Formation Kinetics of the Mixed Cyclopentane—Carbon Dioxide Hydrates in Aqueous Sodium Chloride Solutions
Abstract
:1. Introduction
2. Experimental Section
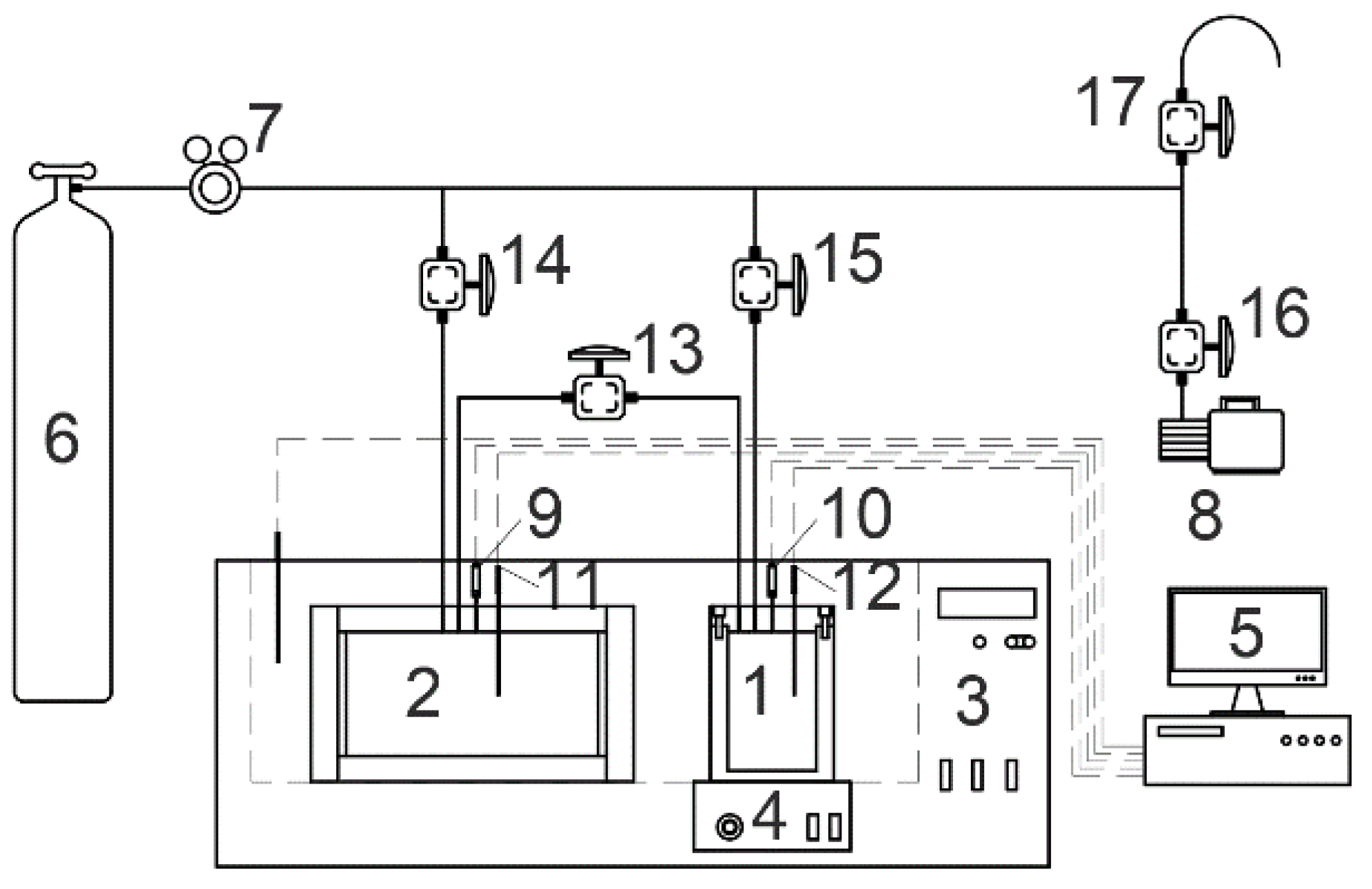
2.1. Materials and Apparatus
2.2. Procedure
3. Results and Discussion
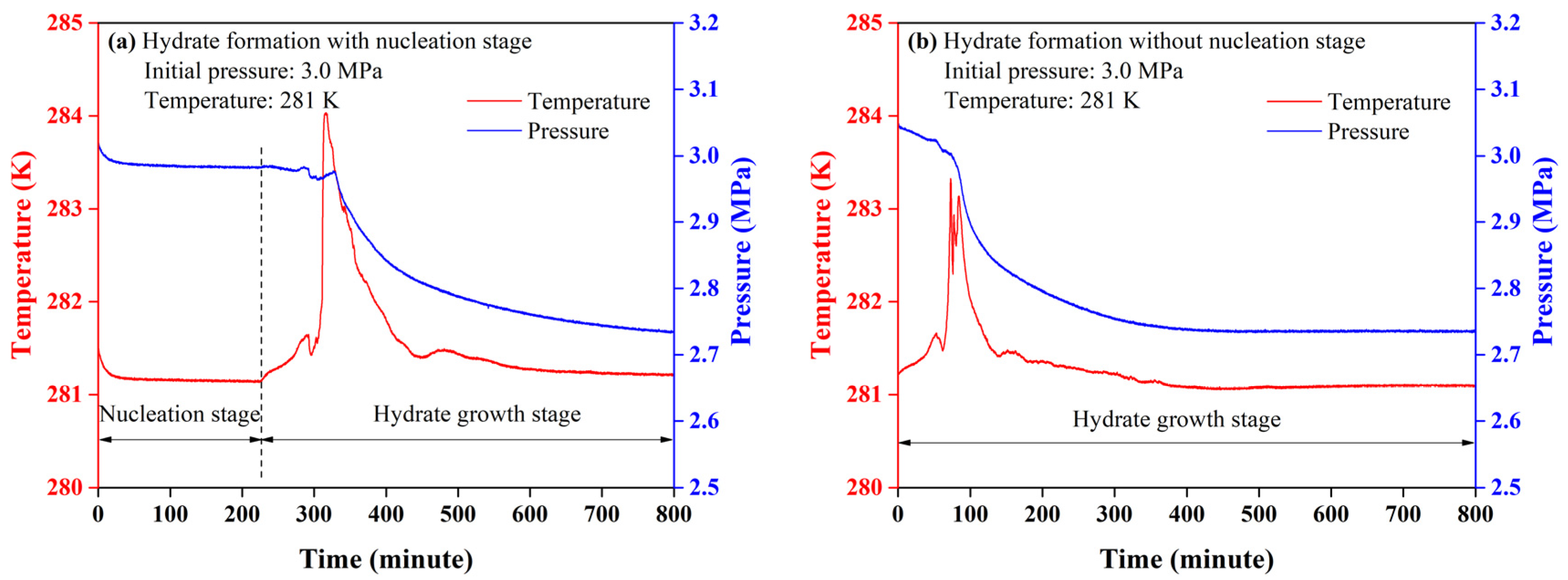
3.1. Kinetic Features
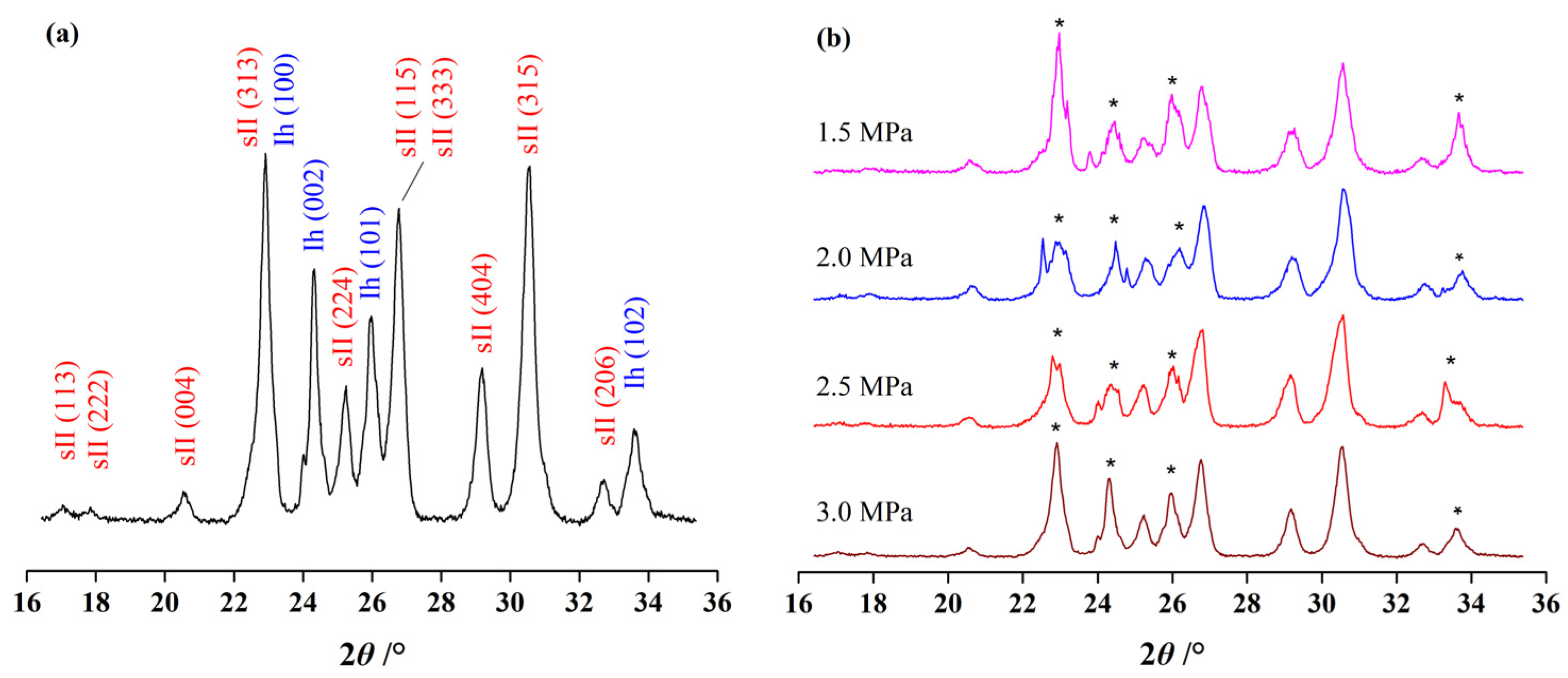
3.2. Effect of Initial Pressure
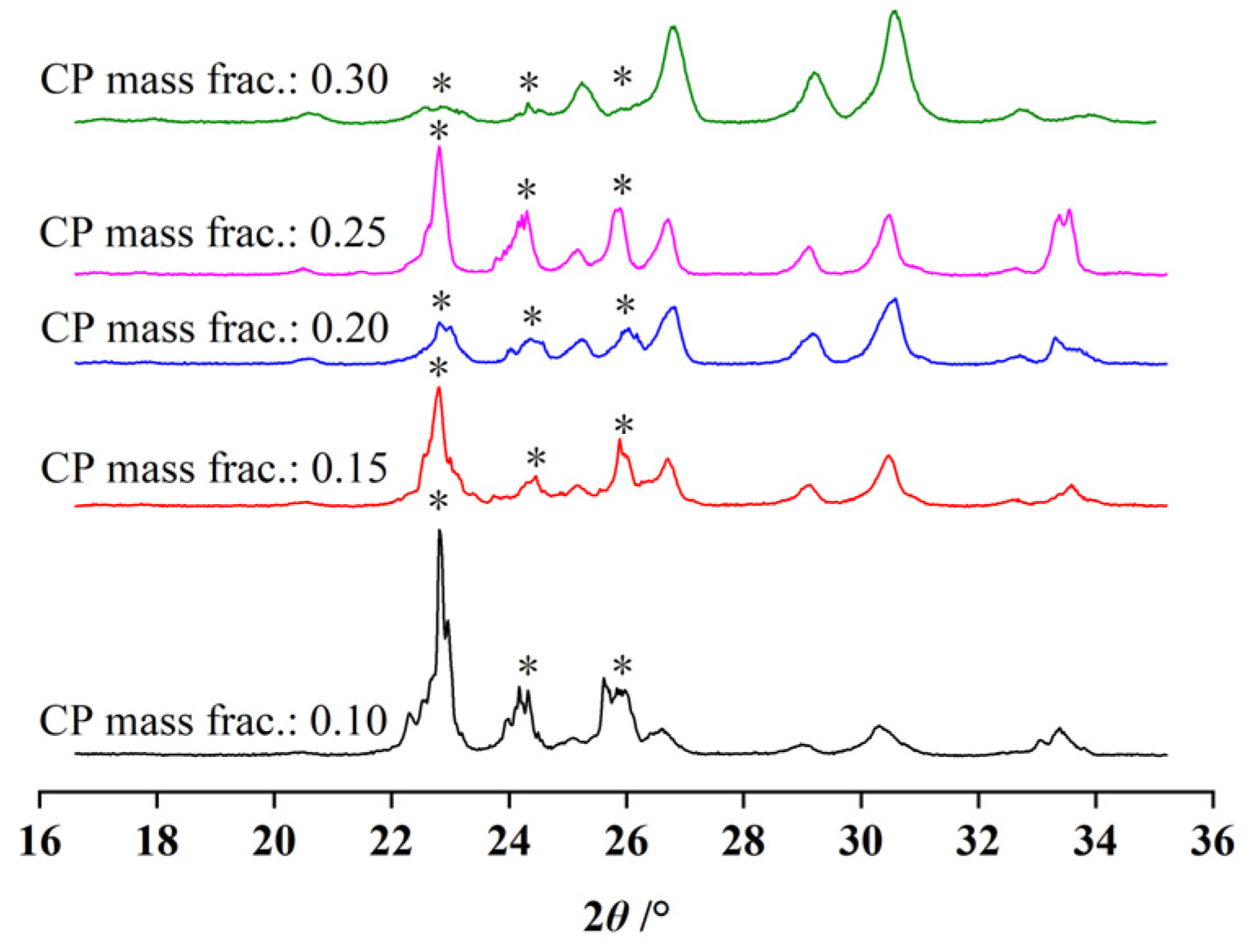
3.3. Effect of the CP Mass Fraction
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sosso, G.C.; Chen, J.; Cox, S.J.; Fitzner, M.; Pedevilla, P.; Zen, A.; Michaelides, A. Crystal Nucleation in Liquids: Open Questions and Future Challenges in Molecular Dynamics Simulations. Chem. Rev. 2016, 116, 7078–7116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.G.; Li, X.S.; Yan, K.F.; Ruan, X.K.; Chen, Z.Y.; Xia, Z.M. Research progress in hydrate-based technologies and processes in China: A review. Chin. J. Chem. Eng. 2019, 27, 1998–2013. [Google Scholar] [CrossRef]
- Li, F.G.; Yuan, Q.; Li, T.D.; Li, Z.; Sun, C.Y.; Chen, G.J. A review: Enhanced recovery of natural gas hydrate reservoirs. Chin. J. Chem. Eng. 2019, 27, 2062–2073. [Google Scholar] [CrossRef]
- Zhou, X.B.; Liang, D.Q. Enhanced performance on CO2 adsorption and release induced by structural transition that occurred in TBAB∙26H2O hydrates. Chem. Eng. J. 2019, 378, 122128. [Google Scholar] [CrossRef]
- Ma, S.H.; Zheng, J.N.; Tian, M.R.; Tang, D.W.; Yang, M.J. NMR quantitative investigation on methane hydrate formation characteristics under different driving forces. Fuel 2020, 261, 116364. [Google Scholar] [CrossRef]
- Han, S.; Rhee, Y.W.; Kang, S.P. Investigation of salt removal using cyclopentane hydrate formation and washing treatment for seawater desalination. Desalination 2017, 404, 132–137. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, J.C.; Li, X.S.; Zhang, Y.; Li, G. Evaluation of Gas Production from Marine Hydrate Deposits at the GMGS2-Site 8, Pearl River Mouth Basin, South China Sea. Energies 2016, 9, 222. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Wang, T.; Zeng, Z.; Jiang, J.H.; Deng, B.; Chen, C.Z.; Li, J.Y.; Li, C.H.; Tao, L.M.; Li, X.; et al. Oleic acid potassium soap: A new potential kinetic promoter for methane hydrate formation. Chem. Eng. J. 2019, 363, 349–355. [Google Scholar] [CrossRef]
- Karanjkar, P.U.; Lee, J.W.; Morris, J.F. Calorimetric investigation of cyclopentane hydrate formation in an emulsion. Chem. Eng. Sci. 2012, 68, 481–491. [Google Scholar] [CrossRef]
- Gao, W.; Habib, M.; Smith, D.W. Removal of organic contaminants and toxiciy from industrial effluents using freezing processes. Desalination 2009, 245, 108–119. [Google Scholar] [CrossRef]
- Cha, J.H.; Seol, Y. Increasing Gas Hydrate Formation Temperature for Desalination of High Salinity Produced Water with Secondary Guests. ACS Sustain. Chem. Eng. 2013, 1, 1218–1224. [Google Scholar] [CrossRef]
- Zheng, J.N.; Yang, M.J.; Liu, Y.; Wang, D.Y.; Song, Y.C. Effects of cyclopentane on CO2 hydrate formation and dissociation as a co-guest molecule for desalination. J. Chem. Thermodyn. 2017, 104, 9–15. [Google Scholar] [CrossRef]
- Galfre, A.; Kwaterski, M.; Brantuas, P.; Cameirao, A.; Herri, J.M. Clathrate Hydrate Equilibrium Data for the Gas Mixture of Carbon Dioxide and Nitrogen in the Presence of an Emulsion of Cyclopentane in Water. J. Chem. Eng. Data 2014, 59, 592–602. [Google Scholar] [CrossRef] [Green Version]
- Herslund, P.J.; Daraboina, N.; Thomsen, K.; Abildskov, J.; von Solms, N. Measuring and modelling of the combined thermodynamic promoting effect of tetrahydrofuran and cyclopentane on carbon dioxide hydrates. Fluid Phase Equilibr. 2014, 381, 20–27. [Google Scholar] [CrossRef]
- Yu, Y.S.; Zhang, Q.Z.; Li, X.S.; Chen, C.; Zhou, S.D. Kinetics, compositions and structures of carbon dioxide/hydrogen hydrate formation in the presence of cyclopentane. Appl. Energy 2020, 265, 114808. [Google Scholar] [CrossRef]
- Zylyftari, G.; Lee, J.W.; Morris, J.F. Salt effects on thermodynamic and rheological properties of hydre forming emulsions. Chem. Eng. Sci. 2013, 95, 148–160. [Google Scholar] [CrossRef]
- Zha, L.; Liang, D.Q.; Li, D.L. Phase equilibria of CO2 hydrate in NaCl-MgCl2 aqueous solutions. J. Chem. Thermodyn. 2012, 55, 110–114. [Google Scholar] [CrossRef]
- Takeya, S.; Hori, A.; Hondoh, T.; Uchida, T. Freezing-memory effect of water on nucleation of CO2 hydrate crystals. J. Phys. Chem. B 2000, 104, 4164–4168. [Google Scholar] [CrossRef]
- Liu, N.; Chen, W.J.; Liu, D.P.; Xie, Y.M. Characterization of CO2 hydrate formation by temperature vibration. Energy Convers. Manag. 2011, 52, 2351–2354. [Google Scholar] [CrossRef]
- He, Y.Y.; Rudolph, E.S.J.; Zitha, P.L.J.; Golombok, M. Kinetics of CO2 and methane hydrate formation: An experimental analysis in the bulk phase. Fuel 2011, 90, 272–279. [Google Scholar] [CrossRef]
- Kang, K.C.; Linga, P.; Park, K.N.; Choi, S.J.; Lee, J.D. Seawater desalination by gas hydrate process and removal characteristics of dissolved ions (Na+, K+, Mg2+, Ca2+, B3+, Cl−, SO42−). Desalination 2014, 353, 84–90. [Google Scholar] [CrossRef]
- Park, K.N.; Hong, S.Y.; Lee, J.W.; Kang, K.C.; Lee, Y.C.; Ha, M.G.; Lee, J.D. A new apparatus for seawater desalination by gas hydrate process and removal characteristics of dissolved minerals (Na+, Mg2+, Ca2+, K+, B3+). Desalination 2011, 274, 91–96. [Google Scholar] [CrossRef]
- Han, S.; Shin, J.Y.; Rhee, Y.W.; Kang, S.P. Enhanced efficiency of salt removal from brine for cyclopentane hydrates by washing, centrifuging, and sweating. Desalination 2014, 354, 17–22. [Google Scholar] [CrossRef]
- Liu, C.L.; Ren, H.B.; Meng, Q.G.; Sun, S.C. An experimental study of CO2 hydrate based-seawater desalination with the R141b as an accelerant. Nat. Gas Ind. 2013, 33, 90–95. [Google Scholar]
- Zhou, X.B.; Liang, D.Q.; Yi, L.Z. Experimental study of mixed CH4/CO2 hydrate formation kinetics and modeling. Asia-Pac. J. Chem. Eng. 2014, 9, 886–894. [Google Scholar] [CrossRef]
- Hong, S.; Moon, S.; Lee, Y.; Lee, S.; Park, Y. Investigation of thermodynamic and kinetic effects of cyclopentane derivatives on CO2 hydrates for potential application to seawater desalination. Chem. Eng. J. 2019, 363, 99–106. [Google Scholar] [CrossRef]
- Lim, Y.A.; Babu, P.; Kumar, R.; Linga, P. Morphology of Carbon Dioxide-Hydrogen-Cyclopentane Hydrates with or without Sodium Dodecyl Sulfate. Cryst. Growth Des. 2013, 13, 2047–2059. [Google Scholar] [CrossRef]
- Cai, L.C.; Pethica, B.A.; Debenedetti, P.G.; Sundaresan, S. Formation of cyclopentane methane binary clathrate hydrate in brine solutions. Chem. Eng. Sci. 2016, 141, 125–132. [Google Scholar] [CrossRef]
- Zhang, Y.; Sheng, S.M.; Shen, X.D.; Zhou, X.B.; Wu, W.Z.; Wu, X.P.; Liang, D.Q. Phase Equilibrium of Cyclopentane plus Carbon Dioxide Binary Hydrates in Aqueous Sodium Chloride Solutions. J. Chem. Eng. Data 2017, 62, 2461–2465. [Google Scholar] [CrossRef]
- Babakhani, S.M.; Ho-Van, S.; Bouillot, B.; Douzet, J.; Herri, J.M. Phase equilibrium measurements and modelling of mixed cyclopentane and carbon dioxide hydrates in presence of salts. Chem. Eng. Sci. 2020, 214, 115442. [Google Scholar] [CrossRef]
- Nakane, R.; Gima, E.; Ohmura, R.; Senaha, I.; Yasuda, K. Phase equilibrium condition measurements in carbon dioxide hydrate forming system coexisting with sodium chloride aqueous solutions. J. Chem. Thermodyn. 2019, 130, 192–197. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Makino, T.; Sugahara, T.; Ohgaki, K. Phase equilibrium relations for binary mixed hydrate systems composed of carbon dioxide and cyclopentane derivatives. Fluid Phase Equilibr. 2014, 362, 379–382. [Google Scholar] [CrossRef]
- Klapproth, A.; Piltz, R.O.; Kennedy, S.J.; Kozielski, K.A. Kinetics of sll and Mixed sI/sII, Gas Hydrate Growth for a Methane/Propane Mixture Using Neutron Diffraction. J. Phys. Chem. C 2019, 123, 2703–2715. [Google Scholar] [CrossRef]
- Nesterov, A.N.; Reshetnikov, A.M. New combination of thermodynamic and kinetic promoters to enhance carbon dioxide hydrate formation under static conditions. Chem. Eng. J. 2019, 378, 122165. [Google Scholar] [CrossRef]
- Zhang, J.S.; Lee, J.W. Enhanced Kinetics of CO2 Hydrate Formation under Static Conditions. Ind. Eng. Chem. Res. 2009, 48, 5934–5942. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C. Clathrate Hydrates of Natural Gases; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Lv, Q.N.; Li, L.; Li, X.S.; Chen, Z.Y. Formation Kinetics of Cyclopentane plus Methane Hydrates in Brine Water Systems and Raman Spectroscopic Analysis. Energy Fuel 2015, 29, 6104–6110. [Google Scholar] [CrossRef]
- Yin, Z.Y.; Khurana, M.; Tan, H.K.; Linga, P. A review of gas hydrate growth kinetic models. Chem. Eng. J. 2018, 342, 9–29. [Google Scholar] [CrossRef]
- Guo, D.D.; Ou, W.J.; Ning, F.L.; Fang, B.; Liu, Z.C.; Fang, X.Y.; Lu, W.J.; Zhang, L.; Din, S.U.; He, Z.J. The effects of hydrate formation and dissociation on the water-oil interface: Insight into the stability of an emulsion. Fuel 2020, 266, 116980. [Google Scholar] [CrossRef]
- Ishida, Y.; Takahashi, Y.; Ohmura, R. Dynamic Behavior of Clathrate Hydrate Growth in Gas/Liquid/Liquid System. Cryst. Growth Des. 2012, 12, 3271–3277. [Google Scholar] [CrossRef]
- Mitarai, M.; Kishimoto, M.; Suh, D.; Ohmura, R. Surfactant Effects on the Crystal Growth of Clathrate Hydrate at the Interface of Water and Hydrophobic-Guest Liquid. Cryst. Growth Des. 2015, 15, 812–821. [Google Scholar] [CrossRef]


| Initial Pressure (MPa) | Temperature (K) | CP Mass Fraction | CO2 Consumption (mmol) |
|---|---|---|---|
| 1.5 ± 0.01 | 281 ± 0.1 | 0.2 | 25.6 ± 1.2 |
| 2.0 ± 0.03 | 281 ± 0.1 | 0.2 | 26.0 ± 2.1 |
| 2.5 ± 0.04 | 281 ± 0.1 | 0.2 | 30.0 ± 5.0 |
| 3.0 ± 0.02 | 281 ± 0.1 | 0.2 | 14.1 ± 3.8 |
| Initial Pressure (MPa) | Temperature (K) | CP Mass Fraction | CO2 Consumption (mmol) |
|---|---|---|---|
| 3.0 ± 0.03 | 281 ± 0.1 | 0.10 | 8.6 ± 3.7 |
| 3.0 ± 0.03 | 281 ± 0.1 | 0.15 | 10.4 ± 5.7 |
| 3.0 ± 0.05 | 281 ± 0.1 | 0.20 | 14.2 ± 5.7 |
| 3.0 ± 0.08 | 281 ± 0.1 | 0.25 | 11.9 ± 3.8 |
| 3.0 ± 0.06 | 281 ± 0.1 | 0.30 | 9.9 ± 4.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Zhang, Y.; Zang, X.; Liang, D. Formation Kinetics of the Mixed Cyclopentane—Carbon Dioxide Hydrates in Aqueous Sodium Chloride Solutions. Energies 2020, 13, 4388. https://doi.org/10.3390/en13174388
Zhou X, Zhang Y, Zang X, Liang D. Formation Kinetics of the Mixed Cyclopentane—Carbon Dioxide Hydrates in Aqueous Sodium Chloride Solutions. Energies. 2020; 13(17):4388. https://doi.org/10.3390/en13174388
Chicago/Turabian StyleZhou, Xuebing, Ye Zhang, Xiaoya Zang, and Deqing Liang. 2020. "Formation Kinetics of the Mixed Cyclopentane—Carbon Dioxide Hydrates in Aqueous Sodium Chloride Solutions" Energies 13, no. 17: 4388. https://doi.org/10.3390/en13174388






