Corrosion Behavior of Al in Ethanol–Gasoline Blends
Abstract
1. Introduction
2. Experimental Procedure
2.1. Synthesis of Ethanol
2.2. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.3. Electrochemical Measurements
2.4. Surface Characterization
3. Results and Discussion
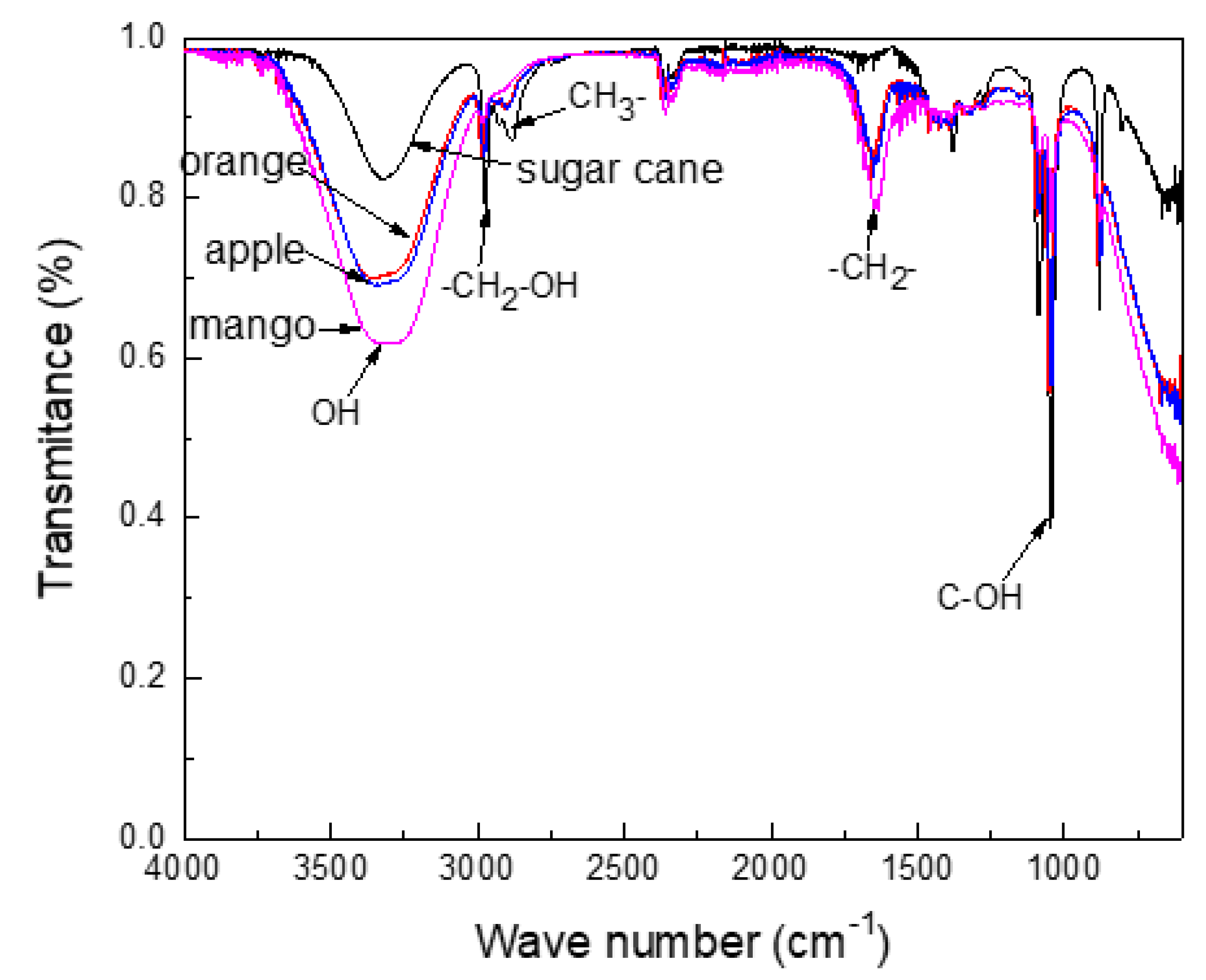
3.1. Infrared Results and Chemical Composition
3.2. OCP Measurements
3.3. LPR Measurements
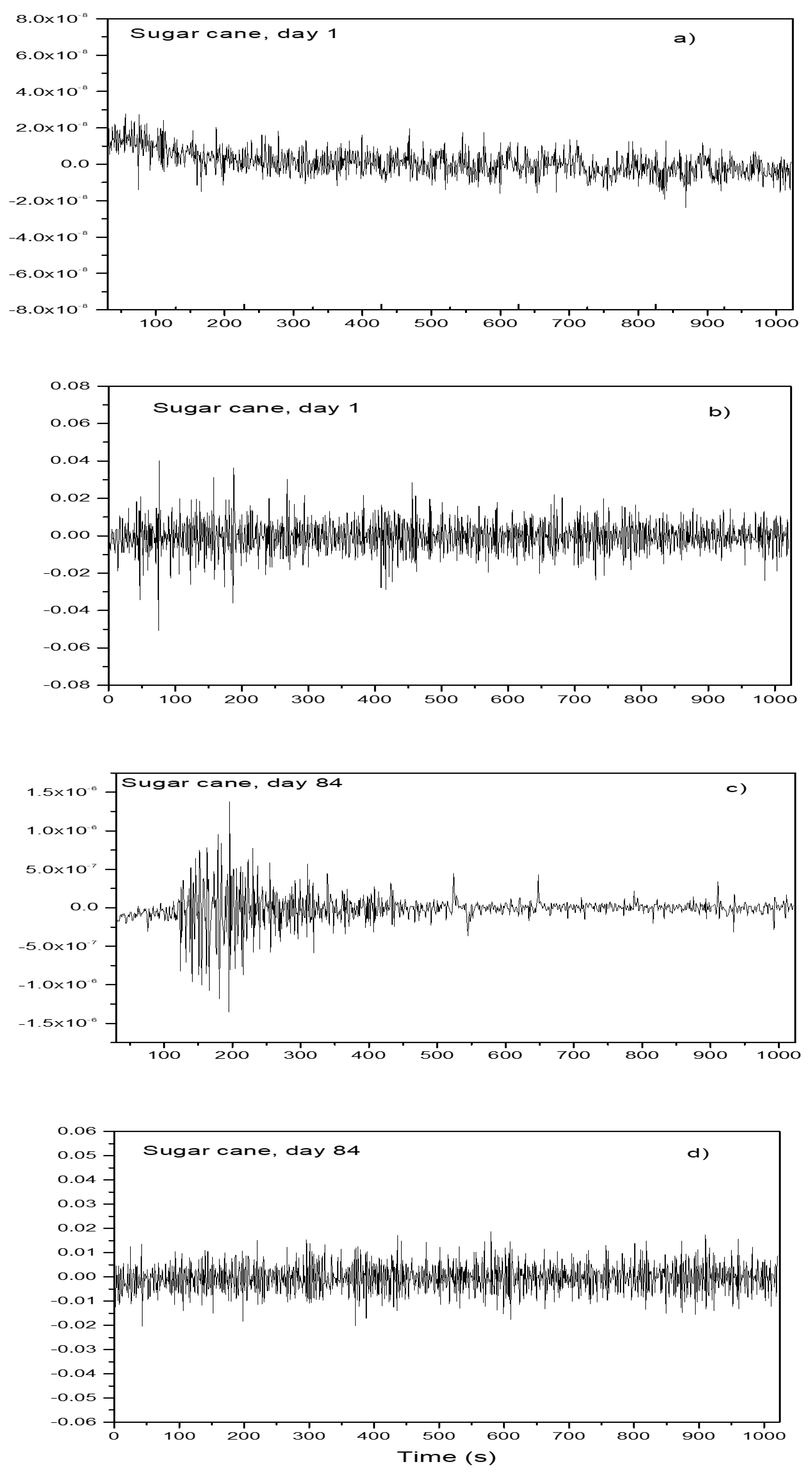
3.4. Electrochemical Noise Measurements
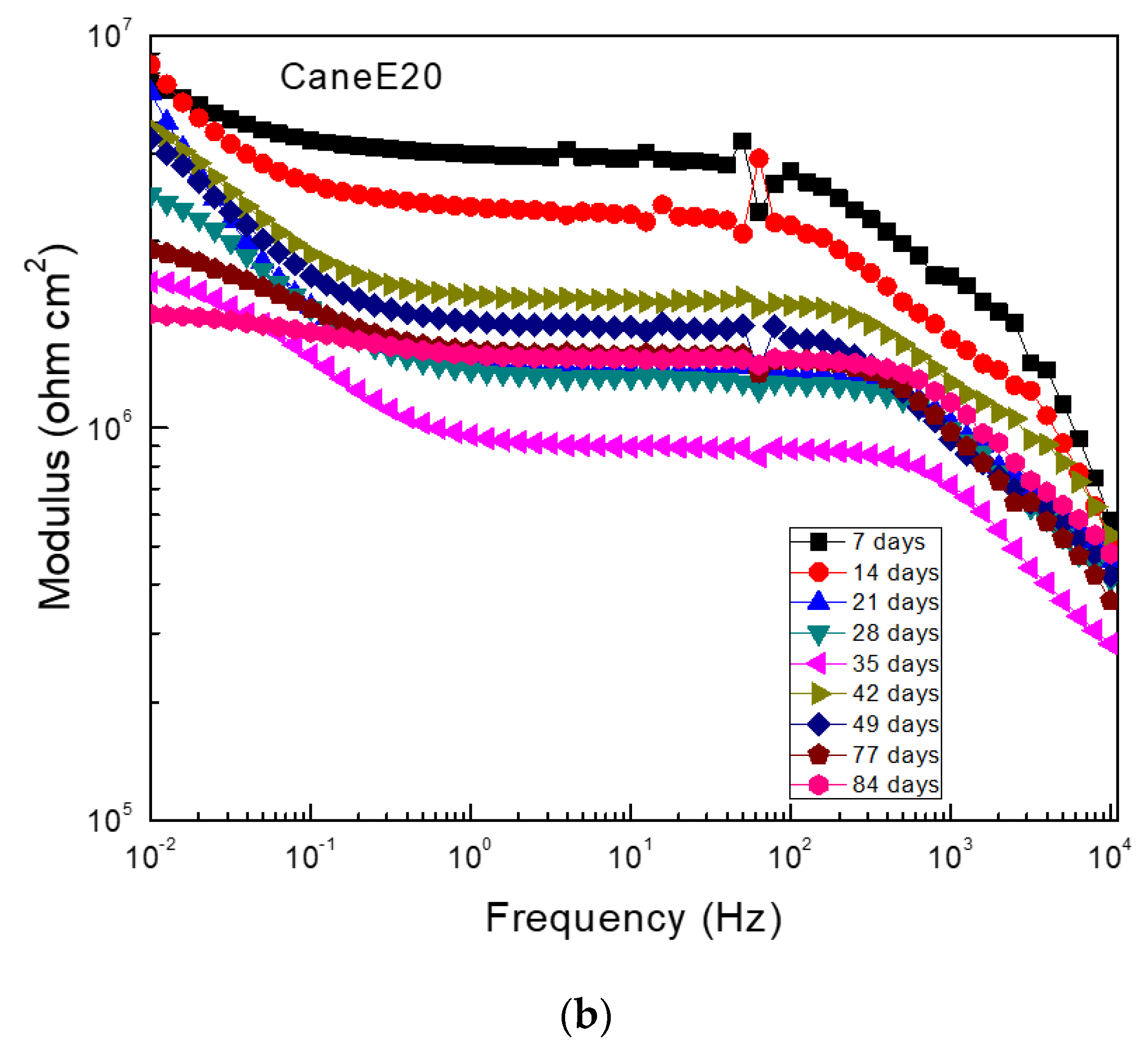
3.5. Electrochemical Impedance Spectroscopy (EIS) Measurements
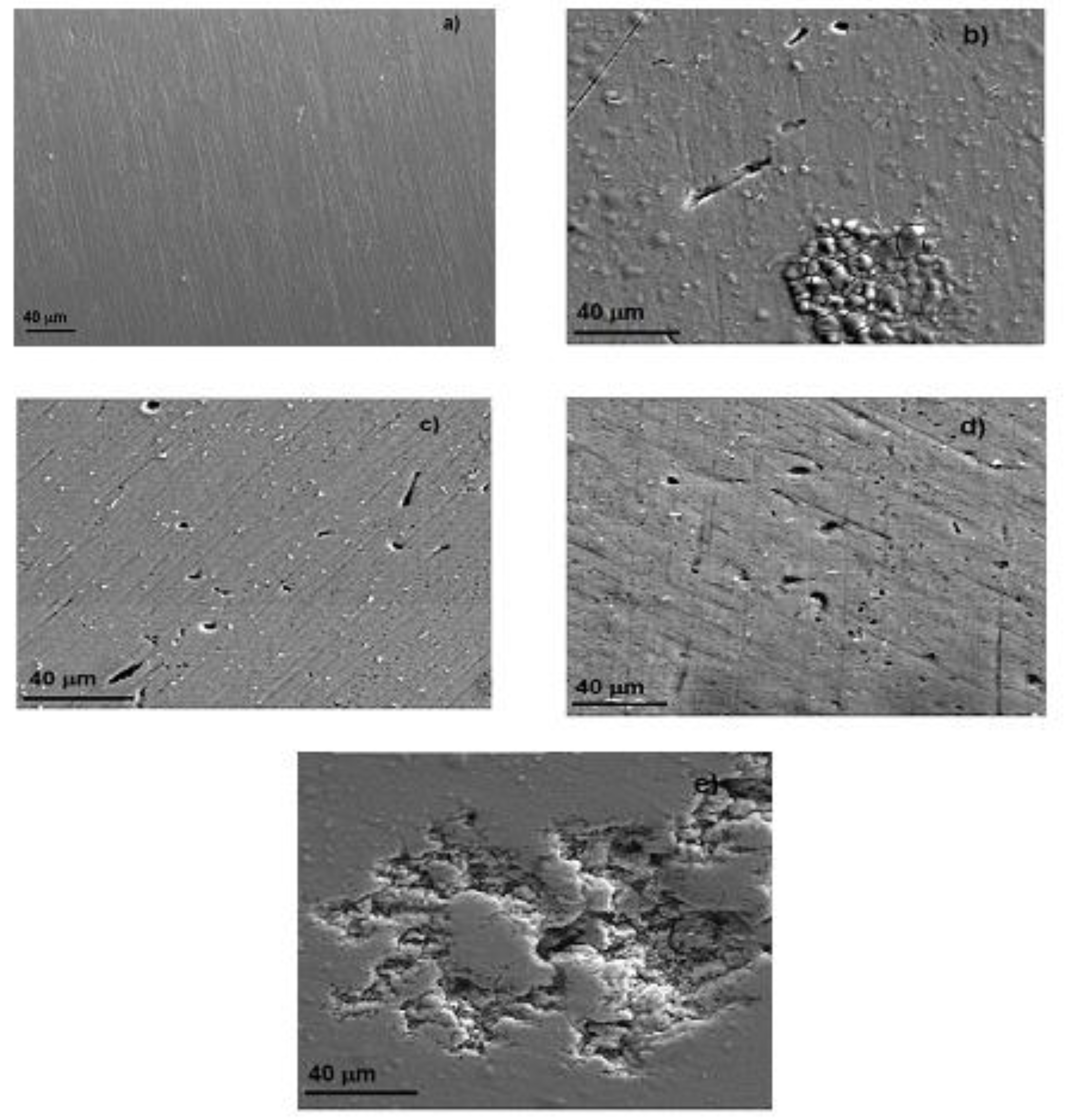
3.6. Surface Characterization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Von Blottnitz, H.; Curran, M.A. A review of assessments conducted on bio-ethanol as a transportation fuel from a net energy, greenhouse gas, and environmental life cycle perspective. J. Clean. Prod. 2007, 15, 607–619. [Google Scholar] [CrossRef]
- Tibaquirá, J.E.; Huertas, J.I.; Ospina, S.; Quirama, L.F.; Niño, J.E. The Effect of Using Ethanol–gasoline Blends on the Mechanical, Energy and Environmental Performance of In-Use Vehicles. Energies 2018, 11, 221. [Google Scholar] [CrossRef]
- Xiaoyuan, L.; Singh, P.M. Cathodic activities of oxygen and hydrogen on carbon steel in simulated fuel-grade ethanol. Electrochim. Acta 2011, 56, 2312–2320. [Google Scholar]
- De souza, J.P.; Mattos, O.R.; Sathler, L.; Takenouti, H. Impedance measurements of corroding mild steel in an automotive fuel ethanol with and without inhibitor in a two and three electrode cell. Corros. Sci. 1987, 27, 1351–1364. [Google Scholar] [CrossRef]
- Scholz, M.; Ellermeier, J. Corrosion behavior of different aluminum alloys in fuels containing ethanol under increased temperatures. Mater. Werkst. 2006, 37, 842–851. [Google Scholar] [CrossRef]
- Eppel, K.; Scholz, M.; Troßmann, T.; Berger, C. Corrosion of metals for automotive applications in ethanol blended biofuels. Energy Mater. 2008, 3, 227–231. [Google Scholar] [CrossRef]
- Del-Pozo, A.; Torres-Islas, A.; Villalobos, J.C.; Sedano, A.; Martínez, H.; Campillo, B.; Serna, S. Stress corrosion cracking of microalloyed pipeline steel in biofuels E-10 and E-85. Corros. Eng. Sci. Technol. 2019, 54, 37–45. [Google Scholar] [CrossRef]
- Jafari, H.; Idris, M.H.; Ourdjini, A.; Rahimi, H.; Ghobadian, B. Effect of ethanol as gasoline additive on vehicle fuel delivery system corrosion. Mater. Corros. 2010, 61, 432–440. [Google Scholar]
- Dimatteo, A.; Lovicu, G.F.; Desanctis, M.; Valentini, R.; Santarelli, S.; Cognetta, F. Compatibility of nickel and silver-based brazing alloys with E85 fuel blends. Mater. Corros. 2015, 66, 158–168. [Google Scholar] [CrossRef]
- Aperador, W.; Caballero-Gómez, J.; Delgado, A. Corrosion Behavior of the AA2124 Aluminium Alloy Exposed to Ethanol Mixtures. Int. J. Electrochem. Sci. 2013, 8, 6154–6161. [Google Scholar]
- Pedraza-Basulto, G.K.; Arizmendi-Morquecho, A.M.; Cabral Miramontes, J.A.; Borunda-Terrazas, A.; Martinez-Villafane, A.; Chacón-Nava, J.G. Effect of Water on the Stress Corrosion Cracking Behavior of API 5L-X52 Steel in E95 Blend. Int. J. Electrochem. Sci. 2013, 8, 5421–5437. [Google Scholar]
- Liu, C.; Frankel, G.S.; Sridhar, N. Effect of Oxygen on Ethanol Stress Corrosion Cracking Susceptibility: Part 1—Electrochemical Response and Cracking-Susceptible Potential Region. Corrosion 2013, 69, 768–780. [Google Scholar]
- Sridhar, N. Knowledge-Based Predictive Analytics in Corrosion. Corrosion 2018, 74, 181–196. [Google Scholar] [CrossRef]
- Skoropinski, D.B. Corrosion of aluminum fuel system components by reaction with EGME icing inhibitor. Energy Fuel 1996, 10, 108–116. [Google Scholar] [CrossRef]
- Yoo, Y.H.; Park, I.J.; Kim, J.G.; Kwak, D.H.; Ji, W.S. Corrosion characteristics of aluminium alloy in bio-ethanol blended gasoline fuel: Part 1. The corrosion properties of aluminum alloy in high temperature fuels. Fuel 2011, 90, 633–639. [Google Scholar] [CrossRef]
- Cavalcanti, E.; Wanderley, V.G.; Miranda, T.R.V.; Uller, L. The effect of water, sulphate and pH on the corrosion behavior of carbon steel in ethanolic solutions. Electrochim. Acta 1987, 32, 935–947. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Ambrozin, A.R.P.; Santos, A.O.; Contri, P.P.; Kuri, S.E. Evaluation of metallic corrosion caused by alcohol fuel and some contaminants. Mater. Sci. Forum 2010, 636, 1024–1029. [Google Scholar] [CrossRef]
- Baena, L.M.; Gomez, M.; Calderon, J.A. Aggressiveness of a 20% bioethanol–80% gasoline mixture on auto parts: I behavior of metallic materials and evaluation of their electrochemical properties. Fuel 2012, 95, 320–328. [Google Scholar] [CrossRef]
- Yoo, Y.H.; Park, I.J.; Kim, J.G.; Kwak, D.H.; Ji, W.S. Corrosion characteristics of aluminium alloy in bio-ethanol blended gasoline fuel: Part 2. The effects of dissolved oxygen in the fuel. Fuel 2011, 90, 1208–1214. [Google Scholar] [CrossRef]
- Hassan, J.; Idris, M.H.; Ourdjini, A.; Rahimi, H.; Ghobadian, B. EIS study of corrosion behavior of metallic materials in ethanol blended gasoline containing water as a contaminant. Fuel 2011, 90, 1181–1187. [Google Scholar]
- Olafsen, A.; Fjellvag, H. Synthesis of rare earth oxide carbonates and thermal stability of Nd2O2CO3. J. Mat. Chem. 1999, 9, 2697–2702. [Google Scholar] [CrossRef]
- Rawat, J.; Rao, P.V.C.; Nettem, V.C. Effect of Ethanol–gasoline Blends on Corrosion Rate in the Presence of Different Materials of Construction used for Transportation, Storage and Fuel Tanks. Open Electrochem. J. 2009, 1, 32–36. [Google Scholar]
- Turgoose, S.; Cottis, R.A. Corrosion Testing Made Easy: Electrochemical Impedance and Noise; NACE International: Houston, TX, USA, 1999; pp. 1–149. [Google Scholar]
- Hladky, K.; Dawson, J.L. The measurement of localized corrosion using electrochemical noise. Corros. Sci. 1981, 21, 317–322. [Google Scholar] [CrossRef]
- Hladky, K.; Dawson, J.L. The measurement of corrosion using electrochemical 1/f noise. Corros. Sci. 1982, 22, 231–237. [Google Scholar] [CrossRef]
- Bommersbach, P.; Alemany-Dumont, C.; Millet, J.P.; Normand, B. Hydrodynamic effect on the behavior of a corrosion inhibitor film: Characterization by electrochemical impedance spectroscopy. Electrochim. Acta 2006, 51, 4011–4018. [Google Scholar] [CrossRef]
- Behpour, M.; Ghoreishi, S.M.; Salavati-Niasari, M.; Ebrahimi, B. Evaluating two new synthesized S–N Schiff bases on the corrosion of copper in 15% hydrochloric acid. Mater. Chem. Phys. 2008, 107, 153–157. [Google Scholar] [CrossRef]
- French, R.; Malone, P. Phase equilibria in ethanol fuel blends. Fluid Phase Equilib. 2005, 229, 27–40. [Google Scholar] [CrossRef]
- MArinov, N. A detailed chemical kinetic model for ethanol oxidation. Int. J. Chem. Kinet. 1999, 31, 183–220. [Google Scholar] [CrossRef]
- Vargel, C. Corrosion of Aluminum; Elsevier Ltd.: Oxford, UK, 2004; pp. 1–120. [Google Scholar]









| Ethanol Source | Water Contents (%) |
|---|---|
| Apple | 0.3 |
| Mango | 0.4 |
| Orange | 0.4 |
| Sugar cane | 0.8 |
| Ethanol Source | RS (ohm cm−2) | CPEdl (ohm−1m−2sn) | nct | Rct (ohm cm−2) | CPEf (ohm−1m−2sn) | nf | Rf (ohm cm−2) |
|---|---|---|---|---|---|---|---|
| Apple | 2.8 × 104 | 4.1 × 10−11 | 0.9 | 2.2 × 109 | 1.1 × 10−9 | 0.8 | 4.4 × 109 |
| Orange | 3.6 × 104 | 3.1 × 10−11 | 0.9 | 1.7 × 109 | 2.3 × 10−9 | 0.8 | 6.6 × 109 |
| Mango | 1.1 × 104 | 2.3 × 10−11 | 0.9 | 2.2 × 109 | 1.3 × 10−10 | 0.8 | 4.7 × 109 |
| Sugar cane | 4.6 × 104 | 6.6 × 10−10 | 0.6 | 2.6 × 106 | 1.6 × 10−6 | 0.7 | 5.9 × 106 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brito-Franco, A.; Uruchurtu, J.; Rosales-Cadena, I.; Lopez-Sesenes, R.; Serna-Barquera, S.A.; Hernandez-Perez, J.A.; Rocabruno-Valdes, C.; Gonzalez-Rodriguez, J.G. Corrosion Behavior of Al in Ethanol–Gasoline Blends. Energies 2020, 13, 5544. https://doi.org/10.3390/en13215544
Brito-Franco A, Uruchurtu J, Rosales-Cadena I, Lopez-Sesenes R, Serna-Barquera SA, Hernandez-Perez JA, Rocabruno-Valdes C, Gonzalez-Rodriguez JG. Corrosion Behavior of Al in Ethanol–Gasoline Blends. Energies. 2020; 13(21):5544. https://doi.org/10.3390/en13215544
Chicago/Turabian StyleBrito-Franco, Alfredo, Jorge Uruchurtu, Isai Rosales-Cadena, Roy Lopez-Sesenes, Sergio Alonso Serna-Barquera, Jose Alfredo Hernandez-Perez, Caroline Rocabruno-Valdes, and Jose Gonzalo Gonzalez-Rodriguez. 2020. "Corrosion Behavior of Al in Ethanol–Gasoline Blends" Energies 13, no. 21: 5544. https://doi.org/10.3390/en13215544
APA StyleBrito-Franco, A., Uruchurtu, J., Rosales-Cadena, I., Lopez-Sesenes, R., Serna-Barquera, S. A., Hernandez-Perez, J. A., Rocabruno-Valdes, C., & Gonzalez-Rodriguez, J. G. (2020). Corrosion Behavior of Al in Ethanol–Gasoline Blends. Energies, 13(21), 5544. https://doi.org/10.3390/en13215544






