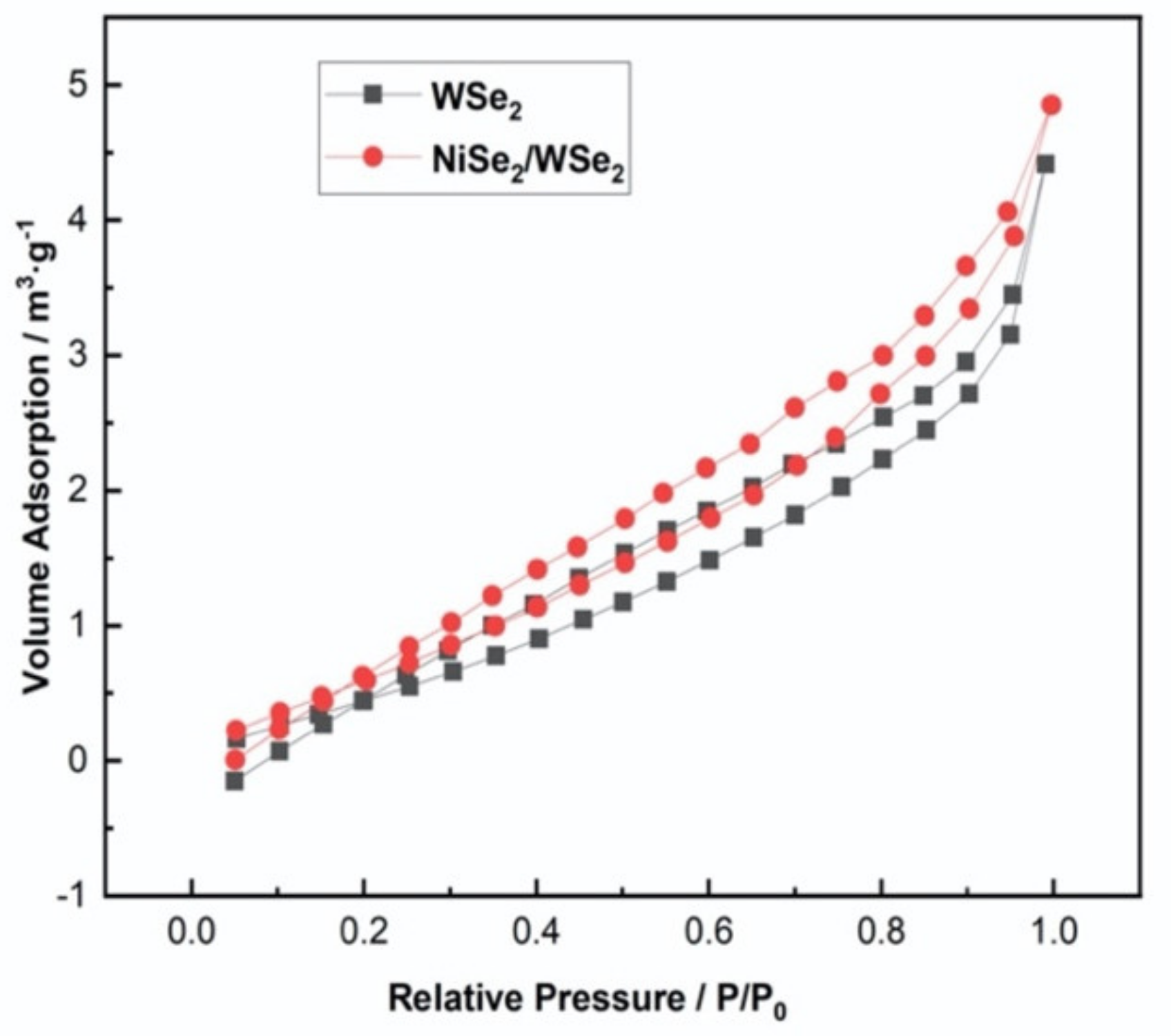

| Sample | Specific Surface Area (m2·g−1) | Pore Volumes (cc·g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| WSe2 | 3.007 | 0.007 | 1.702 |
| NiSe2/WSe2 | 8.522 | 0.008 | 1.916 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Li, Y.; Guo, F.; Zhang, K.; Liu, K.; Jia, W.; Zhao, Y.; Sun, Y. Carbon Dioxide Conversion with High-Performance Photocatalysis into Methanol on NiSe2/WSe2. Energies 2020, 13, 4330. https://doi.org/10.3390/en13174330
Luo Z, Li Y, Guo F, Zhang K, Liu K, Jia W, Zhao Y, Sun Y. Carbon Dioxide Conversion with High-Performance Photocatalysis into Methanol on NiSe2/WSe2. Energies. 2020; 13(17):4330. https://doi.org/10.3390/en13174330
Chicago/Turabian StyleLuo, Zheng, Yinghan Li, Fengbo Guo, Kaizhi Zhang, Kankan Liu, Wanli Jia, Yuxia Zhao, and Yan Sun. 2020. "Carbon Dioxide Conversion with High-Performance Photocatalysis into Methanol on NiSe2/WSe2" Energies 13, no. 17: 4330. https://doi.org/10.3390/en13174330
APA StyleLuo, Z., Li, Y., Guo, F., Zhang, K., Liu, K., Jia, W., Zhao, Y., & Sun, Y. (2020). Carbon Dioxide Conversion with High-Performance Photocatalysis into Methanol on NiSe2/WSe2. Energies, 13(17), 4330. https://doi.org/10.3390/en13174330





