Abstract
Chemical composition influences the calorific power of wood, mainly due to the calorific power of structural compounds and extractives. Heat treatment changes the chemical composition of treated wood. This work studies the relationship between chemical composition and calorific power improvement by heat treatment. Samples were heat-treated by the ThermoWood process ® for 1 h and 2 h. High heating value (HHV) and chemical composition; lignin, cellulose, hemicelluloses and extractives in dichloromethane, ethanol, and water were determined. The HHV of untreated wood ranged between 18.54–19.92 MJ/kg and increased with heat treatment for all the tested species. A positive linear correlation was found between HHV and Klason lignin (R2 = 0.60). A negative trend was observed for holocellulose, cellulose, and hemicelluloses content against HHV, but with low determination coefficients for linear regression. The best adjust for polysaccharides was found for hemicelluloses content. A positive correlation could be found for dichloromethane extractives (R2 = 0.04). The same was obtained in relation to ethanol extractives with R2 = 0.20. For water and total extractives, no clear positive or negative trends could be achieved. The results showed that the HHV of wood increased with heat treatment and that this increase was mainly due to the increase in lignin content.
1. Introduction
Heat treatment is a well-established process used to improve some wood properties like dimensional stability and durability of low-value species allowing them to be used in more demanding use classes like use class 3 [1,2,3,4]. There are several commercial processes like Thermowood ®, Plato ®, Bois Perdure ®, Rectification ®, and Oil Heat Treatment ®, that differ in the way heating is done and in what is used to shield the wood from oxygen like steam, inert gas, or vegetable oil. These treatments are generally made at relatively low temperatures (180–220 °C) compared to torrefaction processes nevertheless are done at a temperature high enough to alter the chemical composition of wood, producing new material with improved properties. Chemically it is known that hemicelluloses are the first compounds to be affected by the treatment, due to their amorphous nature, low molecular weight, and branched structure. Degradation begins with the cleavage of the acetyl groups of hemicelluloses with the consequent release of acetic acid that leads to further depolymerization of polysaccharides [5]. At the same time, dehydration reactions occur with furfural formation in pentoses and hydroxy-methyl-furfural in hexoses [5]. Although cellulose is more resistant than hemicelluloses, there is a degradation of amorphous cellulose, and consequently, an increase in crystalline cellulose. Lignin is affected by the treatment, but its degradation is slower than that of carbohydrates, leading to an increased percentage with treatment [6]. These chemical changes derived from the treatment are known to increase the calorific power of heat-treated woods [7].
The influence of chemical composition on the calorific power of wood is mainly due to the higher calorific power of lignin compared to cellulose [8]. In accordance with Demirbas [9], cellulose, and hemicelluloses have an HHV of 18.60 MJ/kg, while lignin has an HHV of 23.26–25.58 MJ/kg. However, extractives might also play an important role in the value of the calorific power of woods. For instance, Doat [10] studied several tropical woods and concluded that ethanol extractives have high calorific power. In accordance with Rossi et al. [11], that studied the effect of extractive removal on the calorific value of Jatoba (Hymenaea courbaril), Tornillo (Cedrelinga catenaeformis), Ipe (Tabebuia sp.), and Brazilwood (Paubrasilia echinata) wood residues concluded that there was no significant change on the calorific value by the removal of extractives from Brazilwood, while for Tornillo and Jatoba, extractive removal led to a decrease in wood calorific value showing that extractives had a higher calorific value than structural wood compounds. In relation to Ipe, the removal of extractives increased the calorific value, which could be due to the low calorific value of these compounds. Zanuncio et al. [12] stated that the removal of total extractives and of dichloromethane extractives reduced the gross calorific value of most of the wood species tested. Nevertheless, the removal of extractives in cold water did not influence the gross calorific value, while the removal of dichloromethane extracts reduced the gross calorific value of E. urophylla, P. oocarpa, and C. citriodora. This was attributed to the fewer hydroxyl groups and more carbon content of dichloromethane extractives compared to water extractives.
Several studies have been made to test the correlation between lignin content and high heating value (HHV) of wood. For example, White [13], determined the lignin content of four softwoods, Engelmann spruce (Picea engelnzanni), Western redcedar (Thuja plicata), Southern pine (Pinus sp.), Redwood (Sequoia sempervirens) and four hardwoods, Maple (Acer sp.), Yellow-poplar (Liriodendron tulipifera), Red oak (Quercus sp.), and Basswood (Tilia sp.) and concluded that the HHV increased with the lignin content. Hardwoods were hard.
This increase was linear, with a determination coefficient (R2) of 0.70. Likewise, Ngangyo-Heya et al. [14], that studied five timber species of the semi-arid land of Mexico (Helietta parvifolia, Ebenopsis ebano, Acacia berlandieri, Havardia pallens, and Acacia wrightii ) obtained a linear correlation (R2 = 0.44) between lignin and calorific value. Demirbas [15], obtained a stronger correlation between lignin and the HHV of extracted samples (R2 = 0.874), similar to Acar et al. [15], that tried to correlate the HHVs of nine biomass samples with lignin content and obtained a positive linear relation with a determination coefficient (R2) of 0.932. A positive trend was also reported by Enes et al. [16], with several scrubs, agriculture, and forest wastes, but with a lower determination coefficient (0.242), probably due to the mixture of different materials.
Some authors tried to correlate extractive content with the HHV of wood, nevertheless, most of the studies show that this is dependent on the species and on the kind of extractives. In accordance with Ruiz-Aquino et al. [17], the higher heating value of five tree species from Oaxaca, Mexico showed a positive correlation with the extractives content (R2 = 0.339), while Howard [18] reported a positive correlation between the alcohol/benzene extractives of loblolly pine and the higher heating value (R2 = 0.54). Whereas, Ngangyo-Heya et al. [14], that studied the calorific value and chemical composition of five semi-arid Mexican tree species, obtained a weak correlation between the calorific value and the number of extractives with a determination coefficient (R2) of 0.02.
There have also been several attempts to estimate HHV from the lignin content or lignin combined with extractives content. According to White [13], the inclusion of extractive content on the linear regression equation improved the R2 from 0.70 to 0.76. Telmo and Lousada [19] proposed a formula to correlate the HHV with lignin and extractives content and stated that the model explained 83.66% of the variation. These authors also stated that the percentage of HHV explained by lignin was 56.42% and by extractive contents 43.58%.
In accordance with Demirbas [9], which obtained no significant correlations between HHV and the holocellulose content, there are no direct relations between HHV and the holocellulose. Telmo and Lousada [19] mentioned a work reported in Shafizadeh [8] where the correlation between HHV and holocellulose was obtained, but no more information was given.
The main objective of this work was to study the relationship between chemical composition and calorific power improvement by heat treatment of eight wood species from Turkey. This knowledge will allow us to estimate the calorific power of other treated woods and will give us some information about the energy that can be produced at the end of these woods service life.
2. Materials and Methods
2.1. Materials
Samples of eight different wood species from the Duzce city region in Turkey were obtained from a local mill. The species were duka (Tapirira marchandii), afrormosia (Pericopsis elata), black locust (Robinia pseudoacacia L.), wenge (Millettia laurentii), sipo (Entandrophragma utile), santos (Myroxylon balsamum), rose (Dalbergia nigra), and zebrano (Microberlinia brazzavillensis). These samples were heat-treated by the ThermoWood ® method. The treatment was done at 212 °C for 1 and 2 h in the heat treatment kiln. The kiln belonged to a private commercial Novawood Factory in Gerede–Bolu.
2.2. Methods
The average chemical composition of each sample was determined. All samples were grounded in a Retsch SMI mill and sifted with a sieve Retsch AS200 for 20 min at a speed of 50 rpm. The 40–60 mesh fraction was used for the chemical analysis.
The extractive content was determined by successive Soxhlet extraction using about 3 g of each sample and 200 mL dichloromethane (DCM), ethanol, and water as solvents. The extraction time was 6 h for DCM and 16 h for ethanol and water. The extractive content was determined in relation to the dry wood in accordance with TAPPI T 204 “Solvent Extractives of Wood and Pulp” [20]. Insoluble lignin was determined by the Klason method Tappi T 222 om-02 [21] with some changes, as follows. 350 mg of grounded samples were placed in a beaker, and 3 mL of 72% iced sulfuric acid was added to each sample and placed in a 30 °C thermal bath for one hour, stirring the mixture with a glass rod every 10 min. In the end, 84 mL of distilled water were added and transferred to 100 mL thermal glass bottles, which were placed in an autoclave with water at the bottom. This step differs from the standard once the second hydrolysis is done in an autoclave at 120 °C, rather than boiling the solution for 4 h. The samples remained in the autoclave for one hour at a temperature of 120 °C. The bottles of the autoclave were removed and cooled with ice. The mixture was filtered with Nº4 crucibles. The percentage of insoluble lignin was determined in relation to dry wood.
For the determination of Holocellulose, the acid chlorite method was used. A solution A (8.5 g of sodium chlorite dissolved in 250 mL of distilled water) and a solution B (13.5 g of sodium hydroxide dissolved in 50 mL of distilled water to which 37.5 mL of glacial acetic acid is added and filling with water until 250 mL). 2 g of each of the extracted woods was placed in a 1 L flask, to which 160 mL of distilled water is added, 20 mL of solution A and 20 mL of solution B. Flasks were placed in a water bath at 70 °C, under reflux with a condenser to avoid losing liquid, for 3 h, adding 20 mL of each solution after each hour. If, after three hours, the wood still had a brownish color, the procedure continued until the whole sample became whitish, then filtered in a crucible no. 2. The samples were rinsed thoroughly with cold water followed by 15 mL of acetone and dried overnight at 60 °C in the oven, followed by 1 h at 100 °C, cooled, and weighed. The percentage of holocellulose was determined in relation to the dry mass of wood.
A-cellulose was determined by weighing about 0.5 g of dry holocellulose and placing it in a 100 mL glass, adding 2.5 mL of 17.5% sodium hydroxide, and covering with a watch glass. The glass was placed in a thermal bath at 20 °C, stirred, and after 30 min, was removed from the bath, waiting another 15 min. Then, 8.25 mL of water at 20 °C was added and the mixture was shaken, and put in the bath for 1 h. Afterward, the solution was filtered in a pre-weighed crucible by washing with 25 mL of 8.3% sodium hydroxide, ending with distilled water. At the end, 3.75 mL of acetic acid were added to the crucible without the suction waiting for 3 min, then turned on the suction and rinsed again with plenty of distilled water. The crucible was dried at 105 °C, cooled, and weighed. A-cellulose content was determined in relation to dry wood.
The calorific value (high heating value) was determined using a Parr calorimeter–model 6400. The grounded sample after being completely dried at 105 °C, and afterward, pressed (4 ton, 10 s) to form a 1 cm diameter pellet, which was inserted into the heat pump. The calorific value of a material is the amount of heat released when the mass unit of that material is completely burned under certain conditions. Since it is not possible to determine directly the amount of heat released in the combustion, the temperature rise in the water contained in the calorimetric container surrounding the sample is measured. Knowing the temperature rise and the calorific capacity (C) of water (amount of heat required to heat the water 1 °C), the HHV of the material was obtained. Three replicates for each wood sample were made.
Two different one-way ANOVA were done in Microsoft Excel 2016 to test if the differences with heat treatment are significant at 0.05 (P1 value) and to test if there are significant differences between 1 h and 2 h treatment (P2 value).
3. Results and Discussion
Table 1 presents the average and the standard deviation of high heating values (HHV) of eight different species before and after being heat-treated for 1 h and 2 h. The table also presents the P values for one-way ANOVA at 0.05 significance level. The HHV of untreated wood ranged between 18.54 MJ/kg for black locust and 19.92 MJ/kg for afrormosia woods. These values are in accordance with several other studies with hardwoods, such as References [15,22,23,24].

Table 1.
High heating value (HHV) of untreated and heat-treated woods. Standard deviation in brackets.
Heat treatment led to an increase in HHV for all the tested woods. This increase was higher for afrormosia wood from 19.86 MJ/kg to 20.41 MJ/kg and lowest for Duka wood with just an increase from 19.73 MJ/kg to 20.18 MJ/kg corresponding to 10.1% and 2.3% increase, respectively. The changes with heat treatment were all considered to be significant at 0.05 level, as shown in Table 1, with P1 values ranging from 0.000 to 0.024, all under 0.05. Whereas, no significant differences were observed between wood treated during 1h and 2h, with the exception of Black locust (P2 value = 0.000), and rosewood (P2 value = 0.007). Nevertheless, in accordance with Domingos et al. [25], that studied the HHV of Eucalyptus globulus and Pinus pinaster woods treated for 224 h and at temperatures from 170 °C to 190 °C, there was an increase in HHV of wood with heat treatment and this increase was higher for higher treatment temperatures and also for a longer time of treatment at the same temperature. Therefore, there should be a significant increase between 1h and 2 h treatment. This difference might be attributed to the fact that commercial equipment was used for the treatment and it was not possible to open the autoclave in the middle of the cycle, so the samples for 1 h (212 °C) were in a different batch than the samples with 2 h (212 °C). Generally, there is an increase in the HHV with heat treatment, and that increase is higher for higher treatment times. The increase in HHV might be due to the increase in lignin content in relation to the carbohydrates since lignin is known to have a higher HHV than carbohydrates [8]. It is well known that wood heat treatments like ThermoWood ® increases the percentage of lignin, due to the higher degradation rate of polysaccharides [6,7,26].
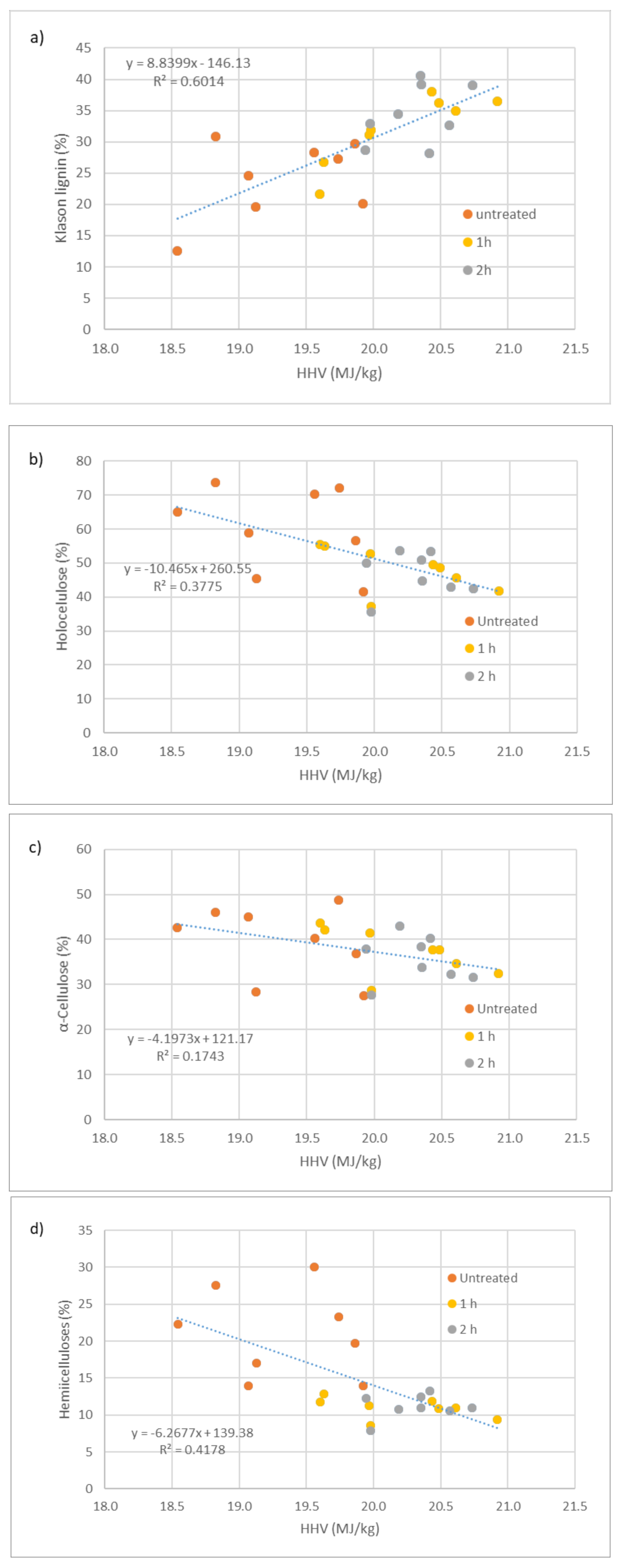
The variation of HHV with structural wood compounds is presented in Figure 1. Regarding Klason lignin (Figure 1a), as shown in the figure, there seems to be a higher dispersion of values for untreated wood in relation to treated wood. As mentioned before, untreated wood HHV is lower than for heat-treated wood, which is clear in the figure. Linear regression was made to determine the relationship between HHV and Klason lignin regardless of being untreated or heat-treated wood. The correlation coefficient was R2 = 0.60, showing that there is a positive linear relation between HHV and Klason lignin. Similar results were reported by White [13], for a mixture of eight species, four softwoods and four hardwoods (R2 = 0.70) or Ngangyo-Heya et al. [14], for five timber species of the semi-arid land of Mexico (Helietta parvifolia, Ebenopsis ebano, Acacia berlandieri, Havardia pallens, and Acacia wrightii) (R2 = 0.44). Stronger correlations for the linear regressions between the amount of lignin and the HHV were obtained by Domingos [25], for heat-treated pine and eucalypt woods with R2 of 0.89 and 0.90 for pine and eucalypt wood, respectively. Nevertheless, these correlations were obtained with just one specie at the same time, which eliminates some of the variability. Then, again, Acar et al. [27] reported a better linear correlation (R2 = 0.93) with nine different biomass samples. When considering treated wood alone, the R2 would be 0.53, while for untreated wood would be only 0.17, which confirms the higher dispersion mentioned for untreated wood. Similar to lignin, holocellulose values show a higher dispersion for untreated wood (Figure 1b), which seems to indicate that heat treatment equalizes wood chemical composition. The partial determination coefficients were R2 = 0.14 for heat-treated and R2 = 0.09 for untreated wood. As expected, holocellulose contents are higher for untreated wood, and therefore, HHV is lower. There is a clear negative trend between holocellulose content and HHV of wood, and this trend is valid for both untreated and heat-treated wood. The linear regression between holocellulose content and HHV has an R2 = 38 showing that at least 38% of the variance in HHV is predictable from Holocellulose content. A-cellulose results (Figure 1c) are similar to that of holocellulose—showing a negative trend with HHV, but with a lower determination coefficient (R2 = 0.17). Once again, there is a higher dispersion on untreated wood values et al. (R2 = 0.11) when compared to heat-treated wood (R2 = 0.17). In relation to hemicelluloses content, results are approximately the same as for cellulose with a higher R2 for the linear regression (0.42). Nevertheless, when considering untreated wood (R2 = 0.03) or heat-treated wood alone (R2 = 0.02), the determination coefficients are much lower. This shows that there is a positive trend for lignin and a negative trend for all the polysaccharide compounds. Only lignin, shows some potential to estimate the HHV of wood by Equation (1), however, there is still a lot of unexplained variation. Equation (1) was obtained by the linear regression between lignin (L) and high heating value (HHV).
HHV (MJ/kg) = 0.068 × L(%) + 17.893
Figure 1.
Variation of HHV with structural compounds of untreated and heat-treated woods: (a) Variation of HHV with Klason lignin; (b) with holocellulose; (c) with α-cellulose. (d) with hemicelluloses.
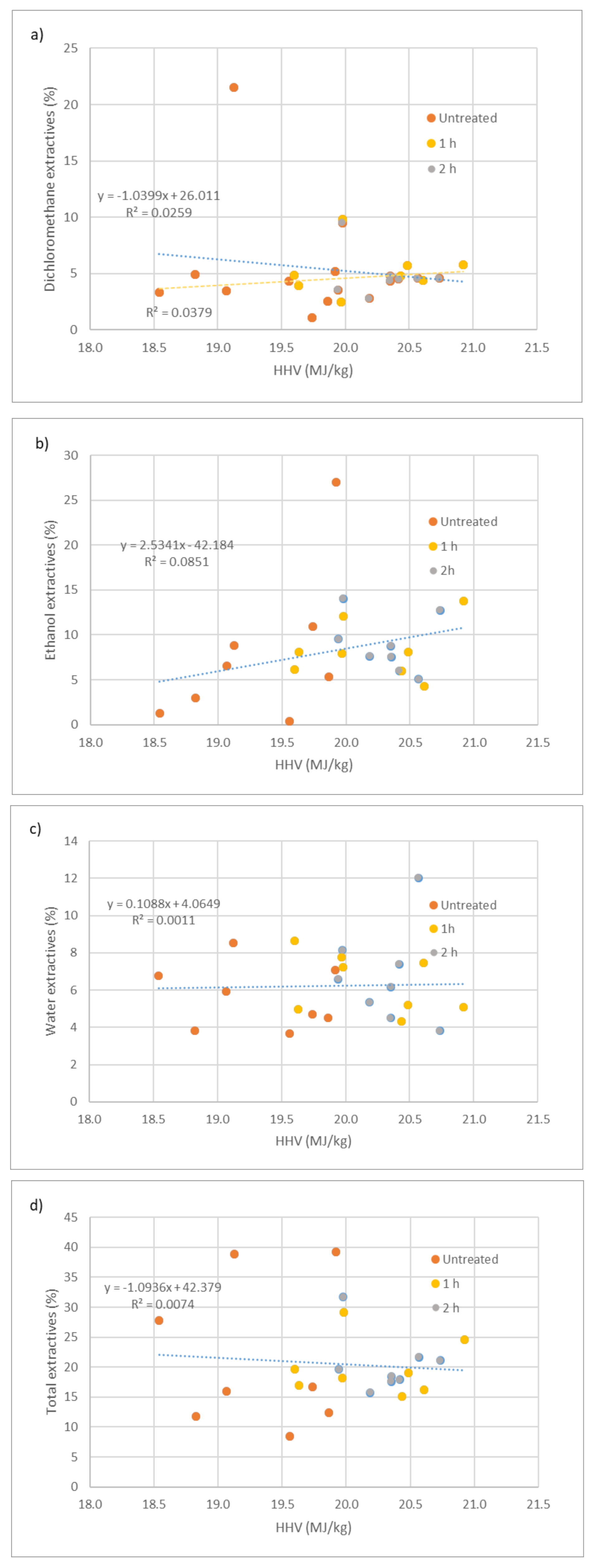
Figure 2 shows the variation of HHV with dichloromethane, ethanol, and water extractives. Generally, heat-treated wood has a higher number of extractives than untreated wood, as shown in Figure 2d. The increase is more noticeable in ethanol extractives (Figure 2b) and somewhat in water extractives (Figure 2c). Only two untreated samples have a higher number of extractives than heat-treated, due to a high percentage of dichloromethane extractives in untreated Santos wood and many ethanol extractives in Wenge wood. The increase in extractive content with heat treatment has been reported before [6,26]. In accordance with Esteves et al. [6], most of the original wood extractives are released or degrade with heat treatment, particularly the most volatile compounds. Whereas, new extractives appear from the degradation products of the structural polymers. Since hemicelluloses and amorphous cellulose are the most affected compounds, due to heat treatment, the major increase is seen in water and ethanol extractives, which is where the degradation products of polysaccharides can be found. Since Santos wood has many dichloromethane extractives, compared to all the remaining samples, a regression analysis was done with and without this sample. Using all the samples, there would be a negative trend (blue line) between dichloromethane extractives and HHV of wood, which clearly is not the case. Excluding this sample, a positive correlation could be found (yellow line), however, with a very low determination coefficient (R2 = 0.04). If we consider heat-treated wood alone, the determination coefficient increases to R2 = 0.52, while untreated wood stays approximately the same (R2 = 0.04). Identical results were obtained in relation to ethanol extractives with R2 = 0.09. Without the sample for untreated wenge, the determination coefficient improves to R2 = 0.20. However, for ethanol extractives, there is a clear positive relation between extractive content and HHV of wood; no significant difference was observed between untreated and heat-treated wood. Surprisingly when considering untreated wood alone, the R2 increases to 0.32, while for heat-treated wood decreases by 0.004. For water and total extractives, no clear positive or negative trends could be achieved. The R2 was 0.001 for water and 0.007 for total extractives. Analyzing water extractives relation for untreated (R2 = 0.04) and heat-treated wood alone (R2 = 0.04) gives higher R2, for total extractives R2 = 0.004 (heat-treated) and R2 = 0.001 (untreated) there isn´t much improvement. Similar results were reported before by Ngangyo-Heya et al. [14], with five Mexican tree species. These authors obtained a weak correlation between the calorific value and the number of total extractives (r = 0.13). According to Moya and Tenorio [28], the difficulty of finding good correlations between extractives and HHV of wood is because once combustion is an oxidation reaction and the heat of combustion of an organic compound is associated with its level of oxidation, species with extractives containing mostly carbon- and hydroge—like some terpenoid hydrocarbons produce much more energy than others containing phenolic compounds with higher oxidation levels. Therefore, once in this study, very different species with different extractives were used, no correlation could be obtained for total extractives. Better results were attained by Ruiz-Aquino et al. [17], with five tree species from the Oaxaca region in Mexico, who reported a positive correlation for the higher heating value with the extractives content (R2 = 0.34), however, this could be due to having species with similar extractives. Since the composition of untreated and heat-treated wood extractives is very different, as stated before [6,26]. This might explain the low correlations obtained for dichloromethane extractives. Most of the original dichloromethane extractives like terpenes and terpenoids, waxes, fats, and fatty acids are known to be released or degraded along with the treatment and are substituted by mostly aldehydes like syringaldehyde and sinapaldehyde [6,26].
Figure 2.
Variation of HHV with extractives of untreated and heat-treated woods: (a) Dichloromethane extractives; (b) ethanol extractives; (c) water extractives; (d) total extractives.
4. Conclusions
The results showed that the HHV of wood increased with the heat treatment and that this increase was mainly due to the increase in lignin content, while dichloromethane and ethanol extractives might also play an important role. The increase in lignin content was due to the higher degradation rate in relation to polysaccharide compounds. Only for lignin content, a positive linear correlation could be found with the HHV of wood, while for holocellulose, cellulose, and hemicelluloses, a negative trend was observed. A positive trend could also be found for dichloromethane and ethanol extractives, but with no linear correlation. For water and total extractives, no clear positive or negative trends could be achieved.
Author Contributions
Conceptualization by B.E.; formal analysis, heat treatment was done by U.A. and S.S., I.D.; performed HHV measurements and most of chemical composition determination helped by U.A., L.C.-L., A.S. and J.F.; resources were provided by U.A. and S.S.; writing, review and editing was done by B.E. with the cooperation of the remaining authors throughout the manuscript; funding acquisition, B.E., I.D., J.F. and L.C.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Funds through the FCT—Foundation for Science and Technology, I.P., within the scope of the project Refª UIDB/00681/2020. Furthermore, we would like to thank the CERNAS Research Centre and the Polytechnic Institute of Viseu for their support.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Esteves, B.M.; Pereira, H. Wood modification by heat treatment: A review. BioResources 2009, 4, 340–404. [Google Scholar]
- Brito, A.F.; Calonego, F.W.; Bond, B.H.; Severo, E.T.D. Color changes, EMC and biological resistance of thermally modified yellow poplar. Wood Fiber Sci. 2018, 50, 439–446. [Google Scholar] [CrossRef]
- Kozakiewicz, P.; Drożdżek, M.; Laskowska, A.; Grześkiewicz, M.; Bytner, O.; Radomski, A.; Zawadzki, J. Effects of thermal modification on selected physical properties of sapwood and heartwood of black poplar (Populus nigra L.). BioResources 2019, 14, 8391–8404. [Google Scholar]
- Tjeerdsma, B.; Boonstra, M.; Militz, H. Thermal modification of non-durable wood species. 2. Improved wood properties of thermal treated wood. The international research group on wood preservation, section 4-processes. In Proceedings of the 29th Annual Meeting, Maastricht, The Netherlands, 9–13 September 2018. [Google Scholar]
- Tjeerdsma, B.F.; Boonstra, M.; Pizzi, A.; Tekely, P.; Militz, H. Characterisation of thermally modified wood: Molecular reasons for wood performance improvement. Holz Roh-Werkst. 1998, 56, 149–153. [Google Scholar] [CrossRef]
- Esteves, B.; Graça, J.; Pereira, H. Extractive composition and summative chemical analysis of thermally treated eucalypt wood. Holzforschung 2008, 62, 344–351. [Google Scholar] [CrossRef]
- Araújo, S.d.O.; Neiva, D.M.; Gominho, J.; Esteves, B.; Pereira, H. Chemical effects of a mild torrefaction on the wood of eight Eucalyptus species. Holzforschung 2017. [Google Scholar] [CrossRef]
- Shafizadeh, F. Thermal Uses and Properties of Carbohydrates and Lignins; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Demirbas, A. Relationships between lignin contents and heating values of biomass. Energy Convers. Manag. 2001, 42, 183–188. [Google Scholar] [CrossRef]
- Doat, J. Le pouvoir calorifique des bois tropicaux. Bois For. Trop. 1977, 172, 33–55. [Google Scholar]
- Rossi, T.; da Moura, L.F.; Torquato, P.R.; Brito, J.O. Effect of extractive removal on the calorific value of Brazilian woods residues. J. Chem. Chem. Eng. 2013, 7, 340. [Google Scholar]
- Zanuncio, A.J.V.; Carvalho, A.G.; Trugilho, P.F.; Monteiro, T.C. Extractives and energetic properties of wood and charcoal. Rev. Árvore 2014, 38, 369–374. [Google Scholar] [CrossRef]
- White, R.H. Effect of lignin content and extractives on the higher heating value of wood. Wood Fiber Sci. 2007, 19, 446–452. [Google Scholar]
- Ngangyo-Heya, M.; Foroughbahchk-Pournavab, R.; Carrillo-Parra, A.; Rutiaga-Quiñones, J.G.; Zelinski, V.; Pintor-Ibarra, L.F. Calorific value and chemical composition of five semi-arid Mexican tree species. Forests 2016, 7, 58. [Google Scholar] [CrossRef]
- Demirbas, A. Calculation of higher heating values of biomass fuels. Fuel 1997, 76, 431–434. [Google Scholar] [CrossRef]
- Enes, T.; Aranha, J.; Fonseca, T.; Lopes, D.; Alves, A.; Lousada, J. Thermal properties of residual agroforestry biomass of northern portugal. Energies 2019, 12, 1418. [Google Scholar] [CrossRef]
- Ruiz-Aquino, F.; Ruiz-Ángel, S.; Feria-Reyes, R.; Santiago-García, W.; Suárez-Mota, M.E.; Rutiaga-Quiñones, J.G. Wood Chemical Composition of Five Tree Species from Oaxaca, Mexico. BioResources 2019, 14, 9826–9839. [Google Scholar]
- Howard, E.T. Heat of combustion of various southern pine materials. Wood Sci. 1972, 5, 194–197. [Google Scholar]
- Telmo, C.; Lousada, J. The explained variation by lignin and extractive contents on higher heating value of wood. Biomass Bioenergy 2011, 35, 1663–1667. [Google Scholar] [CrossRef]
- TAPPI. Solvent Extractives of Wood and Pulp. TAPPI T204 cm-07; TAPPI Press: Atlanta, GA, USA, 2007. [Google Scholar]
- Standard, T. T 222 om-02. Acid-Insoluble Lignin in Wood and Pulp; TAPPI: Atlanta, GA, USA, 2002. [Google Scholar]
- Günther, B.; Gebauer, K.; Barkowski, R.; Rosenthal, M.; Bues, C.-T. Calorific value of selected wood species and wood products. Eur. J. Wood Wood Prod. 2012, 70, 755–757. [Google Scholar] [CrossRef]
- Telmo, C.; Lousada, J. Heating values of wood pellets from different species. Biomass Bioenergy 2011, 35, 2634–2639. [Google Scholar] [CrossRef]
- García, R.; Pizarro, C.; Lavín, A.G.; Bueno, J.L. Characterization of Spanish biomass wastes for energy use. Bioresour. Technol. 2012, 103, 249–258. [Google Scholar] [CrossRef]
- Domingos, I.; Ferreira, J.; Lopes, L.P.C.; Esteves, B. Increase of calorific value of two important feedstocks by mild torrefaction. In Proceedings of the 11th Conference on Sustainable Development of Energy, Water and Environment Systems, Lisbon, Portugal, 4–9 September 2016; pp. 540–541. [Google Scholar]
- Esteves, B.; Videira, R.; Pereira, H. Chemistry and ecotoxicity of heat-treated pine wood extractives. Wood Sci. Technol. 2010. [Google Scholar] [CrossRef]
- Acar, S.; Ayanoglu, A.; Demirbas, A. Determination of higher heating values (HHVs) of biomass fuels. Energy Educ. Sci. Technol. Part A Energy Sci. Res. 2012, 28, 749–758. [Google Scholar]
- Moya, R.; Tenorio, C. Fuelwood characteristics and its relation with extractives and chemical properties of ten fast-growth species in Costa Rica. Biomass Bioenergy 2013, 56, 14–21. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).