Promoting and Inhibitory Effects of Hydrophilic/Hydrophobic Modified Aluminum Oxide Nanoparticles on Carbon Dioxide Hydrate Formation
Abstract
:1. Introduction
2. Materials and Methods
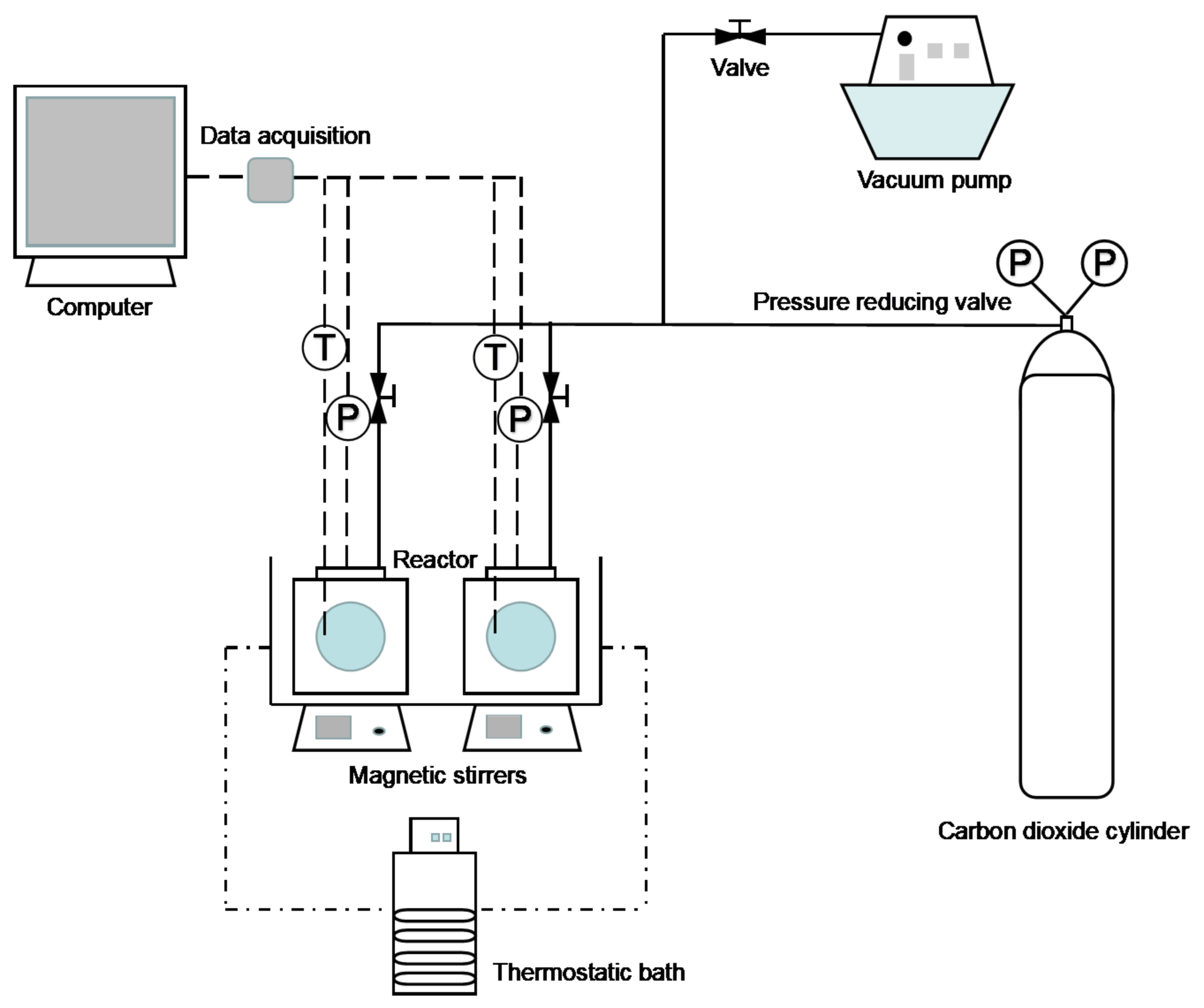
2.1. Apparatus and Materials
2.2. Experimental Procedure
2.2.1. Characterization of the Al2O3 Nanoparticles
2.2.2. Phase Equilibrium Experiments
2.2.3. Hydrate Formation Experiments
2.3. Data Processing
CO2 Consumption
3. Results and Discussion
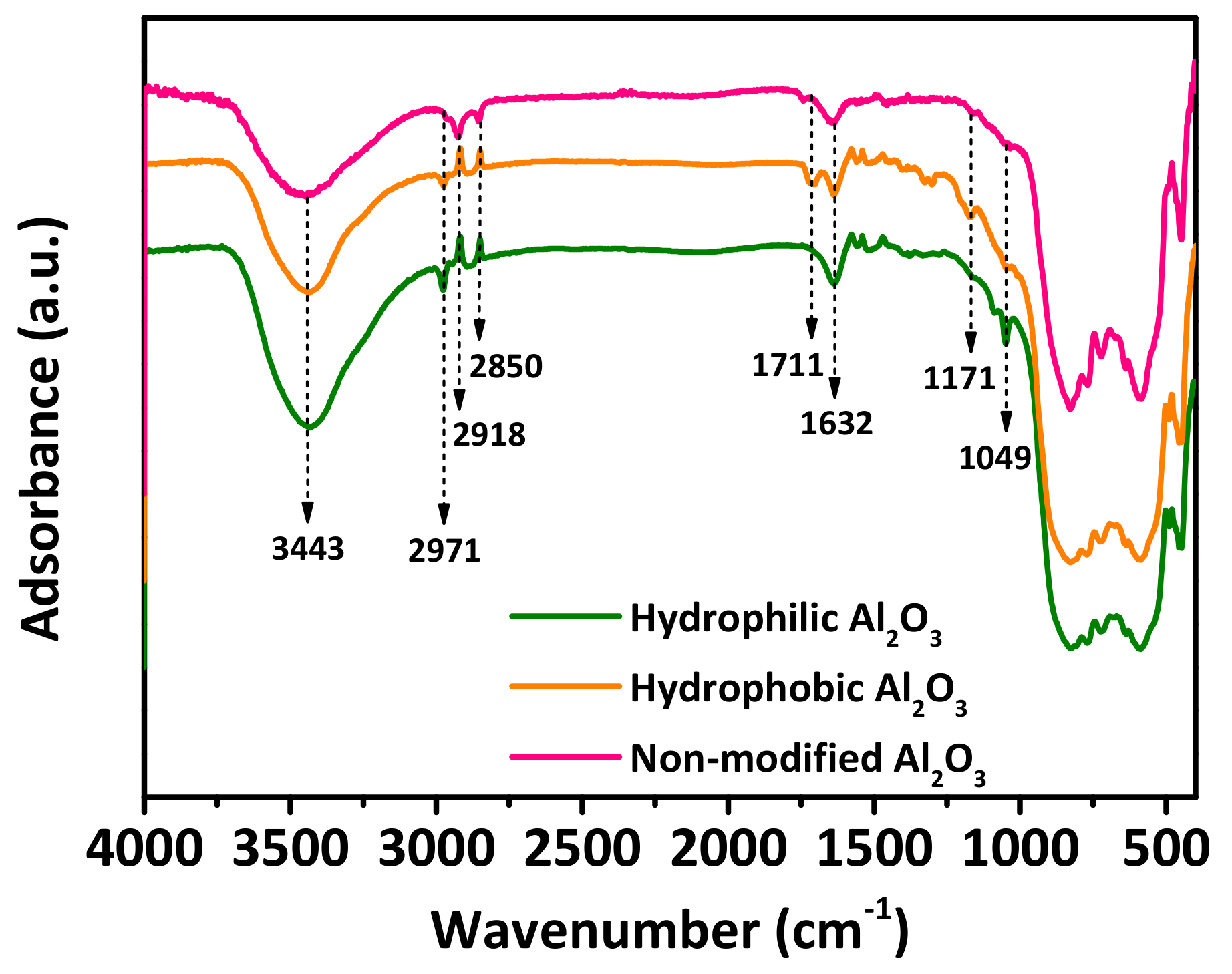
3.1. Characterization Results of the Al2O3 Nanoparticles
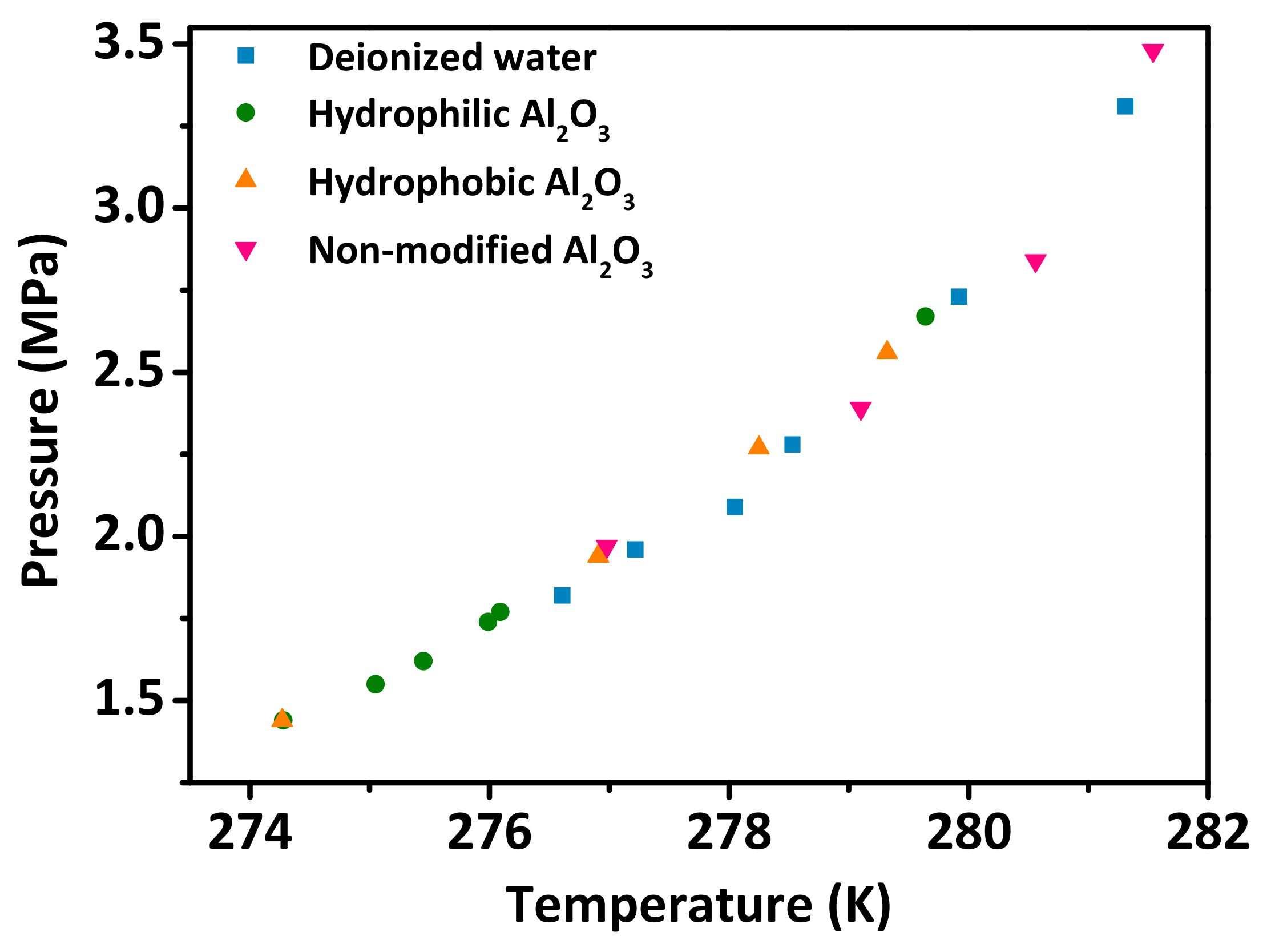
3.2. Effect of the Al2O3 Nanoparticle Additives on the Hydrate Phase Equilibrium
3.3. Effect of the Non-Modified Al2O3 Nanoparticle Additives on the Kinetics of Hydrate Formation
3.4. Effect of the Hydrophilic/Hydrophobic Modified Al2O3 Nanoparticle Additives on the Final Gas Consumption
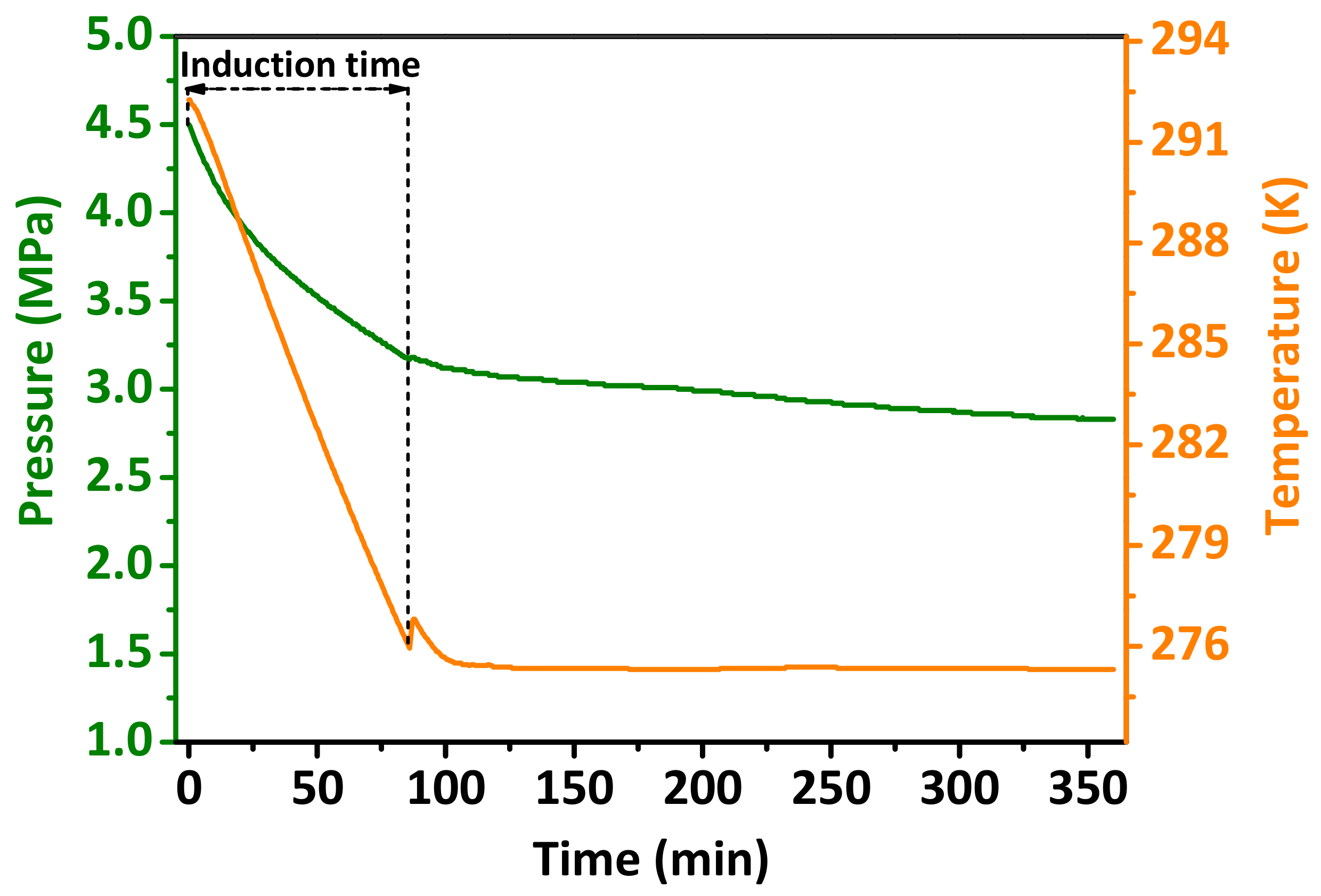
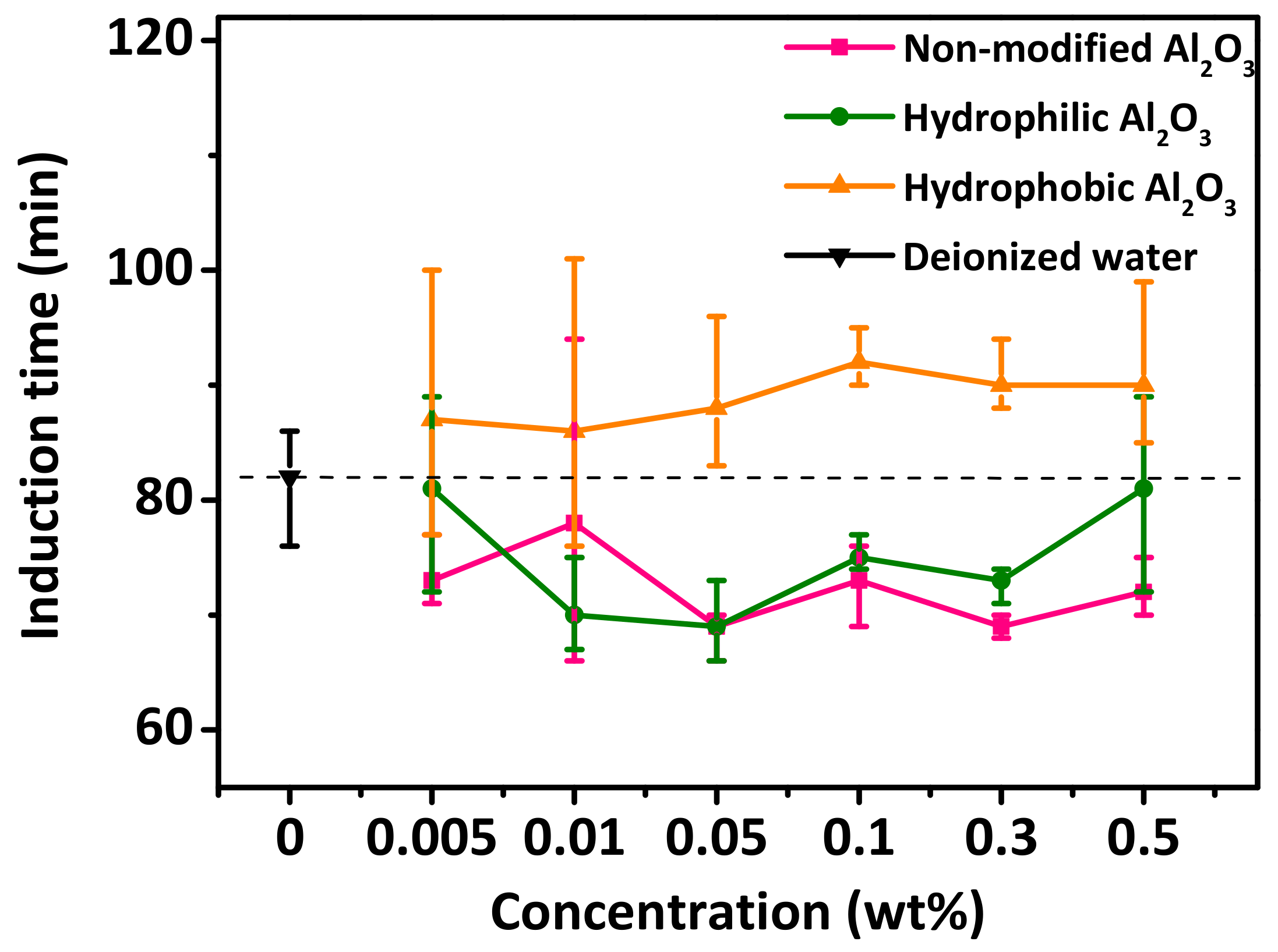
3.5. Effect of the Hydrophilic/Hydrophobic Modified Al2O3 Nanoparticle Additives on the Induction Time
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sloan, E.D., Jr. Fundamental principles and applications of natural gas hydrates. Nature 2003, 426, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.A.; Sloan, E.D. Natural gas hydrates: Recent advances and challenges in energy and environmental applications. AIChE J. 2007, 53, 1636–1643. [Google Scholar] [CrossRef]
- Andres-Garcia, E.; Dikhtiarenko, A.; Fauth, F.; Silvestre-Albero, J.; Ramos-Fernández, E.V.; Gascon, J.; Corma, A.; Kapteijn, F. Methane hydrates: Nucleation in microporous materials. Chem. Eng. J. 2019, 360, 569–576. [Google Scholar] [CrossRef]
- Sloan, E.D.; Koh, C.A. Clathrate Hydrates of Natural Gases, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Yang, M.J.; Song, Y.C.; Jiang, L.L.; Liu, Y.; Li, Y.H. CO2 Hydrate Formation Characteristics in a Water/Brine-Saturated Silica Gel. Ind. Eng. Chem. Res. 2014, 53, 10753–10761. [Google Scholar] [CrossRef]
- Tohidi, B.; Yang, J.H.; Salehabadi, M.; Anderson, R.; Chapoy, A. CO2 Hydrates Could Provide Secondary Safety Factor in Subsurface Sequestration of CO2. Environ. Sci. Technol. 2010, 44, 1509–1514. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.W.; Zhang, P.; Bao, H.S.; Deng, S. Review of fundamental properties of CO2 hydrates and CO2 capture and separation using hydration method. Renew. Sustain. Energy Rev. 2016, 53, 1273–1302. [Google Scholar] [CrossRef]
- Komatsu, H.; Maruyama, K.; Yamagiwa, K.; Tajima, H. Separation processes for carbon dioxide capture with semi-clathrate hydrate slurry based on phase equilibria of CO2+ N2+ tetra-n-butylammonium bromide + water systems. Chem. Eng. Res. Des. 2019, 150, 289–298. [Google Scholar] [CrossRef]
- Bernhardsen, I.M.; Knuutila, H.K. A review of potential amine solvents for CO2 absorption process: Absorption capacity, cyclic capacity and pKa. Int. J. Greenh. Gas Control 2017, 61, 27–48. [Google Scholar] [CrossRef]
- Baker, R.W. Future directions of membrane gas separation technology. Ind. Eng. Chem. Res. 2002, 41, 1393–1411. [Google Scholar] [CrossRef]
- Li, Q.; Fan, S.; Chen, Q.; Yang, G.; Chen, Y.; Li, L.; Li, G. Experimental and process simulation of hydrate-based CO2 capture from biogas. J. Nat. Gas Sci. Eng. 2019, 72, 103008. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.-S.; Seo, Y. Thermodynamic, structural, and kinetic studies of cyclopentane + CO2 hydrates: Applications for desalination and CO2 capture. Chem. Eng. J. 2019, 375, 121974. [Google Scholar] [CrossRef]
- Uilhoorn, F.E. Evaluating the risk of hydrate formation in CO2 pipelines under transient operation. Int. J. Greenh. Gas Control 2013, 14, 177–182. [Google Scholar] [CrossRef]
- Shi, B.-H.; Ding, L.; Li, W.-Q.; Lv, X.-F.; Liu, Y.; Song, S.-F.; Ruan, C.-Y.; Wu, H.-H.; Wang, W.; Gong, J. Investigation on hydrates blockage and restart process mechanisms of CO2 hydrate slurry flow. Asia-Pac. J. Chem. Eng. 2018, 13, e2193. [Google Scholar] [CrossRef]
- Yang, J.; Tohidi, B. Characterization of inhibition mechanisms of kinetic hydrate inhibitors using ultrasonic test technique. Chem. Eng. Sci. 2011, 66, 278–283. [Google Scholar] [CrossRef]
- Kelland, M.A. History of the development of low dosage hydrate inhibitors. Energy Fuels 2006, 20, 825–847. [Google Scholar] [CrossRef]
- Zhao, X.; Qiu, Z.; Zhang, Z.; Zhang, Y. Relationship between the gas hydrate suppression temperature and water activity in the presence of thermodynamic hydrate inhibitor. Fuel 2020, 264, 116776. [Google Scholar] [CrossRef]
- Chong, Z.R.; Chan, A.H.M.; Babu, P.; Yang, M.; Linga, P. Effect of NaCl on methane hydrate formation and dissociation in porous media. J. Nat. Gas Sci. Eng. 2015, 27, 178–189. [Google Scholar] [CrossRef]
- Nasir, Q.; Suleman, H.; Elsheikh, Y.A. A review on the role and impact of various additives as promoters/inhibitors for gas hydrate formation. J. Nat. Gas Sci. Eng. 2020, 76, 103211. [Google Scholar] [CrossRef]
- Said, S.; Govindaraj, V.; Herri, J.-M.; Ouabbas, Y.; Khodja, M.; Belloum, M.; Sangwai, J.S.; Nagarajan, R. A study on the influence of nanofluids on gas hydrate formation kinetics and their potential: Application to the CO2 capture process. J. Nat. Gas Sci. Eng. 2016, 32, 95–108. [Google Scholar] [CrossRef]
- Pahlavanzadeh, H.; Khanlarkhani, M.; Rezaei, S.; Mohammadi, A.H. Experimental and modelling studies on the effects of nanofluids (SiO2, Al2O3, and CuO) and surfactants (SDS and CTAB) on CH4 and CO2 clathrate hydrates formation. Fuel 2019, 253, 1392–1405. [Google Scholar] [CrossRef]
- Yu, Y.-s.; Zhou, S.-d.; Li, X.-s.; Wang, S.-l. Effect of graphite nanoparticles on CO2 hydrate phase equilibrium. Fluid Phase Equilibria 2016, 414, 23–28. [Google Scholar] [CrossRef]
- Mohammadi, M.; Haghtalab, A.; Fakhroueian, Z. Experimental study and thermodynamic modeling of CO2 gas hydrate formation in presence of zinc oxide nanoparticles. J. Chem. Thermodyn. 2016, 96, 24–33. [Google Scholar] [CrossRef]
- Nesterov, A.N.; Reshetnikov, A.M.; Manakov, A.Y.; Rodionova, T.V.; Paukshtis, E.A.; Asanov, I.P.; Bardakhanov, S.P.; Bulavchenko, A.I. Promotion and inhibition of gas hydrate formation by oxide powders. J. Mol. Liq. 2015, 204, 118–125. [Google Scholar] [CrossRef]
- Wang, R.; Liu, T.; Ning, F.; Ou, W.; Zhang, L.; Wang, Z.; Peng, L.; Sun, J.; Liu, Z.; Li, T.; et al. Effect of hydrophilic silica nanoparticles on hydrate formation: Insight from the experimental study. J. Energy Chem. 2019, 30, 90–100. [Google Scholar] [CrossRef] [Green Version]
- Golkhou, F.; Haghtalab, A. Measurement and thermodynamic modeling of carbon dioxide hydrate formation conditions using dry water through hydrophobic nano silica. J. Nat. Gas Sci. Eng. 2019, 68, 102906. [Google Scholar] [CrossRef]
- Pasieka, J.; Coulombe, S.; Servio, P. Investigating the effects of hydrophobic and hydrophilic multi-wall carbon nanotubes on methane hydrate growth kinetics. Chem. Eng. Sci. 2013, 104, 998–1002. [Google Scholar] [CrossRef]
- Najibi, H.; Mirzaee Shayegan, M.; Heidary, H. Experimental investigation of methane hydrate formation in the presence of copper oxide nanoparticles and SDS. J. Nat. Gas Sci. Eng. 2015, 23, 315–323. [Google Scholar] [CrossRef]
- Mohammadi, A.; Manteghian, M.; Haghtalab, A.; Mohammadi, A.H.; Rahmati-Abkenar, M. Kinetic study of carbon dioxide hydrate formation in presence of silver nanoparticles and SDS. Chem. Eng. J. 2014, 237, 387–395. [Google Scholar] [CrossRef]
- Abedi-Farizhendi, S.; Iranshahi, M.; Mohammadi, A.; Manteghian, M.; Mohammadi, A.H. Kinetic study of methane hydrate formation in the presence of carbon nanostructures. Pet. Sci. 2019, 16, 657–668. [Google Scholar] [CrossRef] [Green Version]
- Anderson, G.K. Enthalpy of dissociation and hydration number of carbon dioxide hydrate from the Clapeyron equation. J. Chem. Thermodyn. 2003, 35, 1171–1183. [Google Scholar] [CrossRef]
- Eslamimanesh, A.; Mohammadi, A.H.; Richon, D. Thermodynamic model for predicting phase equilibria of simple clathrate hydrates of refrigerants. Chem. Eng. Sci. 2011, 66, 5439–5445. [Google Scholar] [CrossRef]
- Klauda, J.B.; Sandler, S.I. A fugacity model for gas hydrate phase equilibria. Ind. Eng. Chem. Res. 2000, 39, 3377–3386. [Google Scholar] [CrossRef]
- Nashed, O.; Partoon, B.; Lal, B.; Sabil, K.M.; Shariff, A.M. Review the impact of nanoparticles on the thermodynamics and kinetics of gas hydrate formation. J. Nat. Gas Sci. Eng. 2018, 55, 452–465. [Google Scholar] [CrossRef]
- Zhao, L.; Gao, L. Coating multi-walled carbon nanotubes with zinc sulfide. J. Mater. Chem. 2004, 14, 1001. [Google Scholar] [CrossRef]
- Shen, X.-C.; Fang, X.-Z.; Zhou, Y.-H.; Liang, H. Synthesis and Characterization of 3-Aminopropyltriethoxysilane-Modified Superparamagnetic Magnetite Nanoparticles. Chem. Lett. 2004, 33, 1468–1469. [Google Scholar] [CrossRef]
- Park, S.-S.; An, E.-J.; Lee, S.-B.; Chun, W.-G.; Kim, N.-J. Characteristics of methane hydrate formation in carbon nanofluids. J. Ind. Eng. Chem. 2012, 18, 443–448. [Google Scholar] [CrossRef]
- Kashchiev, D.; Firoozabadi, A. Induction time in crystallization of gas hydrates. J. Cryst. Growth 2003, 250, 499–515. [Google Scholar] [CrossRef]
- Yang, M.; Liu, W.; Song, Y.; Ruan, X.; Wang, X.; Zhao, J.; Jiang, L.; Li, Q. Effects of Additive Mixture (THF/SDS) on the Thermodynamic and Kinetic Properties of CO2/H2 Hydrate in Porous Media. Ind. Eng. Chem. Res. 2013, 52, 4911–4918. [Google Scholar] [CrossRef]
- Liu, G.-Q.; Wang, F.; Luo, S.-J.; Xu, D.-Y.; Guo, R.-B. Enhanced methane hydrate formation with SDS-coated Fe3O4 nanoparticles as promoters. J. Mol. Liq. 2017, 230, 315–321. [Google Scholar] [CrossRef]
- Yan, S.; Dai, W.; Wang, S.; Rao, Y.; Zhou, S. Graphene Oxide: An Effective Promoter for CO2 Hydrate Formation. Energies 2018, 11, 1756. [Google Scholar] [CrossRef] [Green Version]
- McElligott, A.; Uddin, H.; Meunier, J.-L.; Servio, P. Effects of Hydrophobic and Hydrophilic Graphene Nanoflakes on Methane Hydrate Kinetics. Energy Fuels 2019, 33, 11705–11711. [Google Scholar] [CrossRef]
- Farhang, F.; Nguyen, A.V.; Sewell, K.B. Fundamental Investigation of the Effects of Hydrophobic Fumed Silica on the Formation of Carbon Dioxide Gas Hydrates. Energy Fuels 2014, 28, 7025–7037. [Google Scholar] [CrossRef]
- Nguyen, N.N.; Nguyen, A.V.; Steel, K.M.; Dang, L.X.; Galib, M. Interfacial Gas Enrichment at Hydrophobic Surfaces and the Origin of Promotion of Gas Hydrate Formation by Hydrophobic Solid Particles. J. Phys. Chem. C 2017, 121, 3830–3840. [Google Scholar] [CrossRef]
- Veilleux, J.; Coulombe, S. A total internal reflection fluorescence microscopy study of mass diffusion enhancement in water-based alumina nanofluids. J. Appl. Phys. 2010, 108, 104316. [Google Scholar] [CrossRef]




| Properties | VK-L30 | VK-L30H | VK-L30G |
|---|---|---|---|
| modification treatment | non-modified | hydrophilic modified | hydrophobic modified |
| appearance | white powder | ||
| average particle size (nm) | 30 | ||
| packing density (g/cm3) | 0.25–0.35 | ||
| pH of aqueous solution | 7–9.5 | ||
| specific surface area (m2/g) | 30–60 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Liao, X.; Shi, C.; Ling, Z.; Jiang, L. Promoting and Inhibitory Effects of Hydrophilic/Hydrophobic Modified Aluminum Oxide Nanoparticles on Carbon Dioxide Hydrate Formation. Energies 2020, 13, 5380. https://doi.org/10.3390/en13205380
Liu Y, Liao X, Shi C, Ling Z, Jiang L. Promoting and Inhibitory Effects of Hydrophilic/Hydrophobic Modified Aluminum Oxide Nanoparticles on Carbon Dioxide Hydrate Formation. Energies. 2020; 13(20):5380. https://doi.org/10.3390/en13205380
Chicago/Turabian StyleLiu, Yu, Xiangrui Liao, Changrui Shi, Zheng Ling, and Lanlan Jiang. 2020. "Promoting and Inhibitory Effects of Hydrophilic/Hydrophobic Modified Aluminum Oxide Nanoparticles on Carbon Dioxide Hydrate Formation" Energies 13, no. 20: 5380. https://doi.org/10.3390/en13205380





