Improvement of the Pseudocapacitive Performance of Cobalt Oxide-Based Electrodes for Electrochemical Capacitors
Abstract
:1. Introduction
2. Experiment
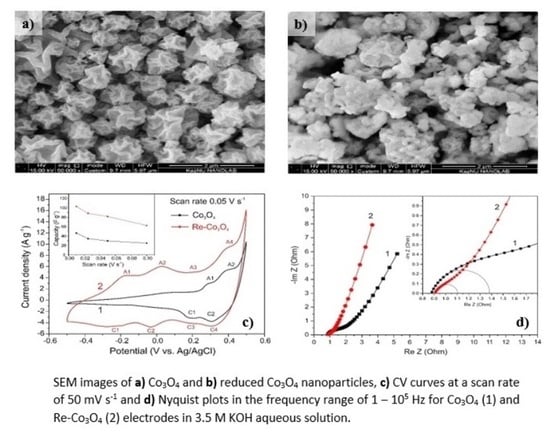
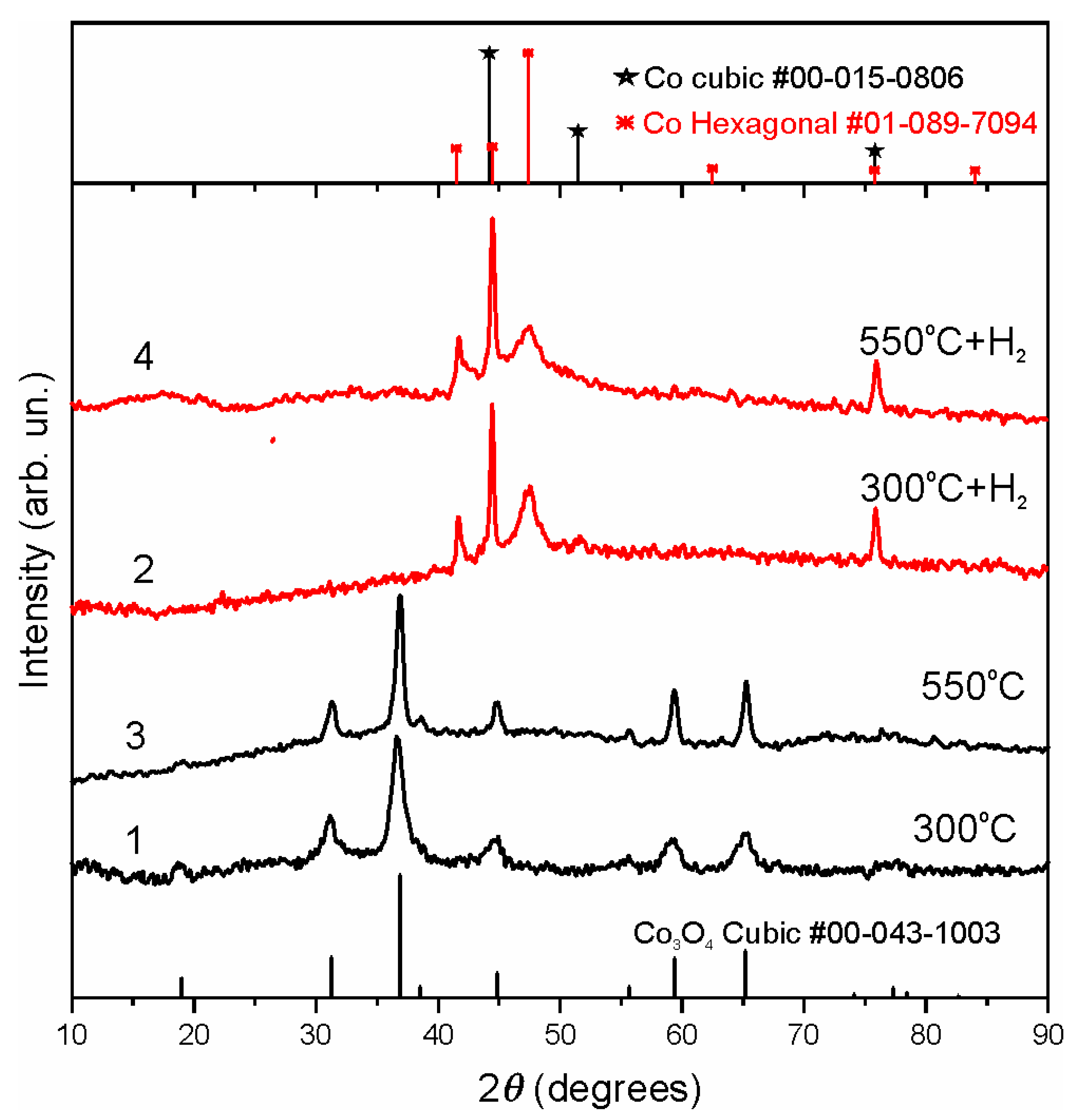
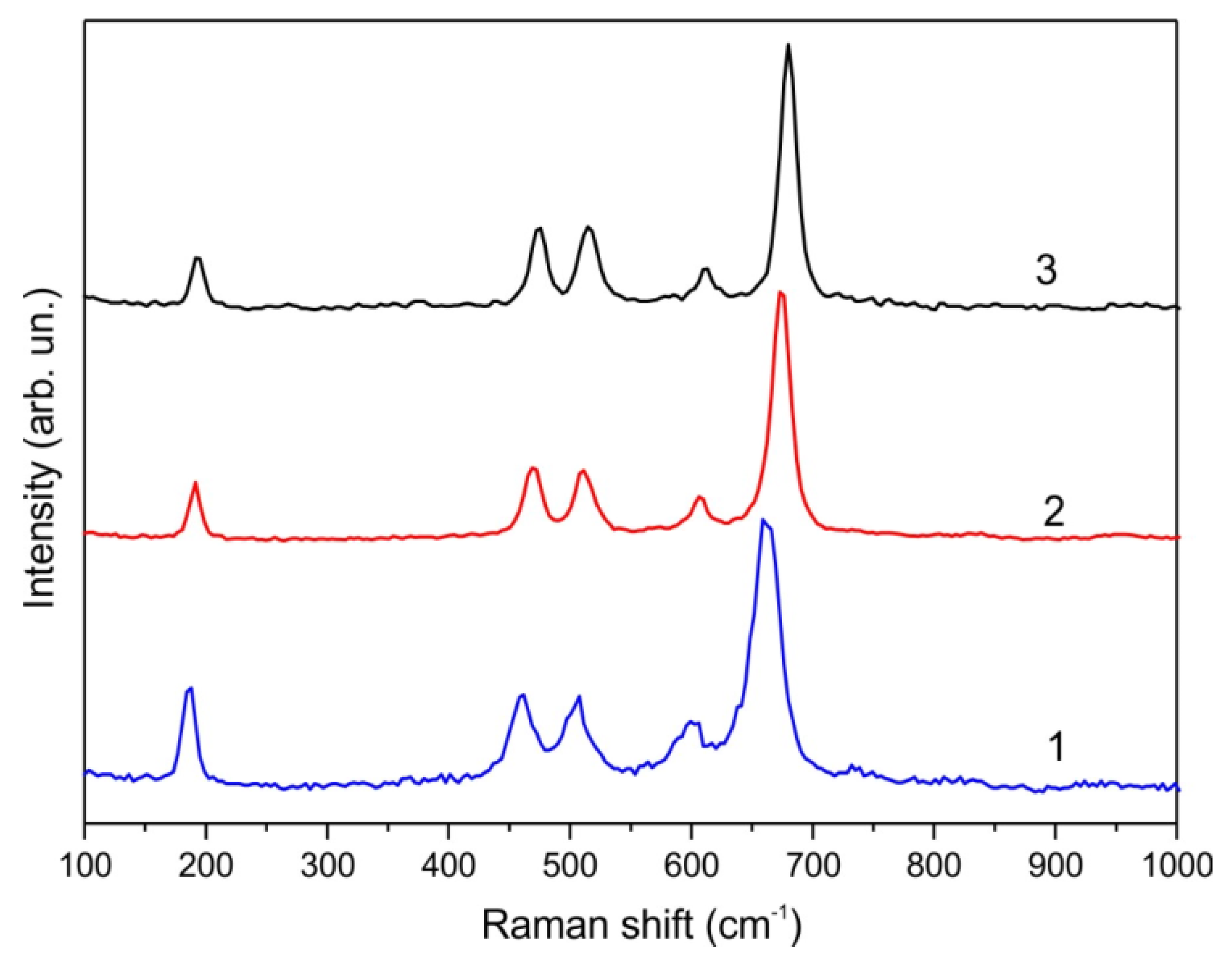
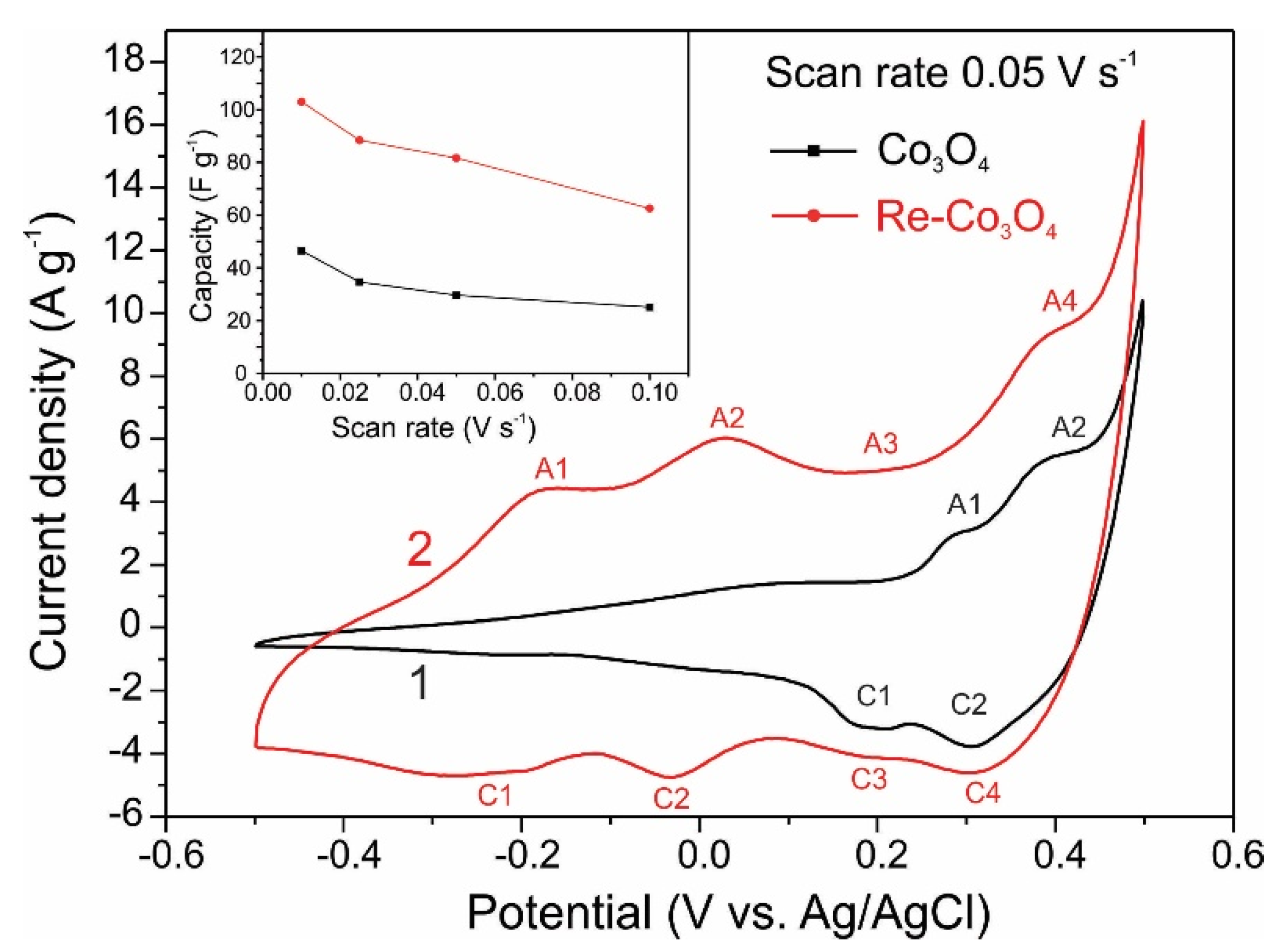
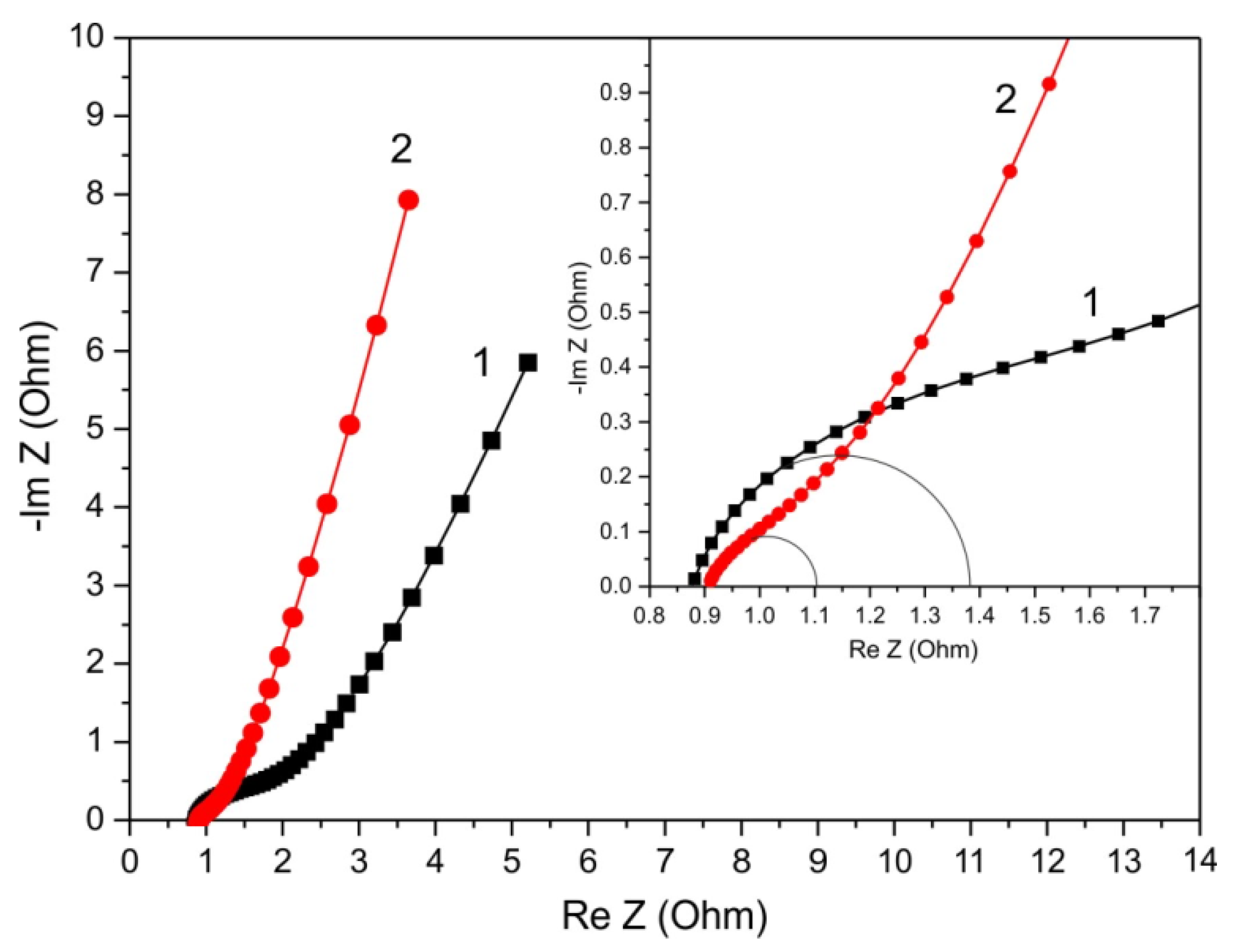
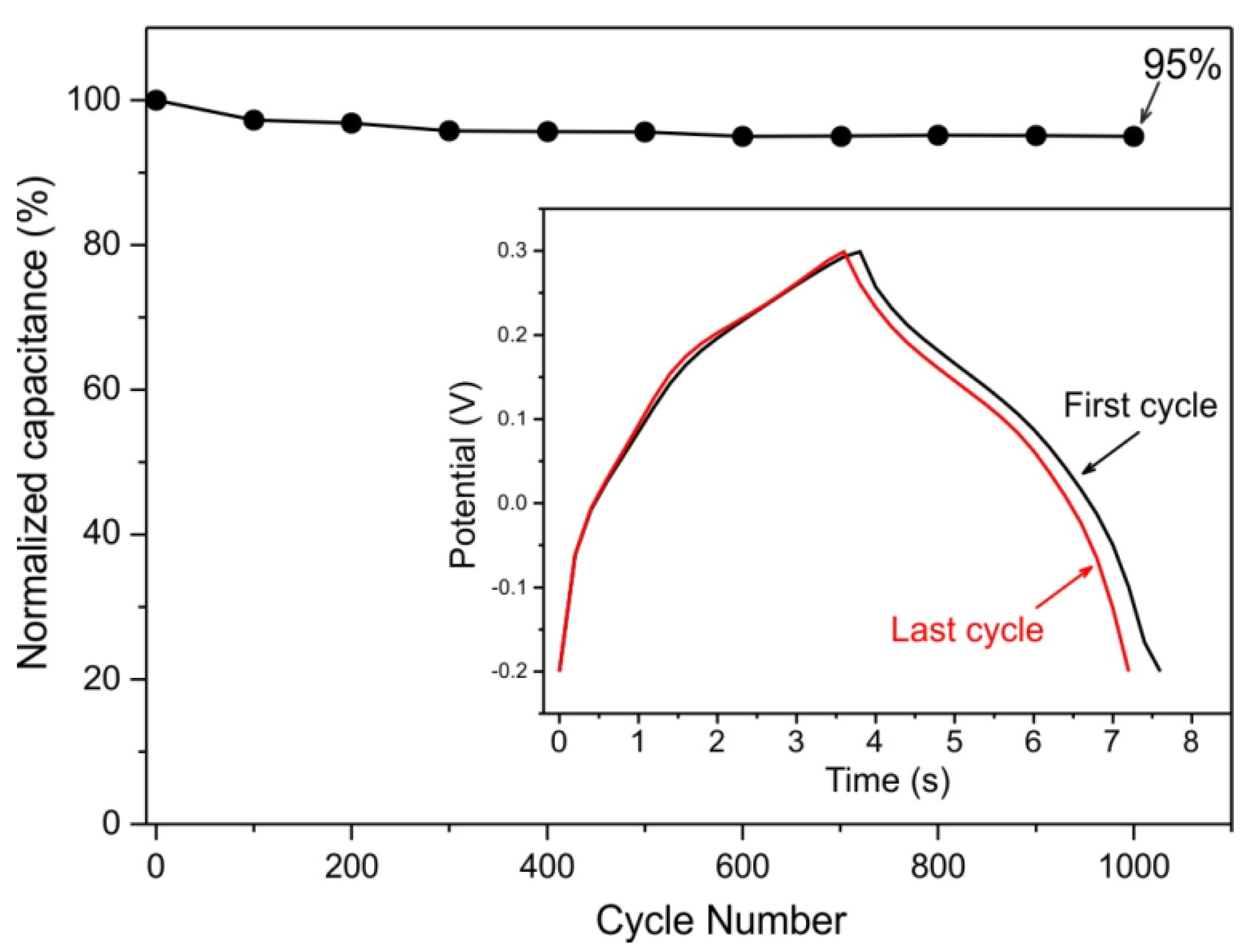
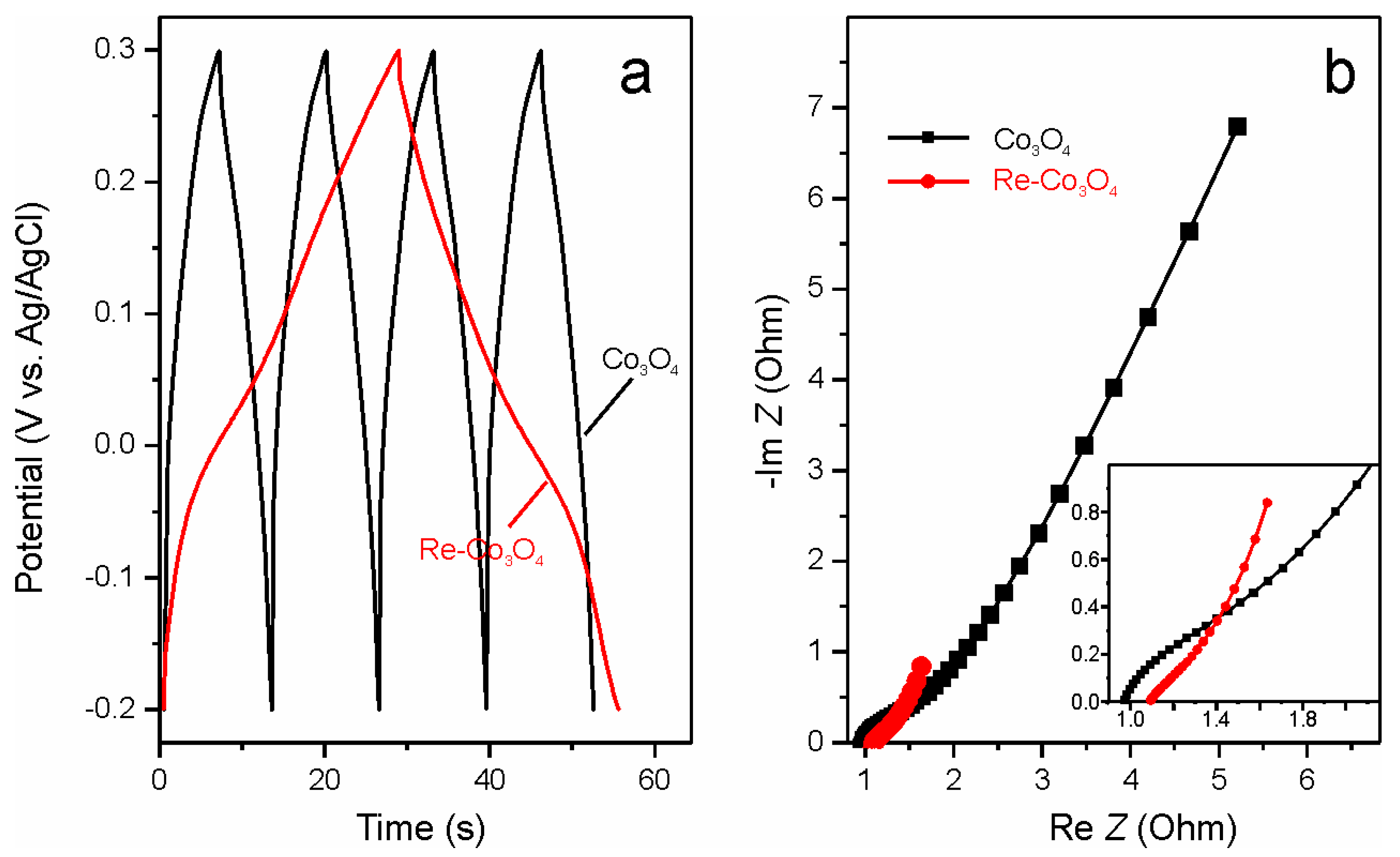
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mirzaeian, M.; Abbas, Q.; Ogwu, A.; Hall, P.J.; Goldin, M.; Mirzaeian, M.; Jirandehi, H.F. Electrode and electrolyte materials for electrochemical capacitors. Int. J. Hydrogen Energy 2007, 42, 25565–25587. [Google Scholar] [CrossRef] [Green Version]
- Mirzaeian, M.; Hall, P.J. High capacity carbon based electrodes for lithium/oxygen batteries. J. Power Syst. Technol. 2007, 31, 90–96. [Google Scholar]
- Da, Y.; Liu, J.; Zhou, L.; Zhu, X.; Chen, X.; Fu, L. Engineering 2D Architectures toward high-performance micro-supercapacitors. Adv. Mater. 2019, 31, 1802793. [Google Scholar] [CrossRef] [Green Version]
- Yu, A.; Chen, Z.; Maric, R.; Zhang, L.; Zhang, J.; Yan, J. Electrochemical supercapacitors for energy storage and delivery-Advanced materials, technologies and applications. Appl. Energy 2015, 153, 1–2. [Google Scholar] [CrossRef]
- González, A.; Goikolea, E.; Barrena, J.A.; Mysyk, R. Review on supercapacitors: Technologies and materials. Renew. Sustain. Energy Rev. 2016, 58, 1189–1206. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, Y.X.; Li, F.; Zhang, Y.; Wen, Z.; Liu, Q. Facile synthesis of hierarchical Co3O4@MnO2 core–shell arrays on Ni foam for asymmetric supercapacitors. J. Power Sources 2014, 252, 98–106. [Google Scholar] [CrossRef]
- Lokhande, C.D.; Dubal, D.P.; Joo, O.-S. Metal oxide thin film based supercapacitors. Curr. Appl. Phys. 2011, 11, 255–270. [Google Scholar] [CrossRef]
- Li, H.; Wang, R.; Cao, R. Physical and electrochemical characterization of hydrous ruthenium oxide/ordered mesoporous carbon composites as supercapacitor. Microporous Mesoporous Mater. 2008, 111, 32–38. [Google Scholar] [CrossRef]
- Wu, M.; Gao, J.; Zhang, S.; Chen, A. Synthesis and characterization of aerogel-like mesoporous nickel oxide for electrochemical supercapacitors. J. Porous Mater. 2006, 13, 407–412. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhang, J. A review of electrode materials for electrochemical supercapacitors. Chem. Soc. Rev. 2012, 41, 797–828. [Google Scholar] [CrossRef] [Green Version]
- Rahmanifar, M.S.; Hemmati, M.; Noori, A.; El-Kady, M.F.; Mousavi, M.F.; Kaner, R.B. Asymmetric supercapacitors: An alternative to activated carbon negative electrodes based on earth abundant elements. Mater. Today Energy 2019, 12, 26–36. [Google Scholar] [CrossRef]
- Oje, A.I.; Ogwu, A.; Mirzaeian, M.; Oje, A.; Tsendzughul, N. Silver thin film electrodes for supercapacitor application. Appl. Surf. Sci. 2019, 488, 142–150. [Google Scholar] [CrossRef]
- Choudhary, N.; Li, C.; Moore, J.; Nagaiah, N.; Zhai, L.; Jung, Y.; Thomas, J. Supercapacitors: Asymmetric Supercapacitor Electrodes and Devices. Adv. Mater. 2017, 29, 1605336. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Ho, K.-C.; Kuo, S.-L.; Wu, N.-L. Investigation on capacitance mechanisms of Fe3O4 electrochemical capacitors. J. Electrochem. Soc. 2006, 153, A75–A80. [Google Scholar] [CrossRef]
- Patil, U.M.; Salunkhe, R.R.; Gurav, K.V.; Lokhande, C.D. Chemically deposited nanocrystalline NiO thin films for supercapacitor application. Appl. Surf. Sci. 2008, 255, 2603–2607. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, F.; Zhang, W.; Mei, Z.; Pei, B.; Zhu, X. Controllable preparation of multishelled NiO hollow nanospheres via layer-by-layer self-assembly for supercapacitor application. J. Power Sources 2014, 246, 24–31. [Google Scholar] [CrossRef]
- Kandalkar, S.G.; Lokhande, C.D.; Mane, R.S.; Han, S.-H. A non-thermal chemical synthesis of hydrophilic and amorphous cobalt oxide films for supercapacitor application. Appl. Surf. Sci. 2007, 253, 3952–3956. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, A.; Zhu, Q.; Nie, Z.; Zhang, Y.; Tang, Y.; Liang, S.; Cao, G. Facile synthesis of nanorod-assembled multi-shelled Co3O4 hollow microspheres for high-performance supercapacitors. J. Power Sources 2014, 272, 107–112. [Google Scholar] [CrossRef]
- Oje, A.I.; Ogwu, A.; Mirzaeian, M.; Tsendzughul, N. Electrochemical energy storage of silver and silver oxide thin films in an aqueous NaCl electrolyte. J. Electroanal. Chem. 2018, 829, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.-Y.; Zhang, Y.; Zhang, W.; Zheng, H.; Su, G. Charge−discharge process of MnO2 supercapacitor. Trans. Nonferrous Met. Soc. China 2007, 17, 649–653. [Google Scholar] [CrossRef]
- Pendashteh, A.; Mousavi, M.F.; Rahmanifar, M. Fabrication of anchored copper oxide nanoparticles on graphene oxide nanosheets via an electrostatic coprecipitation and its application as supercapacitor. Electrochim. Acta 2013, 88, 347357. [Google Scholar] [CrossRef]
- Patake, V.; Lokhande, C.; Joo, O.S. Electrodeposited ruthenium oxide thin films for supercapacitor effect of surface treatments. Appl. Surf. Sci. 2009, 255, 4192–4196. [Google Scholar] [CrossRef]
- Wang, P.; Liu, H.; Xu, Y.; Chen, Y.; Yang, J.; Tan, Q. Supported ultrafine ruthenium oxides with specific capacitance up to 1099 F g-1 for a supercapacitor. Electrochim. Acta 2016, 194, 211–218. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, J.; Wang, T.; Shao, J.; Wang, D.; Yang, Y. Mesoporous transition metal oxides for supercapacitors. Nanomaterials 2015, 5, 1667–1689. [Google Scholar] [CrossRef] [PubMed]
- Jayalakshmi, M.; Rao, M.M.; Venugopal, N.; Kim, K.-B. Hydrothermal synthesis of SnO2-V2O5 mixed oxide and electrochemical screening of carbon nano-tubes (CNT), V2O5, V2O5-CNT, and SnO2-V2O5-CNT electrodes for supercapacitor applications. J. Power Sources 2007, 166, 578–583. [Google Scholar] [CrossRef]
- Li, X.; Xiong, S.; Li, J.; Bai, J.; Qian, Y. Mesoporous NiO ultrathin nanowire networks topotactically transformed from a-Ni(OH)2 hierarchical microspheres and their superior electrochemical capacitance properties and excellent capability for water treatment. J. Mater. Chem. 2012, 22, 14276–14283. [Google Scholar] [CrossRef]
- Pavasupree, S.; Suzuki, Y.; Kitiyanan, A.; Pivsa-Art, S.; Yoshikawa, S. Synthesis and characterization of vanadium oxides nanorods. J. Solid State Chem. 2005, 178, 2152–2158. [Google Scholar] [CrossRef]
- Jia, C.J.; Sun, L.D.; Yan, Z.G.; You, L.P.; Luo, F.; Han, X.D.; Pang, Y.C.; Zhang, Z.; Yan, C.H. Single-crystalline iron oxide nanotubes. Angew. Chem. 2005, 44, 4328–4333. [Google Scholar] [CrossRef]
- Cao, J.; Wang, Y.; Ma, T.; Liu, Y.; Yuan, Z. Synthesis of porous hematite nanorods loaded with CuO nanocrystals as catalysts for CO oxidation. J. Nat. Gas Chem. 2011, 20, 669–676. [Google Scholar] [CrossRef]
- Liu, F.; Yuan, W.; Li, T.; Zhang, Z. Synthesis and electrochemical performance of mesoporous nickel oxide using mixed surfactant template. Mater. Res. Innov. 2015, 19, 70–75. [Google Scholar] [CrossRef]
- Wang, Y.; Chang, B.; Guan, D.; Pei, K.; Chen, Z.; Yang, M.; Dong, X. Preparation of nanospherical porous NiO by a hard template route and its supercapacitor application. Mater. Lett. 2014, 135, 172–175. [Google Scholar] [CrossRef]
- Kim, S.-I.; Lee, J.-S.; Ahn, H.-J.; Song, H.-K.; Jang, J.-H. Facile route to an efficient NiO supercapacitor with a three-dimensional nanonetwork morphology. ACS Appl. Mater. Interfaces 2013, 5, 1596–1603. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.; Lee, H.; Ahn, J.-P.; Park, Y. Sol–Gel Mediated Synthesis of Fe2O3 Nanorods. Adv. Mat. 2003, 15, 1761–1764. [Google Scholar] [CrossRef]
- Jagtap, S.V.; Tale, A.S.; Thakre, S.D. Synthesis by sol gel method and characterization of Co3O4 nanoparticles. Int. J. Res. Eng. Appl. Sci. 2017, 7, 1–6. [Google Scholar]
- Huang, Y.-Y.; Lin, L.-Y. Synthesis of ternary metal oxides for battery-supercapacitor hybrid devices: Influences of metal species on redox reaction and electrical conductivity. ACS Appl. Energy Mater. 2018, 1, 2979–2990. [Google Scholar] [CrossRef]
- Kou, T.; Yao, B.; Liu, T.; Li, Y. Recent advances in chemical methods for activating carbon and metal oxide based electrodes for supercapacitors. J. Mater. Chem. A 2017, 5, 17151. [Google Scholar] [CrossRef]
- Kate, R.S.; Khalate, S.A.; Deokate, R.J. Overview of nanostructured metal oxides and pure nickel oxide (NiO) electrodes for supercapacitors: A review. J. Alloy. Compd. 2018, 734, 89–111. [Google Scholar] [CrossRef]
- Tao, L.; Shengjun, L.; Bowen, Z.; Bei, W.; Dayong, N.; Zeng, C.; Ying, Y.; Ning, W.; Zhang, W.-F. Supercapacitor electrode with a homogeneously Co3O4-coated multiwalled carbon nanotube for a high capacitance. Nanoscale Res. Lett. 2015, 10, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pawar, S.M.; Kim, J.; Inamdar, A.I.; Woo, H.; Jo, Y.; Pawar, B.S.; Cho, S.; Kim, H.; Im, H. Multi-functional reactively-sputtered copper oxide electrodes for supercapacitor and electro-catalyst in direct methanol fuel cell applications. Sci. Rep. 2016, 6, 21310. [Google Scholar] [CrossRef] [Green Version]
- Montemor, M.F.; Eugénio, S.; Tuyen, N.; Silva, R.P.; Silva, T.M.; Carmezim, M.J. Nanostructured transition metal oxides produced by electrodeposition for application as redox electrodes for supercapacitors. In Handbook of Nanoelectrochemistry; Springer: Berlin/Heidelberg, Germany, 2016; pp. 681–714. [Google Scholar]
- Abbas, Q.; Mirzaeian, M.; Ogwu, A.A. Electrochemical performance of controlled porosity resorcinol/formaldehyde based carbons as electrode materials for supercapacitor applications. Int. J. Hydrogen Energy 2017, 42, 25588–25597. [Google Scholar] [CrossRef] [Green Version]
- Mirzaeian, M.; Abbas, Q.; Gibson, D.; Mazur, M. Effect of nitrogen doping on the electrochemical performance of resorcinol-formaldehyde based carbon aerogels as electrode material for supercapacitor applications. Energy 2019, 173, 809–819. [Google Scholar] [CrossRef] [Green Version]
- Abbas, Q.; Mirzaeian, M.; Ogwu, A.A.; Mazur, M.; Gibson, D. Effect of physical activation/surface functional groups on wettability and electrochemical performance of carbon/activated carbon aerogels based electrode materials for electrochemical capacitors. Int. J. Hydrogen Energy 2020, 45, 13586–13595. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.; Ma, L.; Ran, J.; Shen, X.; Tong, H. Incorporation of homogeneous Co3O4 into a nitrogen-doped carbon aerogel via a facile in situ synthesis method: Implications for high performance asymmetric supercapacitors. J. Mater. Chem. A 2016, 4, 9542–9554. [Google Scholar] [CrossRef]
- Raj, R.P.; Ragupathy, P.; Mohan, S. Remarkable capacitive behavior of a Co3O4–polyindole composite as electrode material for supercapacitor applications. J. Mater. Chem. A 2015, 3, 24338–24348. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Dinesh, B.; Rethinasabapathy, M.; Hwang, S.-K.; Jin, C.-S.; Huh, Y.S.; Han, Y.-K. Hexagonal Co3O4 anchored reduced graphene oxide sheets for high-performance supercapacitors and non-enzymatic glucose sensing. J. Mater. Chem. A 2018, 6, 14367–14379. [Google Scholar] [CrossRef]
- Nagajyothi, P.; Pandurangan, M.; Sreekanth, T.V.M.; Shim, J.; Tvm, S. One-step engineered self-assembly Co3O4 nanoparticles to nanocubes for supercapacitors. Mater. Res. Express 2018, 5, 025511. [Google Scholar] [CrossRef]
- Ma, L.; Fan, H.; Wei, X.; Chen, S.; Hu, Q.; Liu, Y.; Zhi, C.; Lu, W.; Zapien, J.A.; Huang, H. Towards high areal capacitance, rate capability and tailorable supercapacitor: Co3O4@Polypyrrole core-shell nanorod bundle arrays electrode. J. Mater. Chem. A 2018, 6, 19058–19065. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, L.; Shi, S.; Xiong, Q.; Zhao, X.; Wang, X.; Gu, C.; Tu, J. Synthesis of porous Co3O4 nanoflake array and its temperature behavior as pseudo-capacitor electrode. J. Power Sources 2014, 256, 200–205. [Google Scholar] [CrossRef]
- Jafarian, M.; Mahjani, M.; Heli, H.; Gobal, F.; Khajehsharifi, H.; Hamedi, M. A study of the electro-catalytic oxidation of methanol on a cobalt hydroxide modified glassy carbon electrode. Electrochim. Acta 2003, 48, 3423–3429. [Google Scholar] [CrossRef]
- Huang, Y.; Liang, J.; Chen, Y. An overview of the applications of graphene-based materials in supercapacitors. Small 2012, 8, 1805–1834. [Google Scholar] [CrossRef]
- An, C.; Zhang, Y.; Guo, H.; Wang, Y. Metal oxide-based supercapacitors: Progress and prospectives. Nanoscale Adv. 2019, 1, 4644–4658. [Google Scholar] [CrossRef] [Green Version]
- Uke, S.J.; Akhare, V.P.; Bambole, D.R.; Bodade, A.B.; Chaudhari, G.N. Recent advancements in the cobalt oxides, manganese oxides, and their composite as an electrode material for supercapacitor: A review. Front. Mater. 2017, 1, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Kebabsa, L.; Kim, J.; Lee, D.; Lee, B. Highly porous cobalt oxide-decorated carbon nanofibers fabricated from starch as free-standing electrodes for supercapacitors. Appl. Surf. Sci. 2020, 511, 145313. [Google Scholar] [CrossRef]
- Numan, A.; Duraisamy, N.; Omar, F.S.; Mahipal, Y.K.; Ramesh, S.; Ramesh, S. Enhanced electrochemical performance of cobalt oxide nanocube intercalated reduced graphene oxide for supercapacitor application. RSC Adv. 2016, 6, 34894–34902. [Google Scholar] [CrossRef]
- Williamson, G.; Hall, W. X-ray line broadening from filed aluminum and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Shults, J.F.; Hofer, L.J.E.; Cohn, E.M.; Stein, K.C.; Anderson, R.B. Synthetic Liquid Fuels from Hydrogenation of Carbon Monoxide; Technical paper 700: 1–15; U.S. Bureau of Mines: Washington, DC, USA, 1959.
- Hadjiev, V.G.; Iliev, M.N.; Vergilov, I.V. The Raman spectra of Co3O4. J. Phys. C Solid State Phys. 1988, 21, L199–L201. [Google Scholar] [CrossRef]
- Gallant, D.; Pézolet, M.; Simard, S. Optical and physical properties of cobalt oxide films electrogenerated in bicarbonate aqueous media. J. Phys. Chem. 2006, 110, 6871–6880. [Google Scholar] [CrossRef]
- Lorite, I.; Romero, J.J.; Fernández, J.F. Effects of the agglomeration state on the Raman properties of Co3O4 nanoparticles. J. Raman Spectrosc. 2012, 43, 1443–1448. [Google Scholar] [CrossRef]
- Samal, R.; Dash, B.; Sarangi, C.K.; Sanjay, K.; Subbaiah, T.; Senanayake, G.; Minakshi, M. Influence of synthesis temperature on the growth and surface morphology of Co3O4 nanocubes for supercapacitor applications. Nanomaterials 2017, 7, 356. [Google Scholar] [CrossRef] [Green Version]
- Premlatha, S.; Chandrasekaran, M.; Bapu, G.N.K.R. Preparation of cobalt-RuO2 nanocomposite modified electrode for highly sensitive and selective determination of hydroxylamine. Sens. Actuators B 2017, 252, 375–384. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, H.; Hu, Z.; Gan, L.; Xu, Z. Facile synthesis of mesoporous cobalt oxide rugby balls for electrochemical energy storage. New J. Chem. 2015, 39, 68–71. [Google Scholar] [CrossRef]
- Naseri, N.; Esfandiar, A.; Qorbani, M.; Moshfegh, A. Selecting support layer for electrodeposited efficient cobalt oxide/hydroxide nanoflakes to split water. ACS Sustain. Chem. Eng. 2016, 4, 3151–3159. [Google Scholar] [CrossRef]
- Qorbani, M.; Naseri, N.; Moshfegh, Z. Hierarchical Co3O4/Co(OH)2 nanoflakes as a supercapacitor electrode: Experimental and semi-empirical model. ACS Appl. Mater. Interfaces 2015, 7, 11172–11179. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Zhou, H.; Shen, X.; Chen, Q.; Zhu, G.-X.; Ji, Z. Facile synthesis of Co3O4 porous nanosheets/reduced graphene oxide composites and their excellent supercapacitor performance. RCS Adv. 2014, 4, 53180–53187. [Google Scholar] [CrossRef]
- Gomez-Meier, H.; Vilche, J.; Arvia, A. The electrochemical behaviour of cobalt in alkaline solutions Part I. The potentiodynamic response in the potential region of the Co/CoO couple. J. Electroanal. Chem. 1982, 134, 251–272. [Google Scholar] [CrossRef] [Green Version]
- Meier, H.; Vilche, J.; Arvia, A. The electrochemical behaviour of cobalt in alkaline solutions part II. The potentiodynamic response of Co(OH)2 electrodes. J. Electroanal. Chem. Interfacial Electrochem. 1982, 138, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Cowling, R.; Riddiford, A. The anodic behaviour of cobalt in alkaline solutions. Electrochim. Acta 1969, 14, 981–989. [Google Scholar] [CrossRef]
- Medway, S.; Lucas, C.; Kowal, A.; Nichols, R.J.; Johnson, D. In situ studies of the oxidation of nickel electrodes in alkaline solution. J. Electroanal. Chem. 2006, 587, 172–181. [Google Scholar] [CrossRef]
- Kessler, T.; Visintin, A.; De Chialvo, M.R.; Triaca, W.E.; Arvia, A.J. The development of a cobalt oxide spinel structure overlayer on cobalt electrodes: A modified electrode surface of electrocatalytic interest. J. Electroanal. Chem. Interfacial Electrochem. 1989, 261, 315–329. [Google Scholar] [CrossRef]
- Grden, M. Impedance study on the capacitance of silver electrode oxidised in alkaline electrolyte. J. Solid State Electrochem. 2017, 21, 3333–3344. [Google Scholar] [CrossRef]
- Wu, X.; Xing, W.; Zhang, L.; Zhou, J.; Zhou, J.; Wang, G.; Qiao, S.Z. Nickel nanoparticles prepared by hydrazine hydrate reduction and their application in supercapacitor. Powder Technol. 2012, 224, 162–167. [Google Scholar] [CrossRef]
- Vittal, R.; Ho, K.-C. Cobalt oxide electrodes-problem and a solution through a novel approach using cetyltrimethylammonium bromide (CTAB). Catal. Rev. 2015, 57, 145–191. [Google Scholar] [CrossRef]
- Augustyn, V.; Simon, P.; Dunn, B.S. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614. [Google Scholar] [CrossRef] [Green Version]
- Ji, X.; Hallam, P.M.; Houssein, S.M.; Kadara, R.; Lang, L.; Banks, C.E. Printable thin film supercapacitors utilizing single crystal cobalt hydroxide nanosheets. RSC Adv. 2012, 2, 1508–1515. [Google Scholar] [CrossRef]
- Xiao, B.; Zhu, W.; Li, Z.; Zhu, J.; Zhu, X.; Pezzotti, G. Tailoring morphology of cobalt –nickel layered double hydroxide via different surfactants for high-performance supercapacitor. R. Soc. Open Sci. 2018, 5, 180867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, H.; Zhu, Y.; Fang, T.; Hua, J.; Qiu, S.; Liu, H.; Qin, L.; Wei, Q.; Zou, Y.; Xiang, C.; et al. Solvothermal synthesis of cobalt nickel layered double hydroxides with a three-dimensional nanopetal structure for high-performance supercapacitors. Sustain. Energy Fuels 2020, 4, 337–346. [Google Scholar] [CrossRef]
- Babar, P.; Lokhande, A.; Pawar, S.M.; Gang, M.; Jo, E.; Go, C.; Suryawanshi, M.P.; Kim, J.-H.; Pawar, S. Electrocatalytic performance evaluation of cobalt hydroxide and cobalt oxide thin films for oxygen evolution reaction. Appl. Surf. Sci. 2018, 427, 253–259. [Google Scholar] [CrossRef]
- Kharade, P.; Thombare, J.V.; Babar, A.; Bulakhe, R.; Kulkarni, S.; Salunkhe, D.J. Electrodeposited nanoflakes like hydrophilic Co3O4 as a supercapacitor electrode. J. Phys. Chem. Solids 2018, 120, 207–210. [Google Scholar] [CrossRef]
- Li, L.; Li, Y.; Gao, S.; Koshizaki, N. Ordered Co3O4 hierarchical nanorod arrays: Tunable superhydrophilicity without UV irradiation and transition to superhydrophobicity. J. Mater. Chem. 2009, 19, 8366–8371. [Google Scholar] [CrossRef]
- Urso, M.; Torrisi, G.; Boninelli, S.; Bongiorno, C.; Priolo, F.; Mirabella, S. Ni(OH)2@Ni core-shell nanochains as low-cost high-rate performance electrode for energy storage applications. Sci. Rep. 2019, 9, 7736. [Google Scholar] [CrossRef] [Green Version]
- Abdullin, K.A.; Kalkozova, Z.K.; Markhabayeva, A.A.; Dupre, R.; Moniruddin; Nuraje, N. Core-shell (W@WO3) nanostructure to improve electrochemical performance. ACS Appl. Energy Mater. 2019, 2, 797–803. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirzaeian, M.; Akhanova, N.; Gabdullin, M.; Kalkozova, Z.; Tulegenova, A.; Nurbolat, S.; Abdullin, K. Improvement of the Pseudocapacitive Performance of Cobalt Oxide-Based Electrodes for Electrochemical Capacitors. Energies 2020, 13, 5228. https://doi.org/10.3390/en13195228
Mirzaeian M, Akhanova N, Gabdullin M, Kalkozova Z, Tulegenova A, Nurbolat S, Abdullin K. Improvement of the Pseudocapacitive Performance of Cobalt Oxide-Based Electrodes for Electrochemical Capacitors. Energies. 2020; 13(19):5228. https://doi.org/10.3390/en13195228
Chicago/Turabian StyleMirzaeian, Mojtaba, Nazym Akhanova, Maratbek Gabdullin, Zhanar Kalkozova, Aida Tulegenova, Shyryn Nurbolat, and Khabibulla Abdullin. 2020. "Improvement of the Pseudocapacitive Performance of Cobalt Oxide-Based Electrodes for Electrochemical Capacitors" Energies 13, no. 19: 5228. https://doi.org/10.3390/en13195228
APA StyleMirzaeian, M., Akhanova, N., Gabdullin, M., Kalkozova, Z., Tulegenova, A., Nurbolat, S., & Abdullin, K. (2020). Improvement of the Pseudocapacitive Performance of Cobalt Oxide-Based Electrodes for Electrochemical Capacitors. Energies, 13(19), 5228. https://doi.org/10.3390/en13195228