Modified Biochar—A Tool for Wastewater Treatment
Abstract
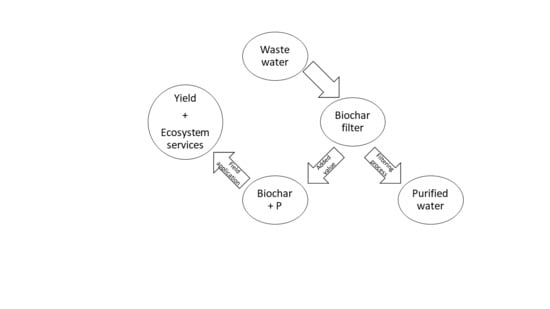
1. Introduction
2. Material and Methods
2.1. Origin and Characteristics of Products 1 and 2
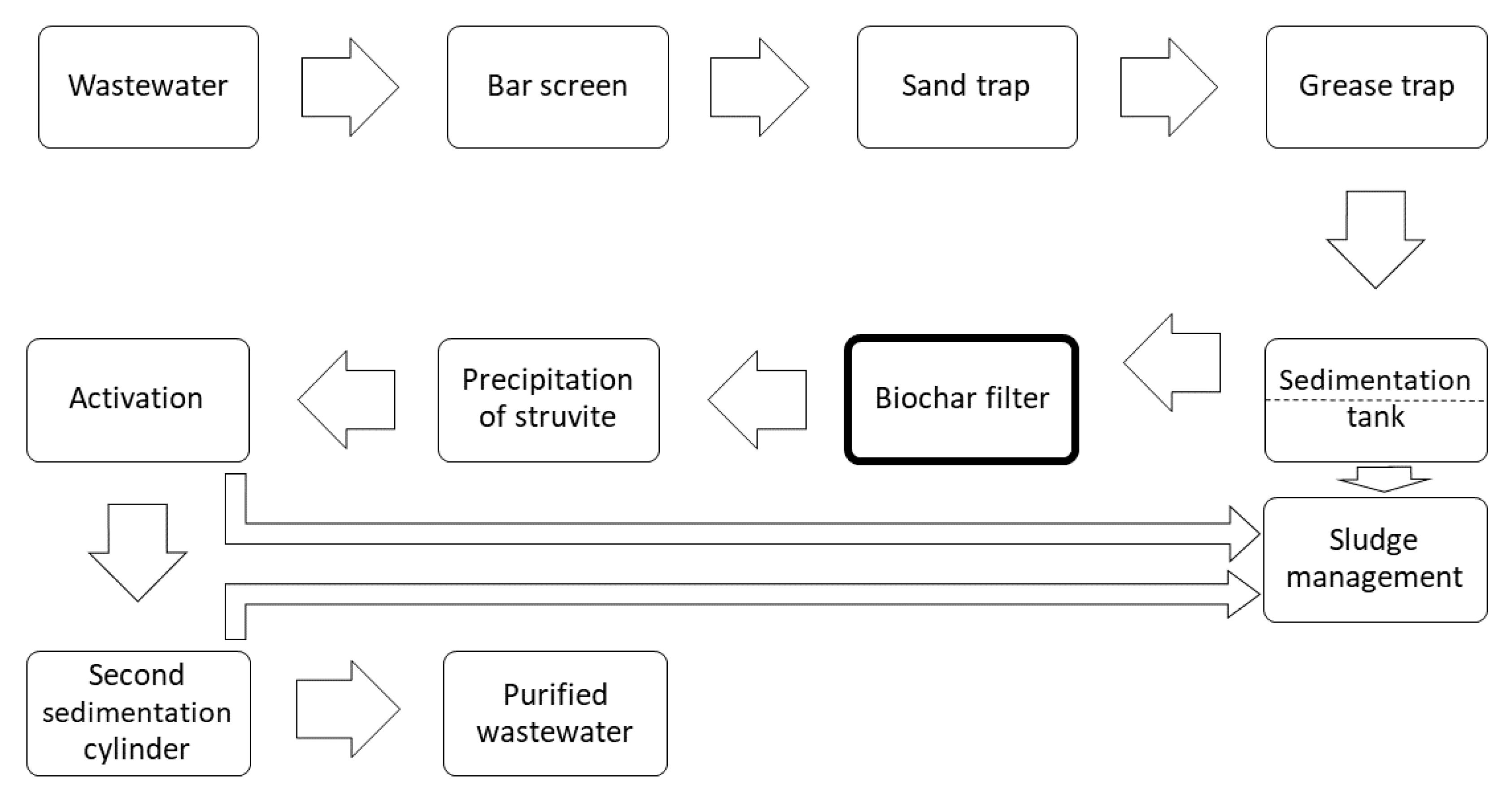
2.2. The Essence of the Technical Method to Obtain Products 3 and 4
2.3. Description of the Practical Implementation of Products 3 and 4
2.4. Evaluation of Fertilizer Product Quality
- Original sludge water,
- Struvite precipitated from sludge water,
- Phosphorus-saturated biochar, and
- Iron(III) phosphate from a reused biochar filter.
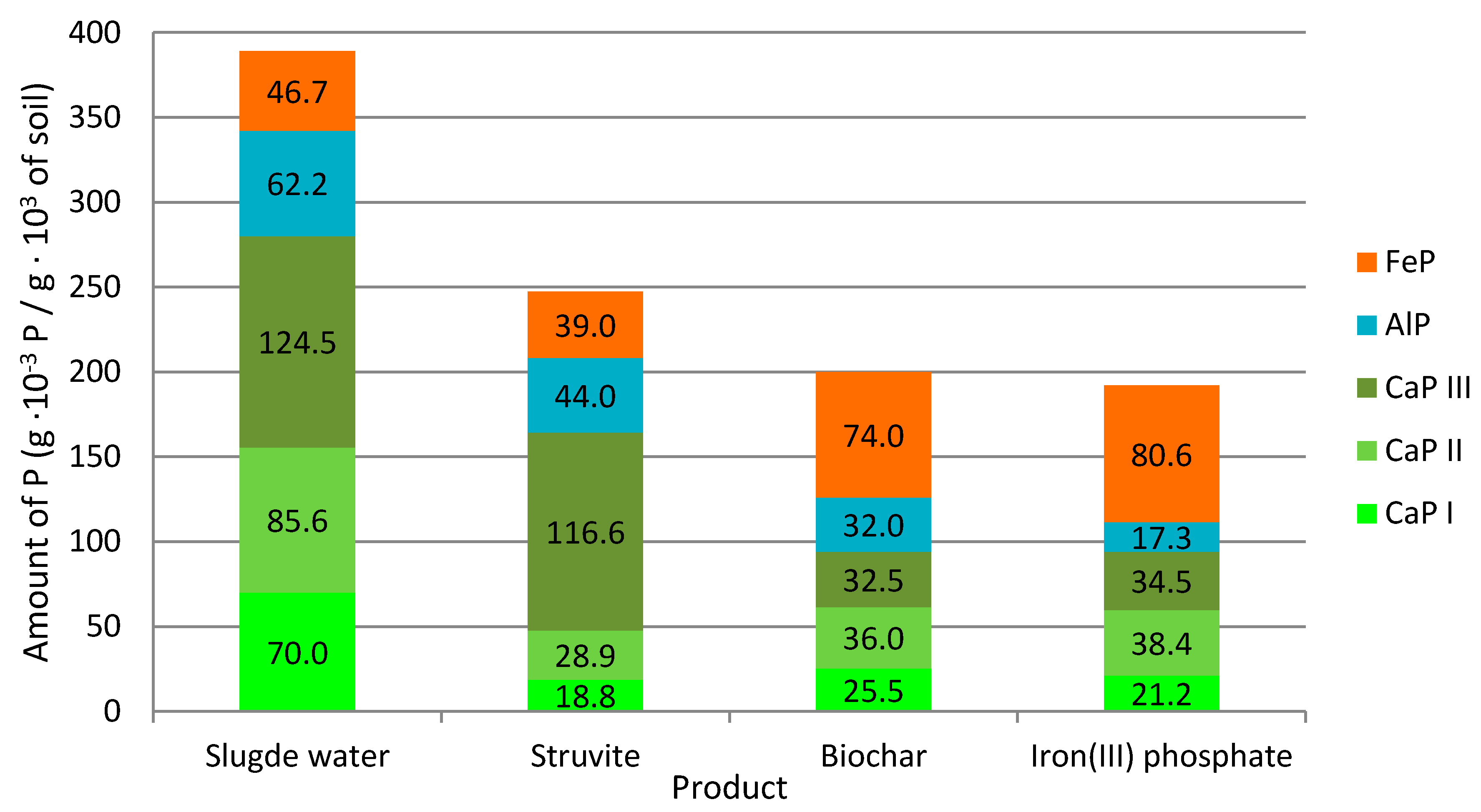
3. Results and Discussion
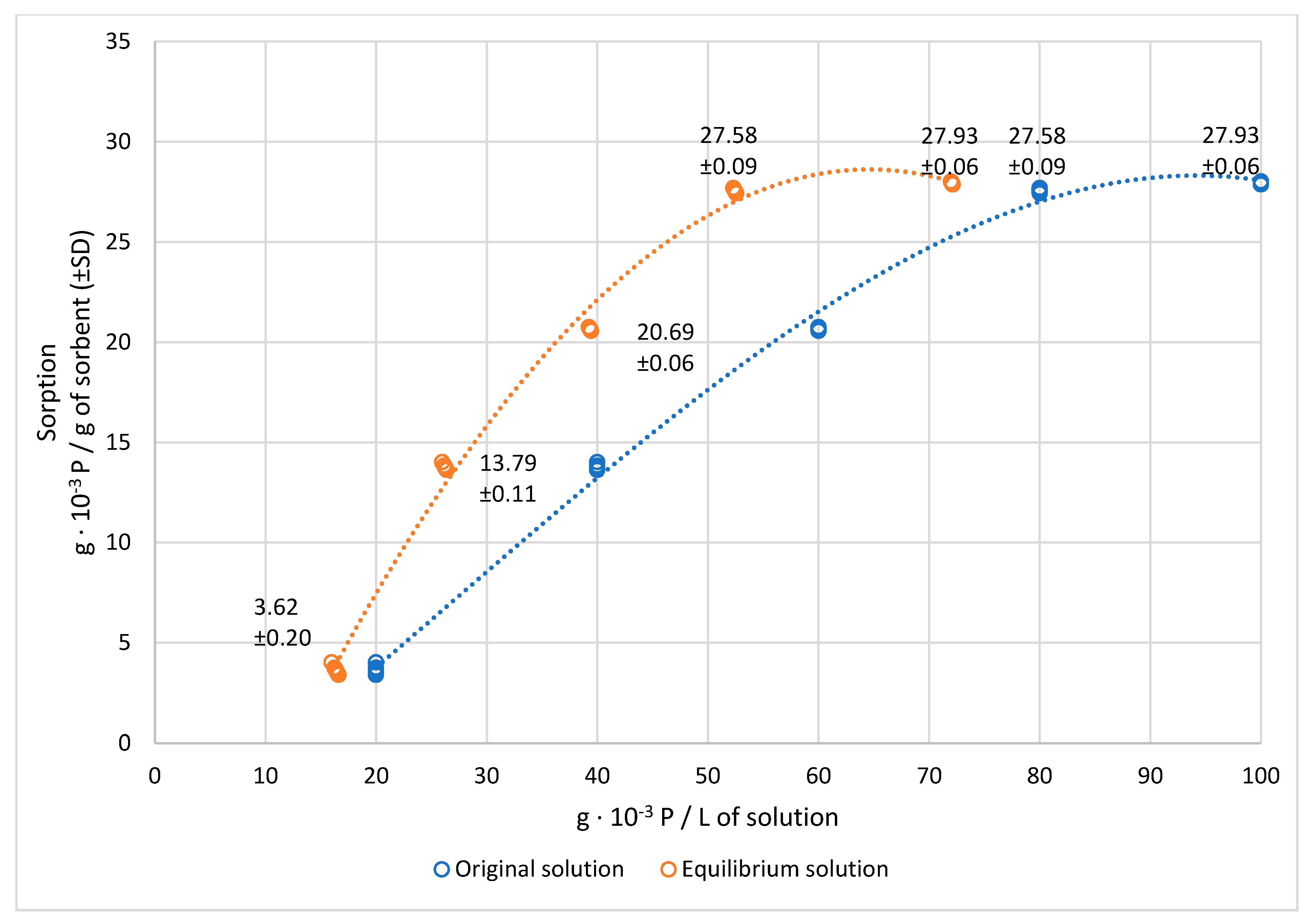
3.1. Efficiency of Sorption
3.2. Quality of Products
4. Conclusions
- Retrieval of phosphorus from wastewater by a biochar filter
- Obtaining a soil improver containing easily plant-accessible phosphorus
- The technology has the potential for use in waste management and agriculture
Author Contributions
Funding
Conflicts of Interest
References
- Cordell, D.; White, S. Peak phosphorus: Clarifying the key issues of a vigorous debate about long-term phosphorus security. Sustainability 2011, 3, 2027. [Google Scholar] [CrossRef]
- Reijnders, L. Phosphorus resources, their depletion and conservation—A review. Resour. Conserv. Recycl. 2014. [Google Scholar] [CrossRef]
- Zangarini, S.; Pepè Sciarria, T.; Tambone, F.; Adani, F. Phosphorus removal from livestock effluents: Recent technologies and new perspectives on low-cost strategies. Environ. Sci. Pollut. Res. 2020. [Google Scholar] [CrossRef]
- El Wali, M.; Golroudbary, S.R.; Kraslawski, A. Impact of recycling improvement on the life cycle of phosphorus. Chin. J. Chem. Eng. 2019. [Google Scholar] [CrossRef]
- Samreen, S.; Kausar, S. Phosphorus fertilizer: The Original and Commercial Sources. In Phosphorus—Recovery and Recycling; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Gupta, D.K.; Chatterjee, S.; Datta, S.; Veer, V.; Walther, C. Role of phosphate fertilizers in heavy metal uptake and detoxification of toxic metals. Chemosphere 2014. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Villalba, G.; Ayres, R.U.; Schroder, H. Global phosphorus flows and environmental impacts from a consumption perspective. J. Ind. Ecol. 2008. [Google Scholar] [CrossRef]
- Van Dijk, K.C.; Lesschen, J.P.; Oenema, O. Phosphorus flows and balances of the European Union Member States. Sci. Total Environ. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Liu, Y.; Liu, J.; Meng, J.; Hu, X.; Tao, S. Improving the imbalanced global supply Chain of phosphorus fertilizers. Earth Futur. 2019. [Google Scholar] [CrossRef]
- Chowdhury, R.B.; Chakraborty, P. Magnitude of anthropogenic phosphorus storage in the agricultural production and the waste management systems at the regional and country scales. Environ. Sci. Pollut. Res. 2016. [Google Scholar] [CrossRef]
- Chien, S.H.; Prochnow, L.I.; Tu, S.; Snyder, C.S. Agronomic and environmental aspects of phosphate fertilizers varying in source and solubility: An update review. Nutr. Cycl. Agroecosyst. 2011. [Google Scholar] [CrossRef]
- Le Moal, M.; Gascuel-Odoux, C.; Ménesguen, A.; Souchon, Y.; Étrillard, C.; Levain, A.; Moatar, F.; Pannard, A.; Souchu, P.; Lefebvre, A.; et al. Eutrophication: A new wine in an old bottle? Sci. Total Environ. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.B.; Moore, G.A.; Weatherley, A.J.; Arora, M. Key sustainability challenges for the global phosphorus resource, their implications for global food security, and options for mitigation. J. Clean. Prod. 2017. [Google Scholar] [CrossRef]
- Westphal, K.; Graeber, D.; Musolff, A.; Fang, Y.; Jawitz, J.W.; Borchardt, D. Multi-decadal trajectories of phosphorus loading, export, and instream retention along a catchment gradient. Sci. Total Environ. 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wiedmann, T.; Hadjikakou, M. Towards meaningful consumption-based planetary boundary indicators: The phosphorus exceedance footprint. Glob. Environ. Chang. 2019. [Google Scholar] [CrossRef]
- Schaller, J.; Faucherre, S.; Joss, H.; Obst, M.; Goeckede, M.; Planer-Friedrich, B.; Peiffer, S.; Gilfedder, B.; Elberling, B. Silicon increases the phosphorus availability of Arctic soils. Sci. Rep. 2019. [Google Scholar] [CrossRef]
- Cordell, D.; Drangert, J.O.; White, S. The story of phosphorus: Global food security and food for thought. Glob. Environ. Chang. 2009. [Google Scholar] [CrossRef]
- Zhu, J.; Li, M.; Whelan, M. Phosphorus activators contribute to legacy phosphorus availability in agricultural soils: A review. Sci. Total Environ. 2018. [Google Scholar] [CrossRef]
- Bindraban, P.S.; Dimkpa, C.O.; Pandey, R. Exploring phosphorus fertilizers and fertilization strategies for improved human and environmental health. Biol. Fertil. Soils 2020. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Giles, C.; Darch, T.; George, T.S.; Blackwell, M.; Stutter, M.; Shand, C.; Lumsdon, D.; Cooper, P.; Wendler, R.; et al. Opportunities for mobilizing recalcitrant phosphorus from agricultural soils: A review. Plant Soil 2018. [Google Scholar] [CrossRef]
- Das, B.; Huth, N.; Probert, M.; Condron, L.; Schmidt, S. Soil phosphorus modeling for modern agriculture requires balance of science and practicality: A perspective. J. Environ. Qual. 2019. [Google Scholar] [CrossRef]
- George, T.S.; Hinsinger, P.; Turner, B.L. Phosphorus in soils and plants—Facing phosphorus scarcity. Plant Soil 2016. [Google Scholar] [CrossRef]
- Scholz, R.W.; Wellmer, F.W. Approaching a dynamic view on the availability of mineral resources: What we may learn from the case of phosphorus? Glob. Environ. Chang. 2013. [Google Scholar] [CrossRef]
- Cabeza, R.; Steingrobe, B.; Römer, W.; Claassen, N. Effectiveness of recycled P products as P fertilizers, as evaluated in pot experiments. Nutr. Cycl. Agroecosyst. 2011. [Google Scholar] [CrossRef]
- Senecal, J.; Vinnerås, B. Urea stabilisation and concentration for urine-diverting dry toilets: Urine dehydration in ash. Sci. Total Environ. 2017. [Google Scholar] [CrossRef] [PubMed]
- Vinnerås, B.; Palmquist, H.; Balmér, P.; Jönsson, H. The characteristics of household wastewater and biodegradable solid waste—A proposal for new Swedish design values. Urban Water J. 2006. [Google Scholar] [CrossRef]
- Comber, S.; Gardner, M.; Georges, K.; Blackwood, D.; Gilmour, D. Domestic source of phosphorus to sewage treatment works. Environ. Technol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Sýkorová, E.; Wanner, J.Ř.; Beneš, O.Ř. Analysis of phosphorus recovery by struvite precipitation from sludge water in selected wastewater treatment plants. Chem. List. 2014, 108, 610–614. [Google Scholar]
- Christodoulou, A.; Stamatelatou, K. Overview of legislation on sewage sludge management in developed countries worldwide. Water Sci. Technol. 2016. [Google Scholar] [CrossRef]
- Kumari, S.; Jose, S.; Tyagi, M.; Jagadevan, S. A holistic and sustainable approach for recovery of phosphorus via struvite crystallization from synthetic distillery wastewater. J. Clean. Prod. 2020. [Google Scholar] [CrossRef]
- Munch, E.V.; Barr, K. Controlled struvite crystallisation for removing phosphorus from anaerobic digester sidestreams. Water Res. 2001. [Google Scholar] [CrossRef]
- Li, B.; Boiarkina, I.; Yu, W.; Huang, H.M.; Munir, T.; Wang, G.Q.; Young, B.R. Phosphorous recovery through struvite crystallization: Challenges for future design. Sci. Total Environ. 2019. [Google Scholar] [CrossRef] [PubMed]
- Min, K.J.; Kim, D.; Lee, J.; Lee, K.; Park, K.Y. Characteristics of vegetable crop cultivation and nutrient releasing with struvite as a slow-release fertilizer. Environ. Sci. Pollut. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Capdevielle, A.; Sýkorová, E.; Biscans, B.; Béline, F.; Daumer, M.L. Optimization of struvite precipitation in synthetic biologically treated swine wastewater—Determination of the optimal process parameters. J. Hazard. Mater. 2013. [Google Scholar] [CrossRef] [PubMed]
- Cornel, P.; Schaum, C. Phosphorus recovery from wastewater: Needs, technologies and costs. Water Sci. Technol. 2009. [Google Scholar] [CrossRef]
- Perera, M.K.; Englehardt, J.D.; Dvorak, A.C. Technologies for recovering nutrients from wastewater: A critical review. Environ. Eng. Sci. 2019. [Google Scholar] [CrossRef]
- Chrispim, M.C.; Scholz, M.; Nolasco, M.A. Phosphorus recovery from municipal wastewater treatment: Critical review of challenges and opportunities for developing countries. J. Environ. Manag. 2019. [Google Scholar] [CrossRef] [PubMed]
- Merino-Martos, A.; de Vicente, J.; Cruz-Pizarro, L.; de Vicente, I. Setting up High Gradient Magnetic Separation for combating eutrophication of inland waters. J. Hazard. Mater. 2011. [Google Scholar] [CrossRef]
- Drenkova-Tuhtan, A.; Schneider, M.; Meyer, C.; Franzreb, M.; Gellermann, C.; Mandel, K.; Steinmetz, H. Polishing of secondary wastewater effluents through elimination and recovery of dissolved phosphorus with reusable magnetic microsorbents. Proc. Water Environ. Fed. 2017. [Google Scholar] [CrossRef]
- Mehta, C.M.; Khunjar, W.O.; Nguyen, V.; Tait, S.; Batstone, D.J. Technologies to recover nutrients from waste streams: A critical review. Crit. Rev. Environ. Sci. Technol. 2015. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, D.Q.; Dong, J.W.; Tan, S.K. Constructed wetlands for wastewater treatment in cold climate—A review. J. Environ. Sci. 2017, 57, 293–311. [Google Scholar] [CrossRef]
- Henriet, O.; Meunier, C.; Henry, P.; Mahillon, J. Improving phosphorus removal in aerobic granular sludge processes through selective microbial management. Bioresour. Technol. 2016. [Google Scholar] [CrossRef]
- Gonzalez-Gil, G.; Holliger, C. Dynamics of microbial community structure of and enhanced biological phosphorus removal by aerobic granules cultivated on propionate or acetate. Appl. Environ. Microbiol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Horáček, J.; Kolář, L.; Čechová, V.; Hřebečková, J. Phosphorus and carbon fraction concentrations in a cambisol soil as affected by tillage. Commun. Soil Sci. Plant Anal. 2008. [Google Scholar] [CrossRef]
- Wollmann, I.; Gauro, A.; Müller, T.; Möller, K. Phosphorus bioavailability of sewage sludge-based recycled fertilizers. J. Plant Nutr. Soil Sci. 2018. [Google Scholar] [CrossRef]
- Eriksson, A.K.; Gustafsson, J.P.; Hesterberg, D. Phosphorus speciation of clay fractions from long-term fertility experiments in Sweden. Geoderma 2015. [Google Scholar] [CrossRef]
- Maroušek, J.; Stehel, V.; Vochozka, M.; Kolář, L.; Maroušková, A.; Strunecký, O.; Peterka, J.; Kopecký, M.; Shreedhar, S. Ferrous sludge from water clarification: Changes in waste management practices advisable. J. Clean. Prod. 2019. [Google Scholar] [CrossRef]
- Kovar, J.L.; Pierzynski, G.M. Methods of Phosphorus Analysis for Soils, Sediments, Residuals, and Waters; Southern Cooperative Series Bulletin; North Carolina State University: Raleigh, NC, USA, 2009. [Google Scholar]
- Ginzburg, K.E.; Lebedeva, L.S. Method to determine mineral forms of soilphosphate. Agrochimija 1971, 1, 125–135. (In Russian) [Google Scholar]
- Zhang, H.; Voroney, R.P.; Price, G.W. Effects of temperature and activation on biochar chemical properties and their impact on ammonium, nitrate, and phosphate sorption. J. Environ. Qual. 2017. [Google Scholar] [CrossRef]
- Kataki, S.; West, H.; Clarke, M.; Baruah, D.C. Phosphorus recovery as struvite: Recent concerns for use of seed, alternative Mg source, nitrogen conservation and fertilizer potential. Resour. Conserv. Recycl. 2016. [Google Scholar] [CrossRef]
- Qian, L.; Wang, S.; Xu, D.; Guo, Y.; Tang, X.; Wang, L. Treatment of municipal sewage sludge in supercritical water: A review. Water Res. 2016. [Google Scholar] [CrossRef]
- Cieślik, B.M.; Świerczek, L.; Konieczka, P. Analytical and legislative challenges of sewage sludge processing and management. Mon. Chem. 2018. [Google Scholar] [CrossRef] [PubMed]
- Seleiman, M.F.; Santanen, A.; Mäkelä, P.S.A. Recycling sludge on cropland as fertilizer—Advantages and risks. Resour. Conserv. Recycl. 2020. [Google Scholar] [CrossRef]
- Hilt, K.; Harrison, J.; Bowers, K.; Stevens, R.; Bary, A.; Harrison, K. Agronomic Response of Crops Fertilized with Struvite Derived from Dairy Manure. Water Air Soil Pollut. 2016. [Google Scholar] [CrossRef]
- Vaneeckhaute, C.; Janda, J.; Vanrolleghem, P.A.; Tack, F.M.G.; Meers, E. Phosphorus use efficiency of bio-based fertilizers: Bioavailability and fractionation. Pedosphere 2016. [Google Scholar] [CrossRef]
- Van Paassen, J.G.; Britton, A.J.; Mitchell, R.J.; Street, L.E.; Johnson, D.; Coupar, A.; Woodin, S.J. Legacy effects of nitrogen and phosphorus additions on vegetation and carbon stocks of upland heaths. New Phytol. 2020. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Houlton, B.Z.; Smith, W.K.; Marklein, A.R.; Reed, S.C.; Parton, W.; Del Grosso, S.J.; Running, S.W. Patterns of new versus recycled primary production in the terrestrial biosphere. Proc. Natl. Acad. Sci. USA 2013. [Google Scholar] [CrossRef] [PubMed]
- Degryse, F.; Baird, R.; da Silva, R.C.; McLaughlin, M.J. Dissolution rate and agronomic effectiveness of struvite fertilizers—Effect of soil pH, granulation and base excess. Plant Soil 2017. [Google Scholar] [CrossRef]
- Capdevielle, A.; Sýkorová, E.; Béline, F.; Daumer, M.L. Effects of organic matter on crystallization of struvite in biologically treated swine wastewater. Environ. Technol. 2016. [Google Scholar] [CrossRef]
- Maroušek, J.; Kolář, L.; Strunecký, O.; Kopecký, M.; Bartoš, P.; Maroušková, A.; Cudlínová, E.; Konvalina, P.; Šoch, M.; Moudrý, J., Jr.; et al. Modified biochars present an economic challenge to phosphate management in wastewater treatment plants. J. Clean. Prod. 2020. [Google Scholar] [CrossRef]
- Kamau, S.; Karanja, N.K.; Ayuke, F.O.; Lehmann, J. Short-term influence of biochar and fertilizer-biochar blends on soil nutrients, fauna and maize growth. Biol. Fertil. Soils 2019. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, C.; Gray, E.M.; Boyd, S.E.; Yang, H.; Zhang, D. Roles of biochar in improving phosphorus availability in soils: A phosphate adsorbent and a source of available phosphorus. Geoderma 2016. [Google Scholar] [CrossRef]
- Wang, D.; Li, C.; Parikh, S.J.; Scow, K.M. Impact of biochar on water retention of two agricultural soils—A multi-scale analysis. Geoderma 2019. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011. [Google Scholar] [CrossRef]
- Leng, L.; Huang, H.; Li, H.; Li, J.; Zhou, W. Biochar stability assessment methods: A review. Sci. Total Environ. 2019. [Google Scholar] [CrossRef] [PubMed]
- Jirka, S.; Tomlinson, T. State of the Biochar Industry 2013—A Survey of Commercial Activity in the Biochar Field; International Biochar Initiative: Philadeplhia, PA, USA, 2014. [Google Scholar]
- Vochozka, M.; Maroušková, A.; Váchal, J.; Straková, J. Biochar pricing hampers biochar farming. Clean Technol. Environ. Policy 2016. [Google Scholar] [CrossRef]
- Latawiec, A.E.; Strassburg, B.B.N.; Junqueira, A.B.; Araujo, E.; Luiz, L.F.; Pinto, H.A.N.; Castro, A.; Rangel, M.; Malaguti, G.A.; Rodrigues, A.F.; et al. Biochar amendment improves degraded pasturelands in Brazil: Environmental and cost-benefit analysis. Sci. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Maroušek, J.; Strunecký, O.; Stehel, V. Biochar farming: Defining economically perspective applications. Clean Technol. Environ. Policy 2019. [Google Scholar] [CrossRef]
- Giwa, A.; Hasan, S.W. Novel thermosiphon-powered reverse osmosis: Techno-economic model for renewable energy and fresh water recovery. Desalination 2018. [Google Scholar] [CrossRef]
- Yacob, T.W.; Fisher, R.; Linden, K.G.; Weimer, A.W. Pyrolysis of human feces: Gas yield analysis and kinetic modeling. Waste Manag. 2018. [Google Scholar] [CrossRef]
- Sri Shalini, S.; Palanivelu, K.; Ramachandran, A.; Raghavan, V. Biochar from biomass waste as a renewable carbon material for climate change mitigation in reducing greenhouse gas emissions—A review. Biomass Convers. Biorefinery 2020. [Google Scholar] [CrossRef]
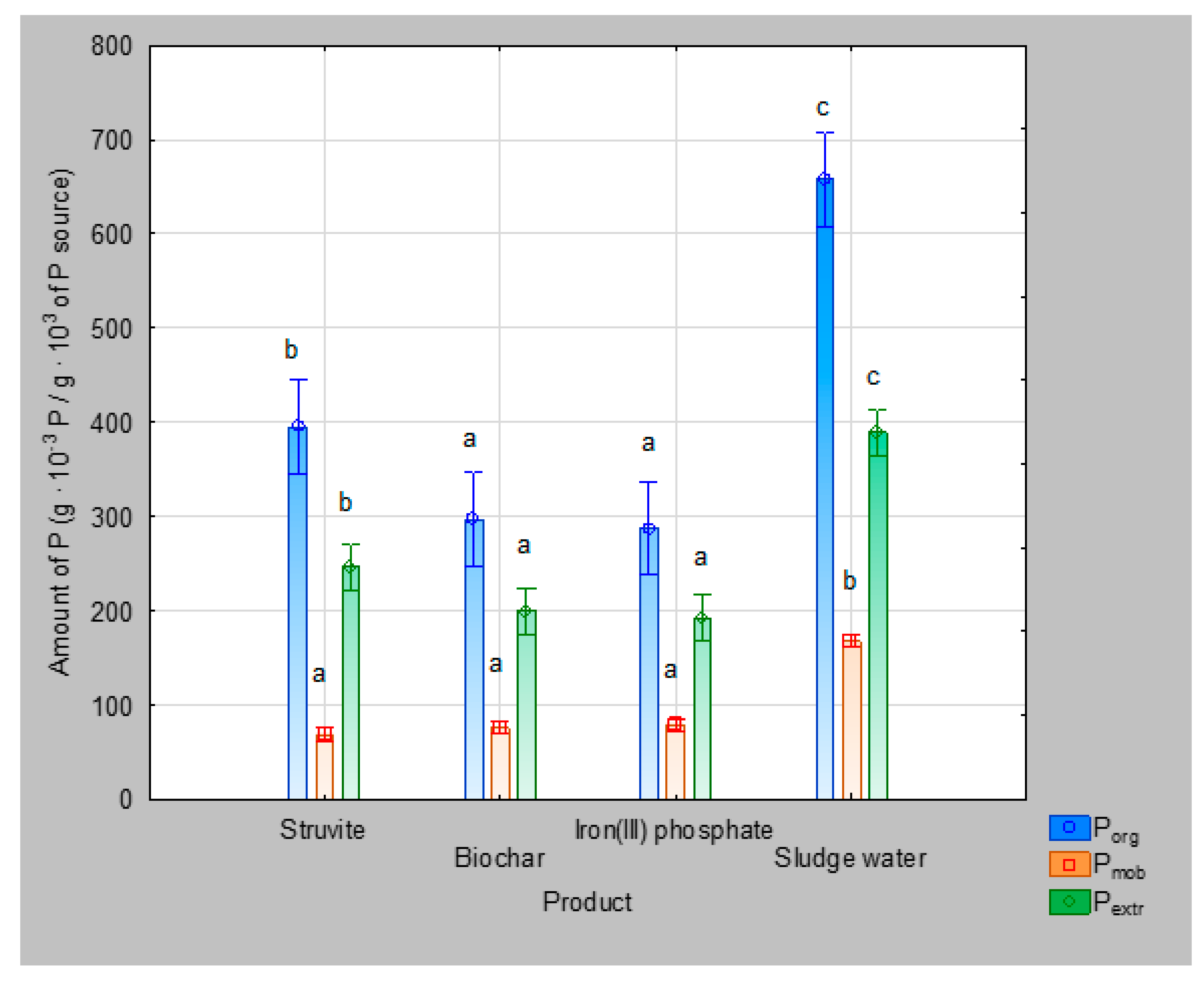
| Pmob (g · 10−3 /g · 103) | Pextr (g · 10−3 /g · 103) | CaP I (%Pextr) | CaP II (%Pextr) | CaP III (%Pextr) | AlP (%Pextr) | FeP (%Pextr) | Porg (g · 10−3 /g · 103) | Ptot (g · 10−3 /g · 103) |
|---|---|---|---|---|---|---|---|---|
| 25 ± 4 | 138 ± 17 | 10 | 14 | 22 | 26 | 28 | 294 ± 23 | 1828 ± 22 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopecký, M.; Kolář, L.; Konvalina, P.; Strunecký, O.; Teodorescu, F.; Mráz, P.; Peterka, J.; Váchalová, R.; Bernas, J.; Bartoš, P.; et al. Modified Biochar—A Tool for Wastewater Treatment. Energies 2020, 13, 5270. https://doi.org/10.3390/en13205270
Kopecký M, Kolář L, Konvalina P, Strunecký O, Teodorescu F, Mráz P, Peterka J, Váchalová R, Bernas J, Bartoš P, et al. Modified Biochar—A Tool for Wastewater Treatment. Energies. 2020; 13(20):5270. https://doi.org/10.3390/en13205270
Chicago/Turabian StyleKopecký, Marek, Ladislav Kolář, Petr Konvalina, Otakar Strunecký, Florina Teodorescu, Petr Mráz, Jiří Peterka, Radka Váchalová, Jaroslav Bernas, Petr Bartoš, and et al. 2020. "Modified Biochar—A Tool for Wastewater Treatment" Energies 13, no. 20: 5270. https://doi.org/10.3390/en13205270
APA StyleKopecký, M., Kolář, L., Konvalina, P., Strunecký, O., Teodorescu, F., Mráz, P., Peterka, J., Váchalová, R., Bernas, J., Bartoš, P., Filipov, F., & Bucur, D. (2020). Modified Biochar—A Tool for Wastewater Treatment. Energies, 13(20), 5270. https://doi.org/10.3390/en13205270