Experimental Study on the Inhibition Effects of Nitrogen and Carbon Dioxide on Coal Spontaneous Combustion
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. C80 Micro-Calorimeter
2.3. Synchronous Thermal Analyzer
3. Results and Discussion
3.1. Inhibition Effects of Inert Gases in the Low-temperature Oxidation Stage
3.1.1. Constant-Temperature Heat Release
3.1.2. Apparent Activation Energy
3.2. Inhibition Effects of Inert Gases in the High-Temperature Combustion Stage
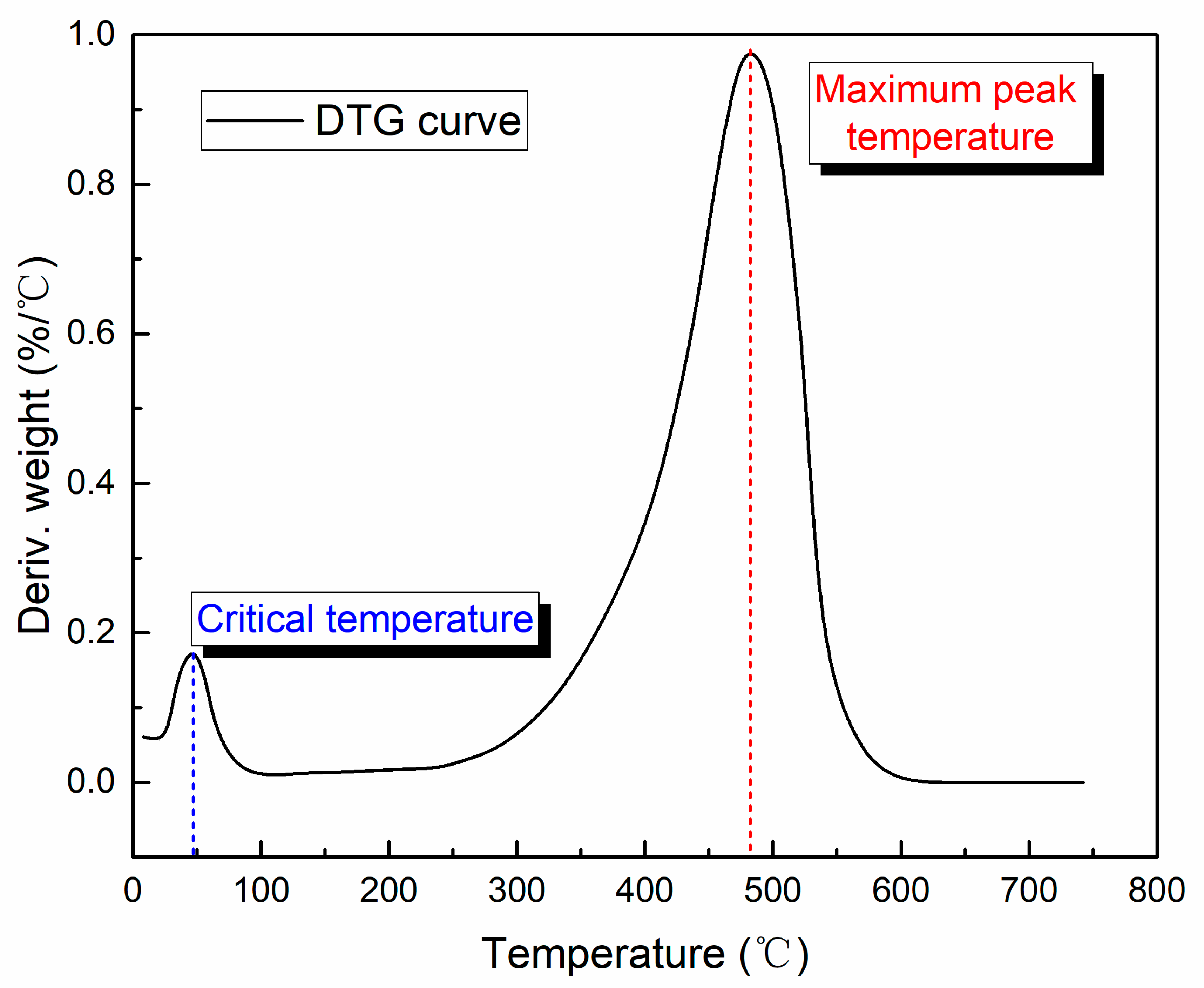
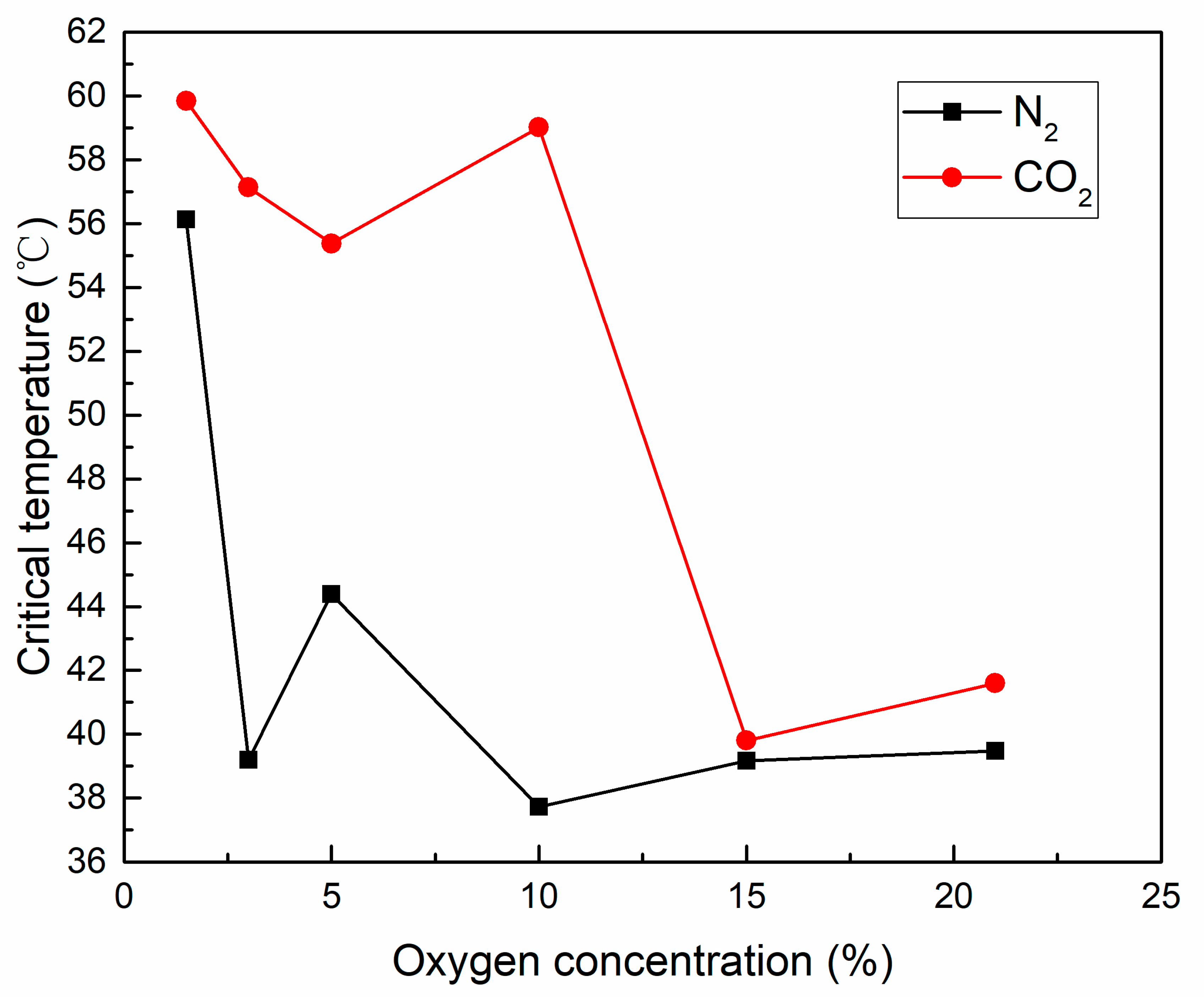
3.2.1. Critical Temperature
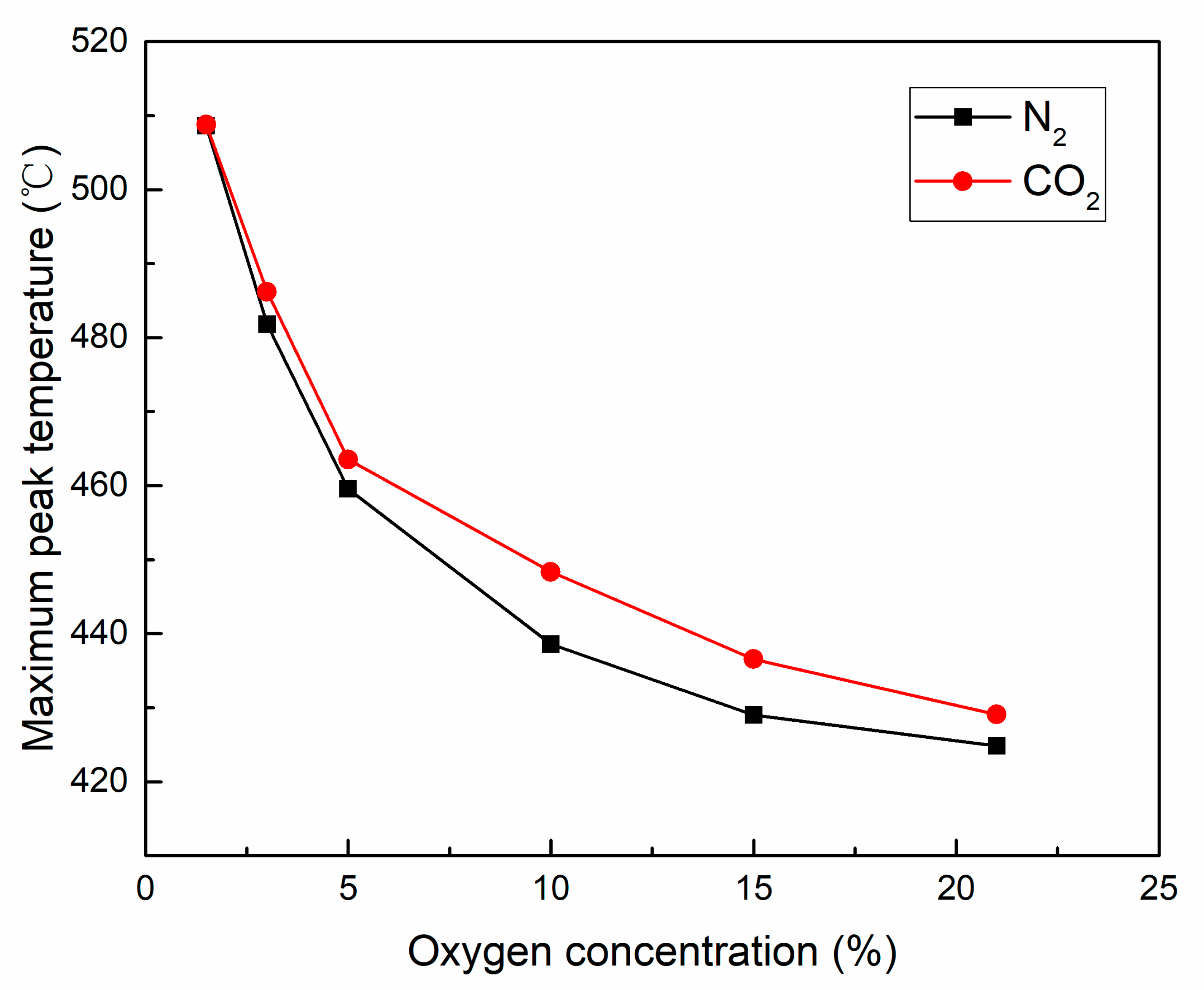
3.2.2. Maximum Peak Temperature
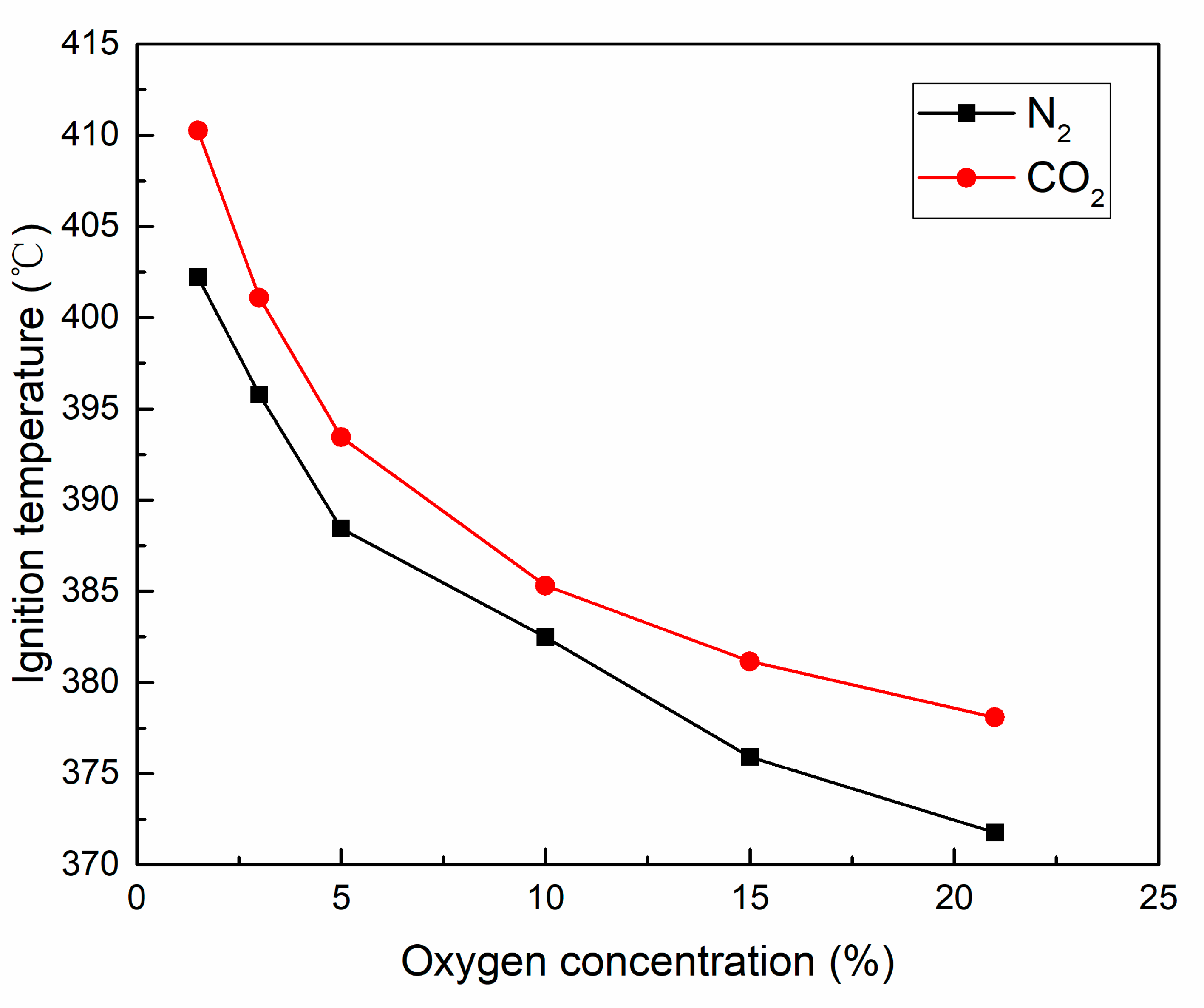
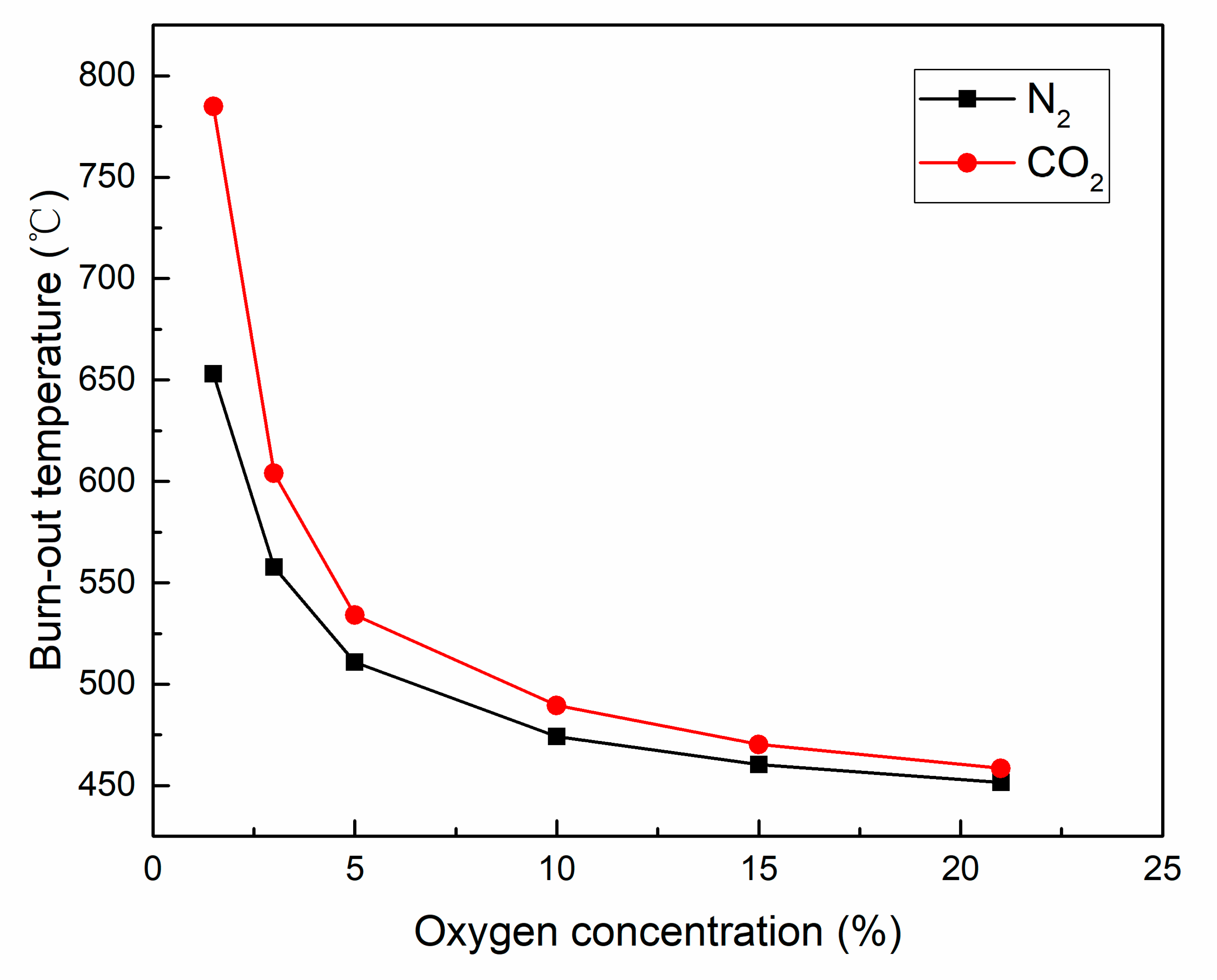
3.2.3. Ignition Temperature and Burn-Out Temperature
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wolde-Rufael, Y. Coal consumption and economic growth revisited. Appl. Energy 2010, 87, 160–167. [Google Scholar] [CrossRef]
- Li, H.; Shi, S.; Lin, B.; Lu, J.; Ye, Q.; Lu, Y.; Wang, Z.; Hong, Y.; Zhu, X. Effects of microwave-assisted pyrolysis on the microstructure of bituminous coals. Energy 2019, 187, 115986. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.; Wang, D.; Zhu, X.; Zhu, Y.; Bai, X.; Yang, Q. Improvement of Foaming Ability of Surfactant Solutions by Water-soluble Polymers: Experiment and Molecular Dynamics Simulation. Polymers 2020, 12, 571. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhang, Y.; Li, Y.; Li, Q.; Zhang, J.; Yang, C. Study on the characteristics of coal spontaneous combustion during the development and decaying processes. Process Saf. Environ. Prot. 2020, 138, 9–17. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, D.; Shao, Z.; Xu, C.; Zhu, X.; Qi, X.; Liu, F. A statistical analysis of coalmine fires and explosions in China. Process Saf. Environ. Prot. 2019, 121, 357–366. [Google Scholar] [CrossRef]
- Deng, J.; Qu, J.; Wang, Q.; Xiao, Y.; Cheng, Y.; Shu, C. Experimental data revealing explosion characteristics of methane, air, and coal mixtures. RSC Adv. 2019, 9, 24627. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Ding, J.; Ma, Y.; Li, S.; Zhang, M. Synthesis and performance characterization of a novel wetting cementing agent for dust control during conveyor transport in coal mines. Powder Technol. 2020, 360, 165–176. [Google Scholar] [CrossRef]
- Zhang, H.; Nie, W.; Yan, J.; Bao, Q.; Wang, H.; Jin, H.; Peng, H.; Chen, D.; Liu, Z.; Liu, Z.; et al. Preparation and performance study of a novel polymeric spraying dust suppression agent with enhanced wetting and coagulation properties for coal mine. Powder Technol. 2020, 364, 901–914. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, H.; Wang, D.; Du, Y.; Zhang, K.; Zhang, J. The influence of pore structure of coal on characteristics of dust generation during the process of conical pick cutting. Powder Technol. 2020, 363, 559–568. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, S.; Xia, T.; Liu, Y.; Pan, Z.; Zhou, F. A novel failure control technology of cross-measure borehole for gas drainage: A case study. Process Saf. Environ. Prot. 2020, 135, 144–156. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, W.; Yang, H.; Yu, S.; Xin, L. Experimental study on the fractal pattern of a coal body pore structure around a water injection bore. J. Energy Resour. Technol. 2020, 142, 012302. [Google Scholar] [CrossRef]
- Zhong, X.; Kan, L.; Xin, H.; Qin, B.; Dou, G. Thermal effects and active group differentiation of low-rank coal during low-temperature oxidation under vacuum drying after water immersion. Fuel 2019, 236, 1204–1212. [Google Scholar] [CrossRef]
- Tang, Y.; Si, S.; Zhong, X. Experimental investigation of the performance of an effective self-suctioning water mist generator for controlling underground coal fires. Process Saf. Environ. Prot. 2019, 126, 98–105. [Google Scholar] [CrossRef]
- Ma, L.; Wang, D.; Kang, W.; Xin, H.; Dou, G. Comparison of the staged inhibitory effects of two ionic liquids on spontaneous combustion of coal based on in situ FTIR and micro-calorimetric kinetic analyses. Process Saf. Environ. Prot. 2019, 121, 326–337. [Google Scholar] [CrossRef]
- Zhuo, H.; Qin, B.; Qin, Q.; Su, Z. Modeling and simulation of coal spontaneous combustion in a gob of shallow buried coal seams. Process Saf. Environ. Prot. 2019, 131, 246–254. [Google Scholar] [CrossRef]
- Lu, W.; Guo, B.; Qi, G.; Cheng, W.; Yang, W. Experimental study on the effect of preinhibition temperature on the spontaneous combustion of coal based on an MgCl2 solution. Fuel 2020, 265, 117032. [Google Scholar] [CrossRef]
- Shi, Q.; Qin, B. Experimental research on gel-stabilized foam designed to prevent and control spontaneous combustion of coal. Fuel 2019, 254, 115558. [Google Scholar] [CrossRef]
- Tian, Z.; Lu, Y.; Liu, S.; Shi, S.; Li, H.; Ye, Q. Application of inorganic solidified foam to control the coexistence of unusual methane emission and spontaneous combustion of coal in the Luwa coal mine, China. Combust. Sci. Technol. 2020, 192, 638–656. [Google Scholar] [CrossRef]
- Ren, X.; Hu, X.; Cheng, W.; Bian, S.; Zhao, Y.; Wu, M.; Xue, D.; Li, Y.; Lu, W.; Wang, P. Study of resource utilization and fire prevention characteristics of a novel gel formulated from coal mine sludge (MS). Fuel 2020, 267, 117261. [Google Scholar] [CrossRef]
- Qi, X.; Chen, L.; Zhang, L.; Bai, C.; Xin, H.; Rao, Z. In situ FTIR study on real-time changes of active groups during lignite reaction under low oxygen concentration conditions. J. Energy Inst. 2019, 92, 1557–1566. [Google Scholar] [CrossRef]
- Ray, S.K.; Singh, R.P. Recent developments and practices to control fire in underground coal mines. Fire Technol. 2007, 43, 285–300. [Google Scholar] [CrossRef]
- Li, T.; Qi, Y.; Li, X.; Wang, J. Coal fire prevention in large areas over long term with a composite inert gas—A case study in Tangkou coal mine, China. Energ. Source Part A 2019. [Google Scholar] [CrossRef]
- Shi, B.; Zhou, G.; Ma, L. Normalizing fire prevention technology and a ground fixed station for underground mine fires using liquid nitrogen: A case study. Fire Technol. 2018, 54, 1887–1893. [Google Scholar] [CrossRef]
- Taraba, B.; Pavelek, Z. Study of coal oxidation behavior in re-opened sealed heating. J. Loss Prevent. Proc. 2016, 40, 433–436. [Google Scholar] [CrossRef]
- Su, H.; Zhou, F.; Song, X.; Qiang, Z. Risk analysis of spontaneous coal combustion in steeply inclined longwall gobs using a sacled-down experimental set-up. Process Saf. Environ. Prot. 2017, 111, 1–12. [Google Scholar] [CrossRef]
- Lei, B.; He, B.; Xiao, B.; Du, P.; Wu, B. Comparative study of single inert gas in confined space inhibiting open flame coal combustion. Fuel 2020, 265, 116976. [Google Scholar] [CrossRef]
- Shao, H.; Jiang, S.; Wu, Z.; Zhang, W.; Wang, K. Comparative research on the influence of dioxide carbon nitrogen on performance of coal spontaneous combustion. J. China Coal Soc. 2014, 39, 2244–2249. [Google Scholar]
- Liu, H.; Wang, F. Thermal characteristics and kinetic analysis of coal-oxygen reaction under the condition of inert gas. Int. J. Coal Prep. Util. 2019. [Google Scholar] [CrossRef]
- Deng, J.; Ren, L.; Ma, L.; Qin, X.; Wang, W.; Liu, C. Low-temperature oxidation and reactivity of coal in O2/N2 and O2/CO2 atmospheres, a case of carboniferous-permian coal in Shaanxi, China. Environ. Earth Sci. 2019, 78, 234. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Shu, C.; Wang, Q.; Ma, T.; Bin, L. Effects of oxygen concentration on the macroscopic characteristic indexes of high-temperature oxidation of coal. J. Energy Inst. 2019, 92, 554–566. [Google Scholar] [CrossRef]
- Ma, L.; Wang, Y.; Wu, R.; Ren, L.; Zou, L. Effect of low oxygen concentrations on the thermokinetics of coal combustion. Combust. Sci. Technol. 2020. [Google Scholar] [CrossRef]
- Ren, L.; Li, Q.; Deng, J.; Xiao, Y.; Ma, L.; Wang, W. Inhibiting effect of CO2 on the oxidative combustion thermodynamics of coal. RSC Adv. 2019, 9, 41126. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, Y.; Huang, Y.; Li, S.; Wang, W. Characteristics of mass, heat and gaseous products during coal spontaneous combustion using TG/DSC-FTIR technology. J. Therm. Anal. Calorim. 2018, 131, 2963–2974. [Google Scholar] [CrossRef]
- Benfell, K.E.; Beamish, B.B.; Rodgers, K.A. Thermogravimetric analytical procedures for characterizing New Zealand and Eastern Australian coals. Thermochim. Acta 1996, 286, 67–74. [Google Scholar] [CrossRef]





| Coal Sample | Mad/(wt%) | Aad/(wt%) | Vad/(wt%) | FCad/(wt%) |
| 12.445 | 6.18 | 29.01 | 52.365 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Xu, J.; Wang, D. Experimental Study on the Inhibition Effects of Nitrogen and Carbon Dioxide on Coal Spontaneous Combustion. Energies 2020, 13, 5256. https://doi.org/10.3390/en13205256
Zhang Y, Xu J, Wang D. Experimental Study on the Inhibition Effects of Nitrogen and Carbon Dioxide on Coal Spontaneous Combustion. Energies. 2020; 13(20):5256. https://doi.org/10.3390/en13205256
Chicago/Turabian StyleZhang, Yi, Jun Xu, and Deming Wang. 2020. "Experimental Study on the Inhibition Effects of Nitrogen and Carbon Dioxide on Coal Spontaneous Combustion" Energies 13, no. 20: 5256. https://doi.org/10.3390/en13205256





