Syngas Composition: Gasification of Wood Pellet with Water Steam through a Reactor with Continuous Biomass Feed System
Abstract
:1. Introduction
2. Materials and Methods
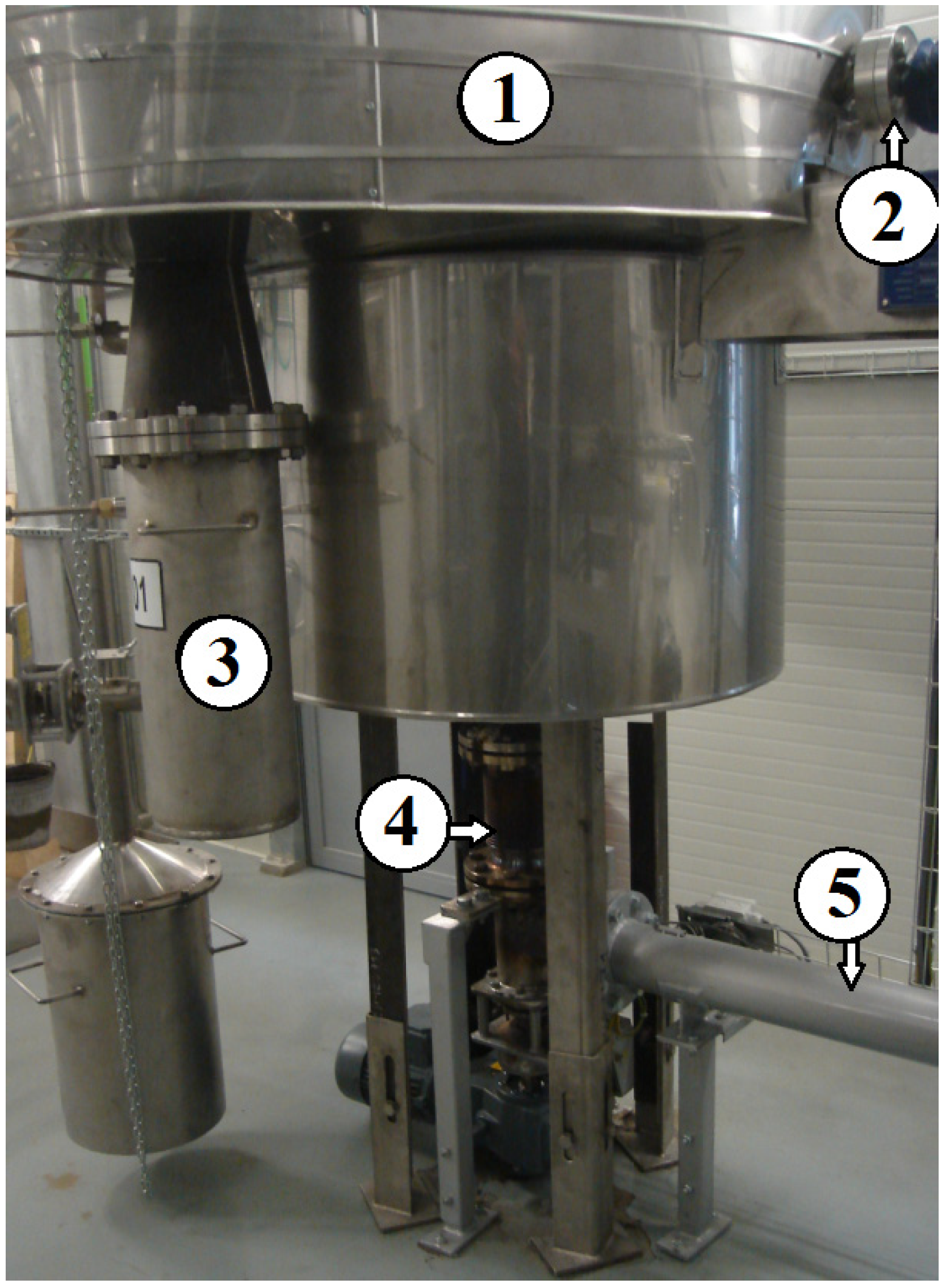
2.1. Gasification Installation
2.2. Materials
2.3. Methods
3. Results and Discussion
3.1. Impact of the Steam Flow Rate on the Concentration of Synthesis Gas Components
3.2. Impact of the Temperature on the Concentration of Synthesis Gas Components
3.3. Impact of Reactor Temperature and the Steam Flow Rate on the Syngas Heat Value
3.4. Correlations between the Content of the Individual Syngas Components
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ates, B.; Koytepe, S.; Ulu, A.; Gurses, C.; Thakur, V.K. Chemistry, Structures, and Advanced Applications of Nanocomposites from Biorenewable Resources. Chem. Rev. 2020. [Google Scholar] [CrossRef] [PubMed]
- Lisy, M.; Lisa, H.; Jecha, D.; Balas, M.; Krizan, P. Characteristic properties of alternative biomass fuels. Energies 2020, 13, 1448. [Google Scholar] [CrossRef] [Green Version]
- Chojnacki, J.; Ondruska, J.; Kuczynski, W.; Soos, L.; Balasz, B. Emissions from the combustion of solid biofuels. In Proceedings of the 9th International Scientific Symposium on Farm Machinery and Process Management in Sustainable Agriculture, Lublin, Poland, 22–24 November 2017; pp. 70–75. [Google Scholar]
- Balas, M.; Lisy, M.; Kracik, P.; Pospisil, J. Municipal solid waste gasification within waste-to-energy processing. MM Sci. J. 2017, 1783–1788. [Google Scholar] [CrossRef]
- Najser, J.; Ochodek, T.; Chlond, R. Functioning of installation for a biomass gasification and economic aspects of electricity generation. Rynek Energii 2009, 6, 68–74. [Google Scholar]
- Gallucci, F.; Liberatore, R.; Sapegno, L.; Volponi, E.; Venturini, P.; Rispoli, F.; Paris, E.; Carnevale, M.; Colantoni, A. Influence of oxidant agent on syngas composition: Gasification of hazelnut shells through an updraft reactor. Energies 2020, 13, 102. [Google Scholar] [CrossRef] [Green Version]
- Hernández, J.J.; Aranda, G.; Barba, J.; Mendoza, J.M. Effect of steam content in the air-steam flow on biomass entrained flow gasification. Fuel Process. Technol. 2012, 99, 43–55. [Google Scholar] [CrossRef]
- Campoy, M.; Gómez-Barea, A.; Vidal, F.B.; Ollero, P. Air-steam gasification of biomass in a fluidised bed: Process optimisation by enriched air. Fuel Process. Technol. 2009, 90, 677–685. [Google Scholar] [CrossRef]
- Lv, P.M.; Xiong, Z.H.; Chang, J.; Wu, C.Z.; Chen, Y.; Zhu, J.X. An experimental study on biomass air-steam gasification in a fluidized bed. Bioresour. Technol. 2004, 95, 95–101. [Google Scholar] [CrossRef]
- Ferreira, S.; Monteiro, E.; Brito, B.; Castro, C.; Calado, L.; Vilarinho, C. Experimental analysis of brewers’ spent grains steam gasification in an allothermal batch reactor. Energies 2019, 12, 912. [Google Scholar] [CrossRef] [Green Version]
- Niu, Y.; Han, F.; Chen, Y.; Lyu, Y.; Wang, L. Experimental study on steam gasification of pine particles for hydrogen-rich gas. J. Energy Inst. 2017, 90, 715–724. [Google Scholar] [CrossRef]
- Sattar, A.; Leeke, G.A.; Hornung, A.; Wood, J. Steam gasification of rapeseed, wood, sewage sludge and miscanthus biochars for the production of a hydrogen-rich syngas. Biomass Bioenergy 2014, 69, 276–286. [Google Scholar] [CrossRef]
- Jangsawang, W.; Klimanek, A.; Gupta, A.K. Enhanced yield of hydrogen from wastes using high temperature steam gasification. J. Energy Resour. Technol. 2006, 128, 179–185. [Google Scholar] [CrossRef] [Green Version]
- Acharya, B.; Dutta, A.; Basu, P. An investigation into steam gasification of biomass for hydrogen enriched gas production in presence of CaO. Int. J. Hydrogen Energy 2010, 35, 1582–1589. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C. A novel reforming method for hydrogen production from biomass steam gasification. Bioresour. Technol. 2009, 100, 4271–4277. [Google Scholar] [CrossRef] [PubMed]
- Valente, A.; Iribarren, D.; Gálvez-Martos, J.L.; Dufour, J. Robust eco-efficiency assessment of hydrogen from biomass gasification as an alternative to conventional hydrogen: A life-cycle study with and without external costs. Sci. Total Environ. 2019, 650, 1465–1475. [Google Scholar] [CrossRef] [PubMed]
- Schmieder, H.; Abeln, J.; Boukis, N.; Dinjus, E.; Kruse, A.; Kluth, M.; Petrich, G.; Sadri, E.; Schacht, M. Hydrothermal gasification of biomass and organic wastes. J. Supercrit. Fluids 2000, 17, 145–153. [Google Scholar] [CrossRef]
- Siwal, S.S.; Zhang, O.; Sun, C.; Thakur, S.; Gupta, V.K.; Thakur, V.K. Energy production from steam gasification processes and parameters that contemplate in biomass gasifier—A review. Bioresour. Technol. 2020, 297, 1–11. [Google Scholar]
- Umeki, K.; Yamamoto, K.; Namioka, T.; Yoshikawa, K. High temperature steam-only gasification of woody biomass. Appl. Energy 2010, 87, 791–798. [Google Scholar] [CrossRef]
- Meng, X.; de Jong, W.; Fu, N.; Verkooijen, A.H.M. Biomass gasification in a 100 kWth steam-oxygen blown circulating fluidized bed gasifier: Effects of operational conditions on product gas distribution and tar formation. Biomass Bioenergy 2011, 35, 2910–2924. [Google Scholar] [CrossRef]
- Howaniec, N.; Smoliński, A. Steam gasification of energy crops of high cultivation potential in Poland to hydrogen-rich gas. Int. J. Hydrogen Energy 2011, 36, 2038–2043. [Google Scholar] [CrossRef]
- Murakami, K.; Kasai, K.; Kato, T.; Sugawarab, K. Conversion of rice straw into valuable products by hydrothermal treatment and steam gasification. Fuel 2012, 93, 37–43. [Google Scholar] [CrossRef]
- Mikeska, M.; Najser, J.; Peer, V.; Frantik, J.; Kielar, J. Quality assessment of gas produced from different types of biomass pellets in gasification process. Energy Explor. Exploit. 2020, 38, 406–416. [Google Scholar] [CrossRef] [Green Version]
- Schweitzer, D.; Gredinger, A.; Schmid, M.; Waizmann, G.; Beirow, M.; Spörl, R.; Scheffknecht, G. Steam gasification of wood pellets, sewage sludge and manure: Gasification performance and concentration of impurities. Bioresour. Technol. 2018, 111, 308–319. [Google Scholar] [CrossRef]
- Hussein, M.S.; Burra, K.G.; Amano, R.S.; Gupta, A.K. Effect of oxygen addition in steam gasification of chicken manure. Fuel 2017, 189, 428–435. [Google Scholar] [CrossRef] [Green Version]
- Roche, E.; de Andrés, J.M.; Narros, A.; Rodríguez, M.E. Air and air-steam gasification of sewage sludge. The influence of dolomite and throughput in tar production and composition. Fuel 2014, 115, 54–61. [Google Scholar] [CrossRef]
- Wei, L.; Xu, S.; Zhang, L.; Liu, C.; Zhu, H.; Liu, S. Steam gasification of biomass for hydrogen-rich gas in a free-fall reactor. Int. J. Hydrogen Energy 2007, 32, 24–31. [Google Scholar] [CrossRef]
- Nipattummakul, N.; Ahmed, I.I.; Gupta, A.K.; Kerdsuwan, S. Hydrogen and syngas yield from residual branches of oil palm tree using steam gasification. Int. J. Hydrogen Energy 2011, 36, 3835–3843. [Google Scholar] [CrossRef]
- Kumar, A.; Eskridge, K.; Jones, D.B.; Hanna, M.A. Steam-air fluidized bed gasification of distillers grains: Effects of steam to biomass ratio, equivalence ratio and gasification temperature. Bioresour. Technol. 2009, 100, 2062–2068. [Google Scholar] [CrossRef]
- Wilson, L.; John, G.R.; Mhilu, C.F.; Yang, W.; Blasiak, W. Coffee husks gasification using high temperature air/steam agent. Fuel Process. Technol. 2010, 91, 1330–1337. [Google Scholar] [CrossRef]
- Howaniec, N.; Smoliński, A.; Stańczyk, K.; Pichlak., M. Steam co-gasification of coal and biomass derived chars with synergy effect as an innovative way of hydrogen-rich gas production. Int. J. Hydrogen Energy 2011, 36, 14455–14463. [Google Scholar] [CrossRef]
- Yu, J.; Tian, F.-J.; Chow, M.C.; McKenzie, L.J.; Li, C.-Z. Effect of iron on the gasification of Victorian brown coal with steam: Enhancement of hydrogen production. Fuel 2006, 85, 127–133. [Google Scholar] [CrossRef]
- Zheng, X.; Ying, Z.; Wang, B.; Chen, C. Hydrogen and syngas production from municipal solid waste (MSW) gasification via reusing CO2. Appl. Therm. Eng. 2018, 144, 242–247. [Google Scholar] [CrossRef]
- Pfeifer, C.; Koppatz, S.; Hofbauer, H. Steam gasification of various feedstocks at a dual fluidised bed gasifier: Impacts of operation conditions and bed materials. Biomass Conv. Bioref. 2011, 1, 39–53. [Google Scholar] [CrossRef]
- Pinto, F.; Franco, C.; André, R.N.; Tavares, C.; Dias, M.; Gulyurtlu, I.; Cabrita, I. Effect of experimental conditions on co-gasification of coal, biomass and plastics wastes with air/steam mixtures in a fluidized bed system. Fuel 2003, 82, 1967–1976. [Google Scholar] [CrossRef]
- Stąsiek, J.; Szkodo, M. Thermochemical conversion of biomass and municipal waste into useful energy using advanced hitag/hitsg technology. Energies 2020, 13, 4218. [Google Scholar] [CrossRef]
- Udomsirichakorn, J.; Basu, P.; Salam, P.A.; Acharya, B. Effect of CaO on tar reforming to hydrogen-enriched gas with in-process CO2 capture in a bubbling fluidized bed biomass steam gasifier. Int. J. Hydrogen Energy 2013, 38, 14495–14504. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Steam gasification of biomass in a conical spouted bed reactor with olivine and γ-alumina as primary catalysts. Fuel Process. Technol. 2013, 116, 292–299. [Google Scholar] [CrossRef]
- Chang, A.C.C.; Chang, H.F.; Lin, F.J.; Lin, K.H.; Chen, C.H. Biomass gasification for hydrogen production. Int. J. Hydrogen Energy 2011, 36, 14252–14260. [Google Scholar] [CrossRef]
- Karmakar, M.K.; Datta, A.B. Generation of hydrogen rich gas through fluidized bed gasification of biomass. Bioresour. Technol. 2011, 102, 1907–1913. [Google Scholar] [CrossRef]
- Salman, C.A.; Omer, C.B. Process modelling and simulation of waste gasification-based flexible polygeneration facilities for power, heat and biofuels production. Energies 2020, 13, 4264. [Google Scholar] [CrossRef]
- Schuster, G.; Löffler, G.; Weigl, K.; Hofbauer, H. Biomass steam gasification—An extensive parametric modeling study. Bioresour. Technol. 2001, 77, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Sheth, P.N. Air-steam biomass gasification: Experiments, modeling and simulation. Energy Convers. Manag. 2016, 110, 307–318. [Google Scholar] [CrossRef]
- Kuo, P.C.; Wu, W. Design, optimization and energetic efficiency of producing hydrogen-rich gas from biomass steam gasification. Energies 2015, 8, 94–110. [Google Scholar] [CrossRef]
- Najser, J.; Buryan, P.; Skoblia, S.; Frantik, J.; Kielar, J.; Peer, V. Problems related to gasification of biomass—Properties of solid pollutants in raw gas. Energies 2019, 12, 963. [Google Scholar] [CrossRef] [Green Version]











| Parameter | Size | Symbol |
|---|---|---|
| Diameter | 6 | mm |
| Length | 10–30 | mm |
| Bulk density | 650 | kg∙m−3 |
| Humidity | 8.67 | % hm. |
| Calorific value | 17.5 | MJ∙kg−1 |
| Ash content | <0.6 | % |
| Attrition | 2 | % |
| Ingredients | Contents % |
|---|---|
| C | 47.43 |
| H | 6.10 |
| N | 0.04 |
| O | 40.00 |
| S | 0.05 |
| Cl | 0.013 |
| Ash | 0.39 |
| Temp °C | Steam Flow Ratio kg∙h−1 | H2% | CH4% | CO2% | CO% | ||||
|---|---|---|---|---|---|---|---|---|---|
| Cont. | Stand. Dev. | Cont. | Stand. Dev. | Cont. | Stand. Dev. | Cont. | Stand. Dev. | ||
| 750 | 10 | 44.56 | 0.24 | 13.44 | 0.13 | 22.34 | 0.21 | 19.66 | 0.33 |
| 750 | 15 | 43.34 | 0.49 | 13.72 | 0.29 | 21.64 | 0.75 | 21.30 | 0.88 |
| 750 | 20 | 42.60 | 0.42 | 14.15 | 0.28 | 22.88 | 0.74 | 20.37 | 0.51 |
| 800 | 10 | 38.30 | 5.10 | 15.56 | 1.54 | 23.87 | 3.74 | 22.27 | 7.55 |
| 800 | 15 | 35.92 | 1.79 | 16.90 | 0.58 | 22.78 | 0.21 | 24.40 | 1.29 |
| 800 | 20 | 33.87 | 0.78 | 17.08 | 0.39 | 23.69 | 0.73 | 25.37 | 1.19 |
| 850 | 10 | 36.36 | 2.17 | 12.34 | 1.52 | 29.06 | 2.50 | 22.24 | 3.17 |
| 850 | 15 | 35.34 | 0.45 | 11.02 | 0.47 | 31.40 | 0.86 | 22.24 | 0.85 |
| 850 | 20 | 35.58 | 0.27 | 10.99 | 0.16 | 34.00 | 0.42 | 19.43 | 0.44 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chojnacki, J.; Najser, J.; Rokosz, K.; Peer, V.; Kielar, J.; Berner, B. Syngas Composition: Gasification of Wood Pellet with Water Steam through a Reactor with Continuous Biomass Feed System. Energies 2020, 13, 4376. https://doi.org/10.3390/en13174376
Chojnacki J, Najser J, Rokosz K, Peer V, Kielar J, Berner B. Syngas Composition: Gasification of Wood Pellet with Water Steam through a Reactor with Continuous Biomass Feed System. Energies. 2020; 13(17):4376. https://doi.org/10.3390/en13174376
Chicago/Turabian StyleChojnacki, Jerzy, Jan Najser, Krzysztof Rokosz, Vaclav Peer, Jan Kielar, and Bogusława Berner. 2020. "Syngas Composition: Gasification of Wood Pellet with Water Steam through a Reactor with Continuous Biomass Feed System" Energies 13, no. 17: 4376. https://doi.org/10.3390/en13174376
APA StyleChojnacki, J., Najser, J., Rokosz, K., Peer, V., Kielar, J., & Berner, B. (2020). Syngas Composition: Gasification of Wood Pellet with Water Steam through a Reactor with Continuous Biomass Feed System. Energies, 13(17), 4376. https://doi.org/10.3390/en13174376






