N Evolution and Physiochemical Structure Changes in Chars during Co-Pyrolysis: Effects of Abundance of Glucose in Fiberboard
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Pyrolysis in the Fixed-Bed Reactor
2.3. Characterization of N Functional Groups in Chars
2.4. Measurements of Physiochemical Structures in Chars
3. Results and Discussion
3.1. Effects of FB:Glucose Ratio on N Contents and Retention in Chars
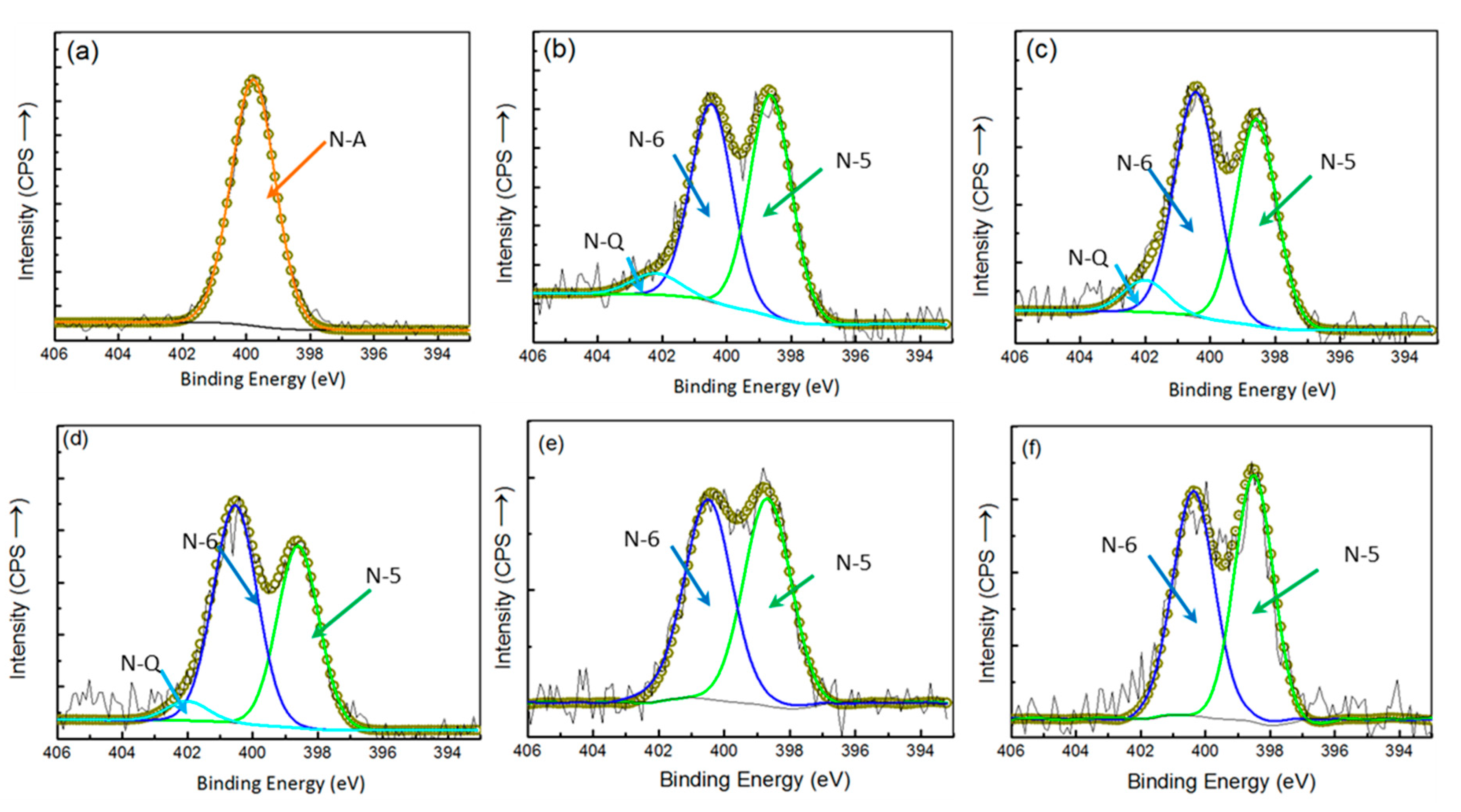
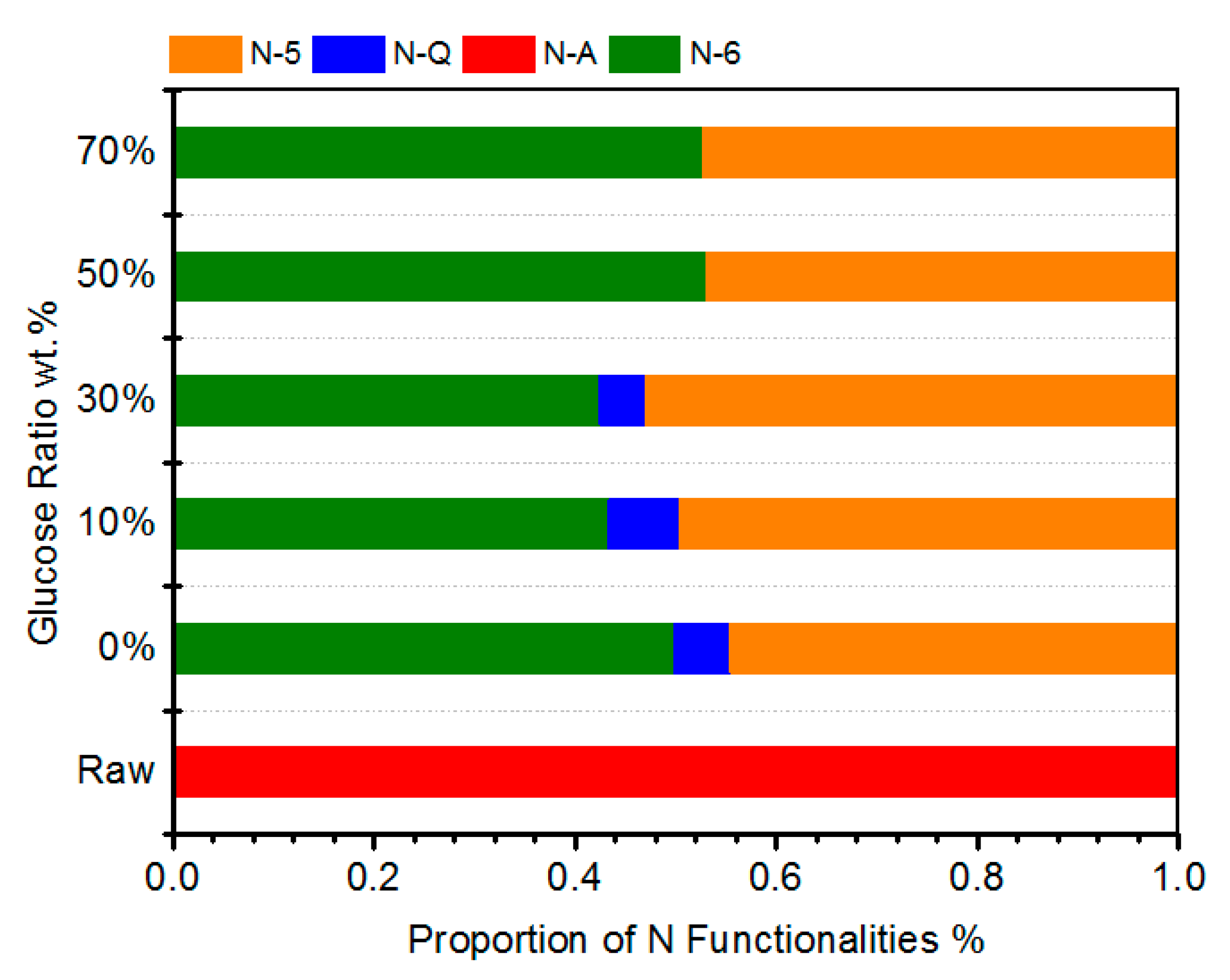
3.2. Effects of FB:Glucose Ratio on N Transformations in Chars
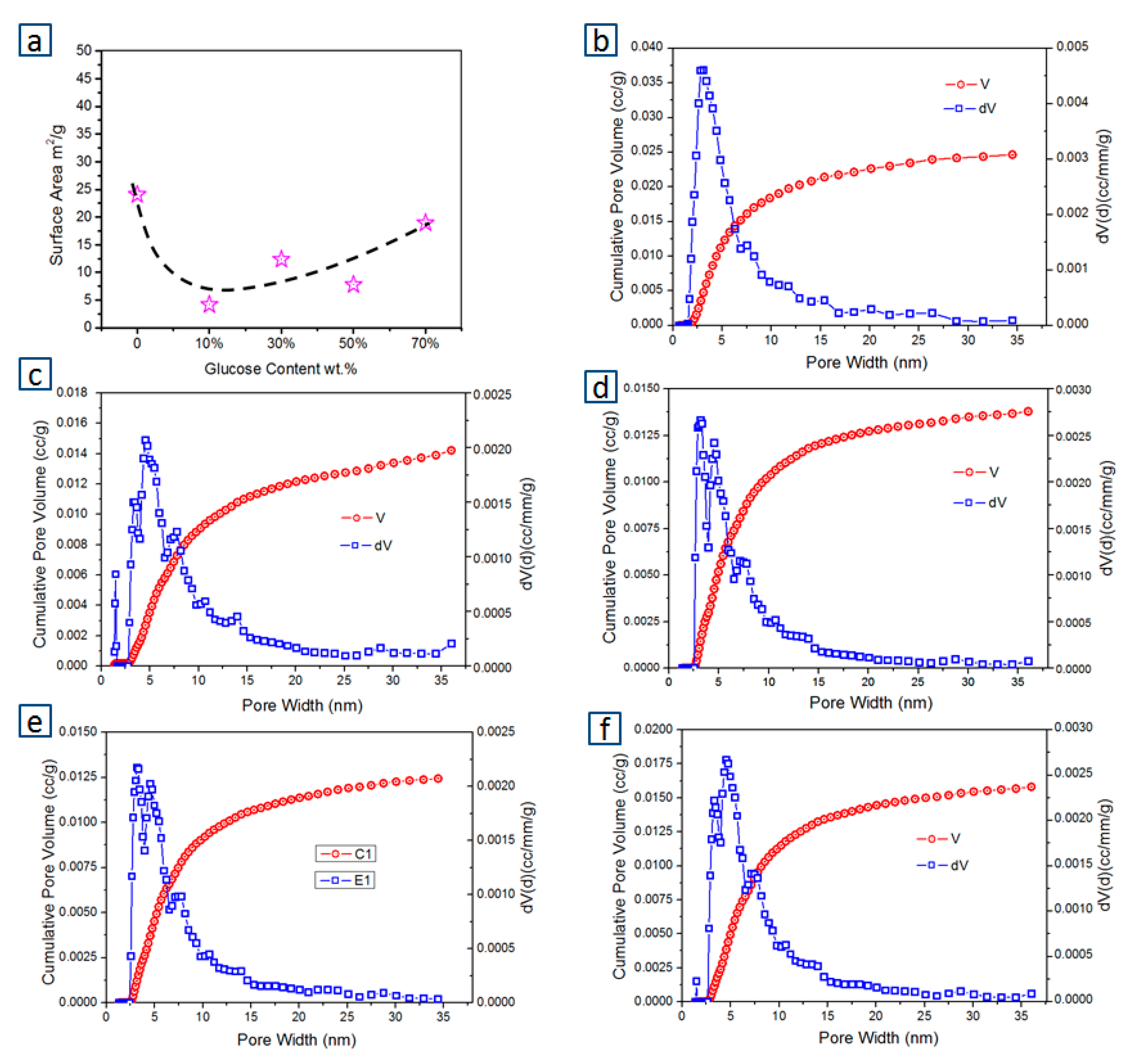
3.3. Effects of FB:Glucose Ratio on Physiochemical Property in Chars
4. Conclusions
- The increase in glucose in the feedstock has significantly increased the N fixation in chars, which was a result of both enhanced volatile–char and volatile–volatile interactions with the involvement of O-containing radicals derived from the glucose devolatilizations.
- The N-containing functional groups in chars were apparently varied with the continual increase in the addition of glucose, and mainly N-5 and N-6 were identified when over 30% glucose in the feedstock was used, indicating the deep amorphous nature of volatile-derived N-O-C compounds; thus, the N species were only located at the edges of less fused aromatic rings.
- The carbon structures revealed by Raman spectroscopy for the chars derived from the co-pyrolysis at various ratios of glucose to FB were very similar, while the porous structures of chars changed obviously.
Author Contributions
Funding
Conflicts of Interest
References
- FAOSTAT-Food and Agriculture Organistation of the United Nations. Forestry Database, Forestry Production and Trade. 2018. Available online: http://www.fao.org/faostat/en/#data/FO/visualize (accessed on 26 July 2020).
- Ferreira, S.D.; Altafini, C.R.; Perondi, D.; Godinho, M. Pyrolysis of Medium Density Fiberboard (MDF) wastes in a screw reactor. Energy Convers. Manag. 2015, 92, 223–233. [Google Scholar] [CrossRef]
- Xu, D.; Yang, L.; Zhao, M.; Guo, M.; Hu, X.; Gholizadeh, M.; Zhang, H.; Zhang, S. Effects of glucose on nitrogen retention and transformation during copyrolysis with fiberboard waste. Energy Fuels 2020, 34, 11083–11090. [Google Scholar] [CrossRef]
- Zhuang, X.; Huang, Y.; Song, Y.; Zhan, H.; Yin, X.; Wu, C. The transformation pathways of nitrogen in sewage sludge during hydrothermal treatment. Bioresour. Technol. 2017, 245, 463–470. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Z.; Qiao, Y.; Wang, B.; Xu, M. Transformation of nitrogen during hydrothermal carbonization of sewage sludge: Effects of temperature and Na/Ca acetates addition. Proc. Combust. Inst. 2020, in press. [Google Scholar] [CrossRef]
- Cheng, S.; Qiao, Y.; Huang, J.; Wang, W.; Wang, Z.; Yu, Y.; Xu, M. Effects of Ca and Na acetates on nitrogen transformation during sewage sludge pyrolysis. Proc. Combust. Inst. 2019, 37, 2715–2722. [Google Scholar] [CrossRef]
- Zhan, H.; Zhuang, X.; Song, Y.; Huang, Y.; Liu, H.; Yin, X.; Wu, C. Evolution of nitrogen functionalities in relation to NOx precursors during low-temperature pyrolysis of biowastes. Fuel 2018, 218, 325–334. [Google Scholar] [CrossRef]
- Li, K.; Zhang, L.; Zhu, L.; Zhu, X. Comparative study on pyrolysis of lignocellulosic and algal biomass using pyrolysis-gas chromatography/mass spectrometry. Bioresour. Technol. 2017, 234, 48–52. [Google Scholar] [CrossRef]
- Chen, W.; Yang, H.; Chen, Y.; Chen, X.; Fang, Y.; Chen, H. Biomass pyrolysis for nitrogen-containing liquid chemicals and nitrogen-doped carbon materials. J. Anal. Appl. Pyrol. 2016, 120, 186–193. [Google Scholar] [CrossRef]
- Mayer, F.M.; Teixeira, C.M.; Pacheco, J.G.A.; de Souza, C.T.; Bauer, D.V.; Caramão, E.B.; Espíndola, J.S.; Trierweiler, J.O.; Perez-Lopez, O.W.; Zini, C.A. Characterization of analytical fast pyrolysis vapors of Medium-Density Fiberboard (MDF) using metal-modified HZSM-5. J. Anal. Appl. Pyrol. 2018, 136, 87–95. [Google Scholar] [CrossRef]
- Nakai, T.; Kartal, S.N.; Hata, T.; Imamura, Y. Chemical characterization of pyrolysis liquids of wood-based composites and evaluation of their bio-efficiency. Build. Sci. 2007, 42, 1236–1241. [Google Scholar] [CrossRef]
- Chen, H.; Xie, Y.; Chen, W.; Xia, M.; Li, K.; Chen, Z.; Chen, Y.; Yang, H. Investigation on co-pyrolysis of lignocellulosic biomass and amino acids using TG-FTIR and Py-GC/MS. Energy Convers. Manag. 2019, 196, 320–329. [Google Scholar] [CrossRef]
- Xu, D.; Gao, Y.; Lin, Z.; Gao, W.; Zhang, H.; Karnowo, K.; Hu, X.; Sun, H.; Syedhassan, S.S.A.; Zhang, S. Application of biochar derived from pyrolysis of waste fiberboard on tetracycline adsorption in aqueous solution. Front. Chem. 2020, 7, 943. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Deng, Y.; Li, X.; Wang, L. Promising nitrogen-rich porous carbons derived from one-step calcium chloride activation of biomass-based waste for high performance supercapacitors. ACS Sustain.Chem. Eng. 2016, 4, 177–187. [Google Scholar] [CrossRef]
- Yuan, S.; Tan, Z.; Huang, Q. Migration and transformation mechanism of nitrogen in the biomass–biochar–plant transport process. Renew. Sustain. Energy Rev. 2018, 85, 1–13. [Google Scholar] [CrossRef]
- Chen, W.; Fang, Y.; Li, K.; Chen, Z.; Xia, M.; Gong, M.; Chen, Y.; Yang, H.; Tu, X.; Chen, H. Bamboo wastes catalytic pyrolysis with N-doped biochar catalyst for phenols products. Appl. Energy 2020, 260, 114242. [Google Scholar] [CrossRef]
- Leng, L.; Xu, S.; Liu, R.; Yu, T.; Zhuo, X.; Leng, S.; Xiong, Q.; Huang, H. Nitrogen containing functional groups of biochar: An overview. Bioresour. Technol. 2020, 298, 122286. [Google Scholar] [CrossRef]
- Darvell, L.I.; Brindley, C.; Baxter, X.C.; Jones, J.M.; Williams, A. Nitrogen in biomass char and its fate during combustion: A model compound approach. Energy Fuels 2012, 26, 6482–6491. [Google Scholar] [CrossRef]
- Liu, W.; Li, W.; Jiang, H.; Yu, H. Fates of chemical elements in biomass during its pyrolysis. Chem. Rev. 2017, 117, 6367–6398. [Google Scholar] [CrossRef]
- Wei, F.; Cao, J.; Zhao, X.; Ren, J.; Wang, J.; Fan, X.; Wei, X. Nitrogen evolution during fast pyrolysis of sewage sludge under inert and reductive atmospheres. Energy Fuels 2017, 31, 7191–7196. [Google Scholar] [CrossRef]
- Chen, W.; Yang, H.; Chen, Y.; Xia, M.; Chen, X.; Chen, H. Transformation of Nitrogen and evolution of N-containing species during algae pyrolysis. Environ. Sci. Technol. 2017, 51, 6570–6579. [Google Scholar] [CrossRef]
- Zhang, X.; Zhan, H.; Yin, X.; Wu, C. Release characteristic of NOx precursors during the pyrolysis of nitrogen-rich biomass. J. Fuel Chem. Technol. 2016, 44, 1464–1472. [Google Scholar]
- Liu, X.; Luo, Z.; Yu, C.; Xie, G. Conversion mechanism of fuel-N during pyrolysis of biomass wastes. Fuel 2019, 246, 42–50. [Google Scholar] [CrossRef]
- Gao, P.; Guo, D.; Liang, C.; Liu, G.; Yang, S. Nitrogen conversion during the rapid pyrolysis of raw/torrefied wheat straw. Fuel 2020, 259, 116227. [Google Scholar] [CrossRef]
- Wei, F.; Cao, J.; Zhao, X.; Ren, J.; Gu, B.; Wei, X. Formation of aromatics and removal of nitrogen in catalytic fast pyrolysis of sewage sludge: A study of sewage sludge and model amino acids. Fuel 2018, 218, 148–154. [Google Scholar] [CrossRef]
- Li, W.; Zhao, Y.; Yao, C.; Lu, J.; Li, R.; Wu, Y. Migration and transformation of nitrogen during hydrothermal liquefaction of penicillin sludge. J. Supercrit. Fluids 2020, 157, 104714. [Google Scholar] [CrossRef]
- Sebestyen, Z.; Jakab, E.; Doman, A.; Bokrossy, P.; Bertoti, I.; Madarasz, J.; Laszlo, K. Thermal degradation of crab shell biomass, a nitrogen-containing carbon precursor. J. Therm. Anal. Calorim. 2020, 142, 301–308. [Google Scholar] [CrossRef] [Green Version]
- Zhan, H.; Zhuang, X.; Song, Y.; Liu, J.; Li, S.; Chang, G.; Yin, X.; Wu, C.; Wang, X. A review on evolution of nitrogen-containing species during selective pyrolysis of waste wood-based panels. Fuel 2019, 253, 1214–1228. [Google Scholar] [CrossRef]
- Becidan, M.; Skreiberg, Ø.; Hustad, J.E. NOx and N2O precursors (NH3 and HCN) in pyrolysis of biomass residues. Energy Fuels 2007, 21, 1173–1180. [Google Scholar] [CrossRef]
- Li, C.; Tan, L. Formation of NOx and SOx precursors during the pyrolysis of coal and biomass. Part III. Further discussion on the formation of HCN and NH3 during pyrolysis. Fuel 2000, 79, 1899–1906. [Google Scholar] [CrossRef]
- Zhuang, X.; Song, Y.; Wang, X.; Zhan, H.; Yin, X.; Wu, C.; Wang, P. Pyrolysis of hydrothermally pretreated biowastes: The controllability on the formation of NOx precursors. Chem. Eng. J. 2020, 393, 124727. [Google Scholar] [CrossRef]
- Liu, X.; Luo, Z.; Yu, C.; Jin, B.; Tu, H. Release mechanism of fuel-N into NOx and N2O precursors during pyrolysis of rice straw. Energies 2018, 11, 520. [Google Scholar] [CrossRef] [Green Version]
- Zheng, A.; Li, L.; Tippayawong, N.; Huang, Z.; Zhao, K.; Wei, G.; Zhao, Z.; Li, H. Reducing emission of NOx and SOx precursors while enhancing char production from pyrolysis of sewage sludge by torrefaction pretreatment. Energy 2020, 192, 116620. [Google Scholar] [CrossRef]
- Zhan, H.; Zhuang, X.; Song, Y.; Yin, X.; Cao, J.; Shen, Z.; Wu, C. Step pyrolysis of N-rich industrial biowastes: Regulatory mechanism of NOx precursor formation via exploring decisive reaction pathways. Chem. Eng. J. 2018, 344, 320–331. [Google Scholar] [CrossRef]
- Girods, P.; Dufour, A.; Rogaume, Y.; Rogaume, C.; Zoulalian, A. Comparison of gasification and pyrolysis of thermal pre-treated wood board waste. J. Anal. Appl. Pyrol. 2009, 85, 171–183. [Google Scholar] [CrossRef]
- Girods, P.; Dufour, A.; Rogaume, Y.; Rogaume, C.; Zoulalian, A. Thermal removal of nitrogen species from wood waste containing urea formaldehyde and melamine formaldehyde resins. J. Hazard. Mater. 2008, 159, 210–221. [Google Scholar] [CrossRef]
- Ma, Z.; Zhang, Y.; Li, C.; Yang, Y.; Zhang, W.; Zhao, C.; Wang, S. N-doping of biomass by ammonia (NH3) torrefaction pretreatment for the production of renewable N-containing chemicals by fast pyrolysis. Bioresour. Technol. 2019, 292, 122034. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.; Bandosz, T.J. Combined effect of nitrogen- and oxygen-containing functional groups of microporous activated carbon on its electrochemical performance in supercapacitors. Adv. Funct. Mater. 2009, 19, 438–447. [Google Scholar] [CrossRef]
- Hulicova-Jurcakova, D.; Seredych, M.; Lu, G.; Kodiweera, N.K.A.C.; Stallworth, P.E.; Greenbaum, S.; Bandosz, T.J. Effect of surface phosphorus functionalities of activated carbons containing oxygen and nitrogen on electrochemical capacitance. Carbon 2009, 47, 1576–1584. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xing, B.; Wang, X.; Wang, K.; Zhu, L.; Wang, S. Nitrogen-doped hierarchical porous biochar derived from corn stalks for phenol-enhanced adsorption. Energy Fuels 2019, 33, 12459–12468. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Yang, H.; Xia, M.; Li, K.; Chen, X.; Chen, H. Co-pyrolysis of lignocellulosic biomass and microalgae: Products characteristics and interaction effect. Bioresour. Technol. 2017, 245, 860–868. [Google Scholar] [CrossRef]
- Chen, W.; Yang, H.; Chen, Y.; Li, K.; Xia, M.; Chen, H. Influence of biochar addition on nitrogen transformation during copyrolysis of algae and lignocellulosic biomass. Environ. Sci. Technol. 2018, 52, 9514–9521. [Google Scholar] [CrossRef] [PubMed]
- Falco, C.; Sevilla, M.; White, R.J.; Rothe, R.; Titirici, M.M. Renewable nitrogen-doped hydrothermal carbons derived from microalgae. ChemSusChem 2012, 5, 1834–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jing, H.; Kitts, D.D. Chemical characterization of different sugar-casein Maillard reaction products and protective effects on chemical-induced cytotoxicity of Caco-2 cells. Food Chem. Toxicol. 2004, 42, 1833–1844. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Xu, P.; Qiu, Y.; Yu, Q.; Ma, J.; Wu, H.; Luo, G.; Xu, M.; Yao, H. Increasing oxygen functional groups of activated carbon with non-thermal plasma to enhance mercury removal efficiency for flue gases. Chem. Eng. J. 2015, 263, 1–8. [Google Scholar] [CrossRef]
- Chen, W.; Chen, Y.; Yang, H.; Li, K.; Chen, X.; Chen, H. Investigation on biomass nitrogen-enriched pyrolysis: Influence of temperature. Bioresour. Technol. 2018, 249, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Xu, M.; Liu, X.; Zhan, Z.; Li, Z.; Yao, H. Effect of cellulose, lignin, alkali and alkaline earth metallic species on biomass pyrolysis and gasification. Fuel Process. Technol. 2010, 91, 903–909. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, Z.; Zhang, H.; Wang, Y.; Xu, X.; Cheng, L.; Zhang, Y. The catalytic reforming of tar from pyrolysis and gasification of brown coal: Effects of parental carbon materials on the performance of char catalysts. Fuel Process. Technol. 2018, 174, 142–148. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, S.; Wu, Y.; Zhu, X.; Xu, Z.; Li, B.; Hu, X.; Sun, H.; Zhou, J.; Zhang, S. Volatile-char interactions during biomass pyrolysis: Contribution of amino group on graphitized carbon nanotube to xylose evolution based on experimental and theoretical studies. Fuel 2020, 282, 118921. [Google Scholar] [CrossRef]
- Zhang, S.; Min, Z.; Tay, H.L.; Asadullah, M.; Li, C.Z. Effects of volatile–char interactions on the evolution of char structure during the gasification of Victorian brown coal in steam. Fuel 2011, 90, 1529–1535. [Google Scholar] [CrossRef]
- Wang, B.; Huang, J.; Gao, X.; Qiao, Y. Effects of Secondary Vapor-Phase Reactions on the Distribution of Chlorine Released from the Pyrolysis of KCl-Loaded Wood. Energy Fuels 2020, 34, 11717–11721. [Google Scholar] [CrossRef]
- Hirata, T.; Kawamoto, S.; Okuro, A. Pyrolysis of melamine–formaldehyde and urea–formaldehyde resins. J. Appl. Polym. Sci. 1991, 42, 3147–3163. [Google Scholar] [CrossRef]
- Maliutina, K.; Tahmasebi, A.; Yu, J. The transformation of nitrogen during pressurized entrained-flow pyrolysis of Chlorella vulgaris. Bioresour. Technol. 2018, 262, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wen, L.; Yang, T.; Zhang, N. Nitrogen Transformation during Sewage Sludge Pyrolysis. Energ. Fuel. 2015, 29, 5088–5094. [Google Scholar] [CrossRef]
- Yu, W.; Lian, F.; Cui, G.; Liu, Z. N-doping effectively enhances the adsorption capacity of biochar for heavy metal ions from aqueous solution. Chemosphere 2018, 193, 8–16. [Google Scholar] [CrossRef]
- Ding, T.; Zhao, J.; Zhu, N.; Wang, C. A comparative study of morphological characteristics of medium-density fiberboard dust by sieve and image analyses. J. Wood Sci. 2020, 66, 1–9. [Google Scholar] [CrossRef]
- Smith, M.W.; Pecha, B.; Helms, G.; Scudiero, L.; Garciaperez, M. Chemical and morphological evaluation of chars produced from primary biomass constituents: Cellulose, xylan, and lignin. Biomass Bioenergy 2017, 104, 17–35. [Google Scholar] [CrossRef]
- Guizani, C.; Haddad, K.; Limousy, L.; Jeguirim, M. New insights on the structural evolution of biomass char upon pyrolysis as revealed by the Raman spectroscopy and elemental analysis. Carbon 2017, 119, 519–521. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Yang, L.; Zhao, M.; Song, Y.; Karnowo; Zhang, H.; Hu, X.; Sun, H.; Zhang, S. N Evolution and Physiochemical Structure Changes in Chars during Co-Pyrolysis: Effects of Abundance of Glucose in Fiberboard. Energies 2020, 13, 5105. https://doi.org/10.3390/en13195105
Xu D, Yang L, Zhao M, Song Y, Karnowo, Zhang H, Hu X, Sun H, Zhang S. N Evolution and Physiochemical Structure Changes in Chars during Co-Pyrolysis: Effects of Abundance of Glucose in Fiberboard. Energies. 2020; 13(19):5105. https://doi.org/10.3390/en13195105
Chicago/Turabian StyleXu, Deliang, Liu Yang, Ming Zhao, Yu Song, Karnowo, Hong Zhang, Xun Hu, Hongqi Sun, and Shu Zhang. 2020. "N Evolution and Physiochemical Structure Changes in Chars during Co-Pyrolysis: Effects of Abundance of Glucose in Fiberboard" Energies 13, no. 19: 5105. https://doi.org/10.3390/en13195105






