Dispersion Effects of Particulate Lead (Pb) from the Stack of a Lead Battery Recycling Plant
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Dispersion Modelling
2.2.1. Meteorological Data
2.2.2. Receptors
2.2.3. Stack Operation Parameters
2.3. Topsoil
2.3.1. Elemental Analysis
2.3.2. Influence of Lead Emissions on Topsoil
3. Results
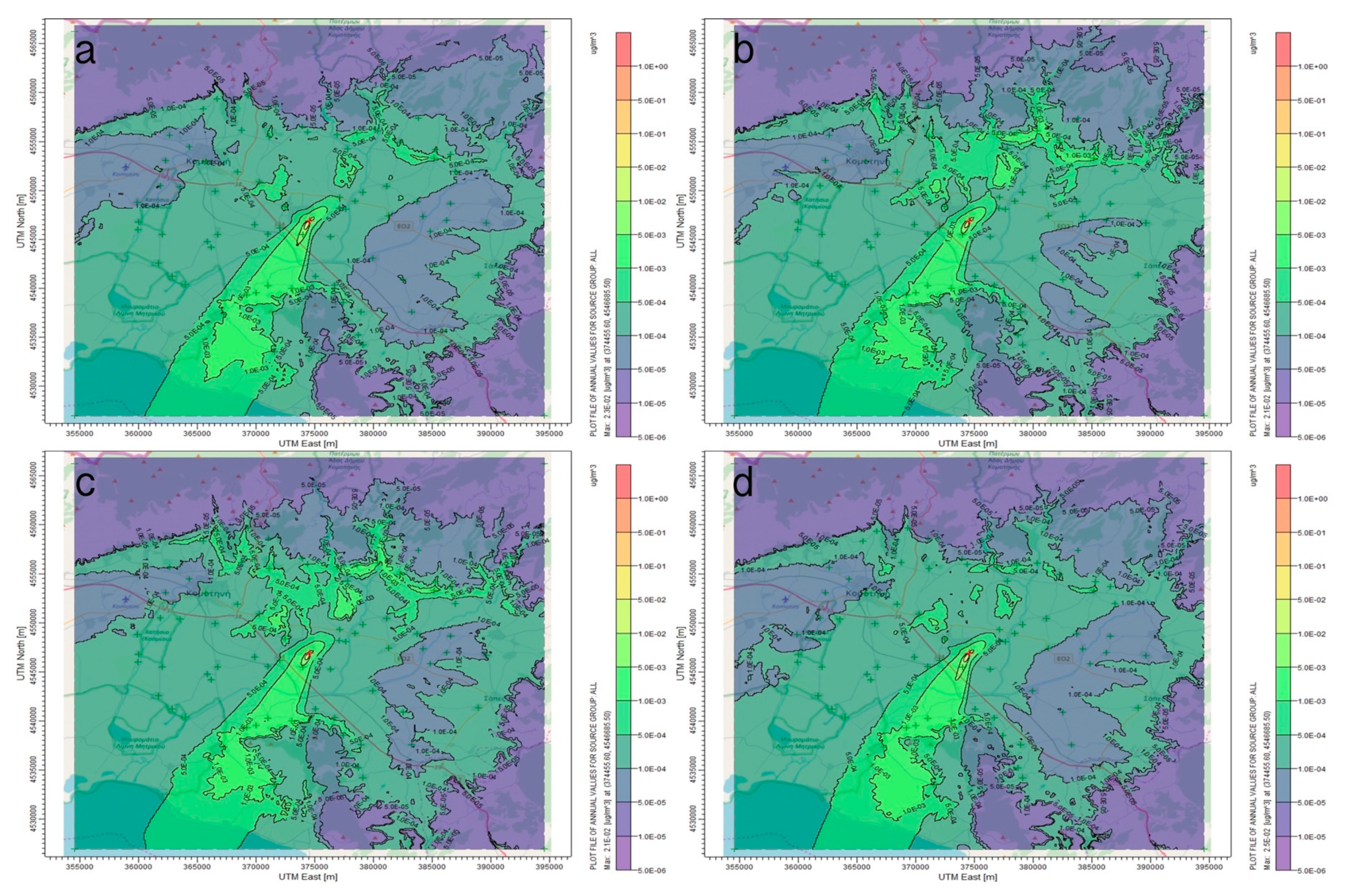
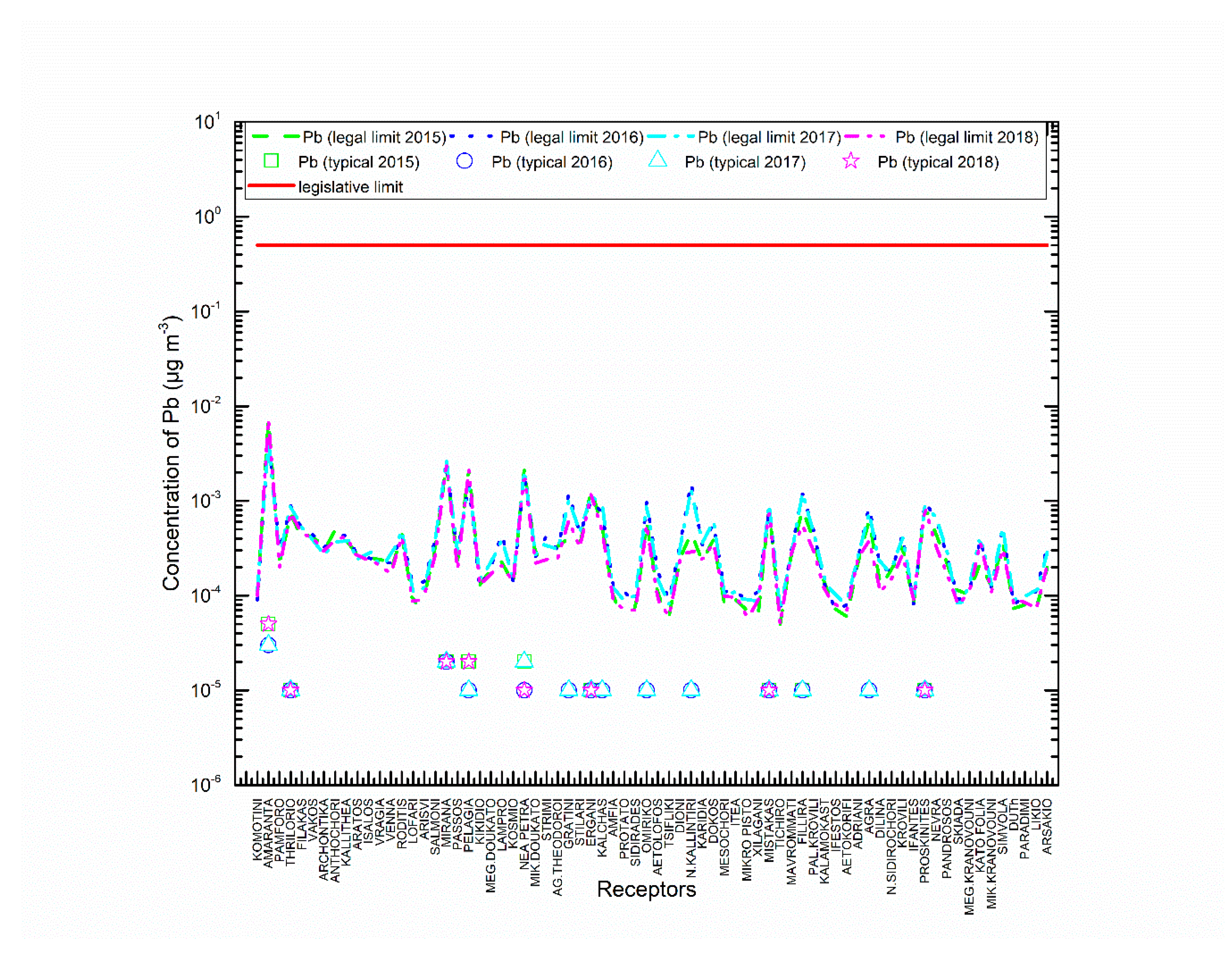
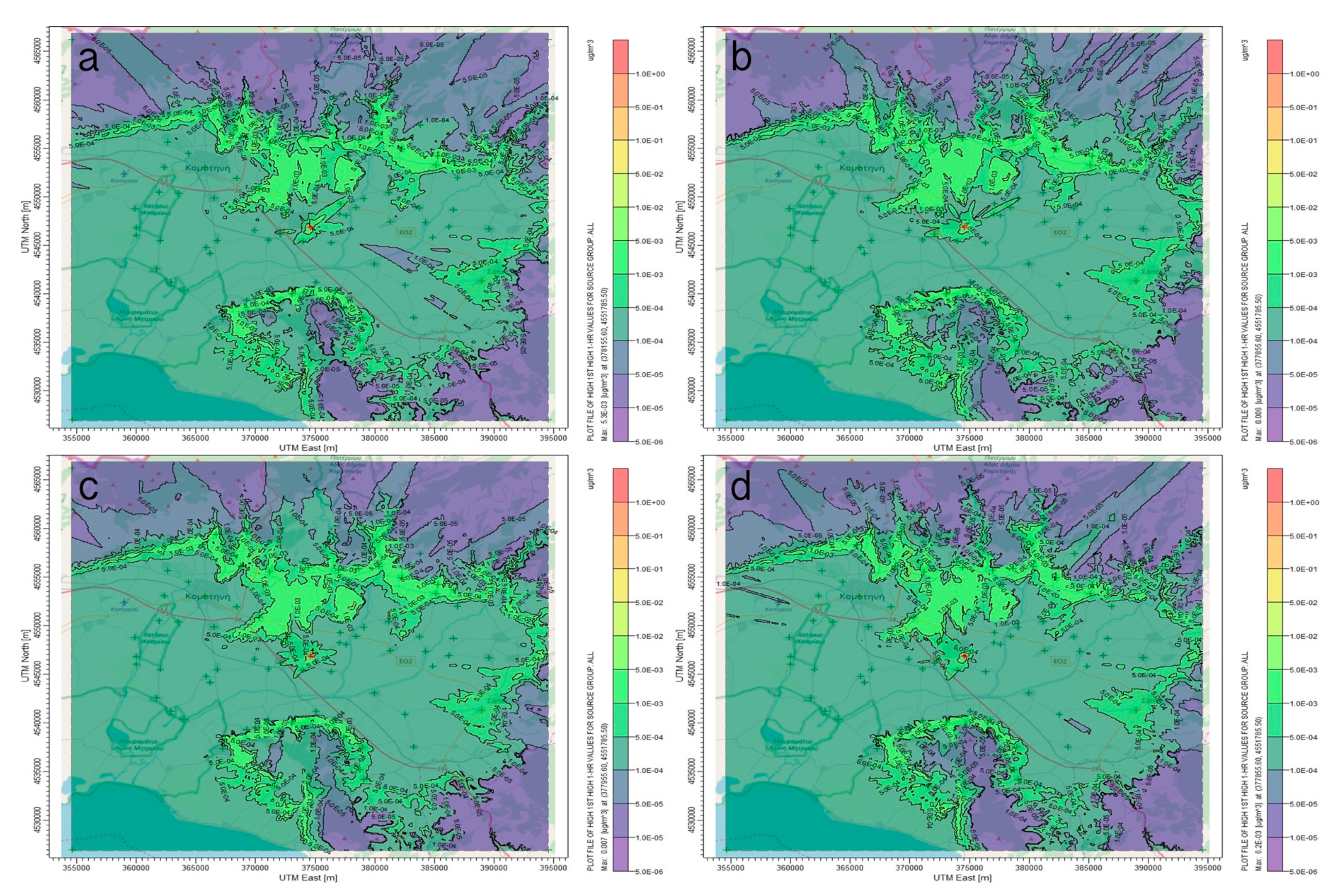
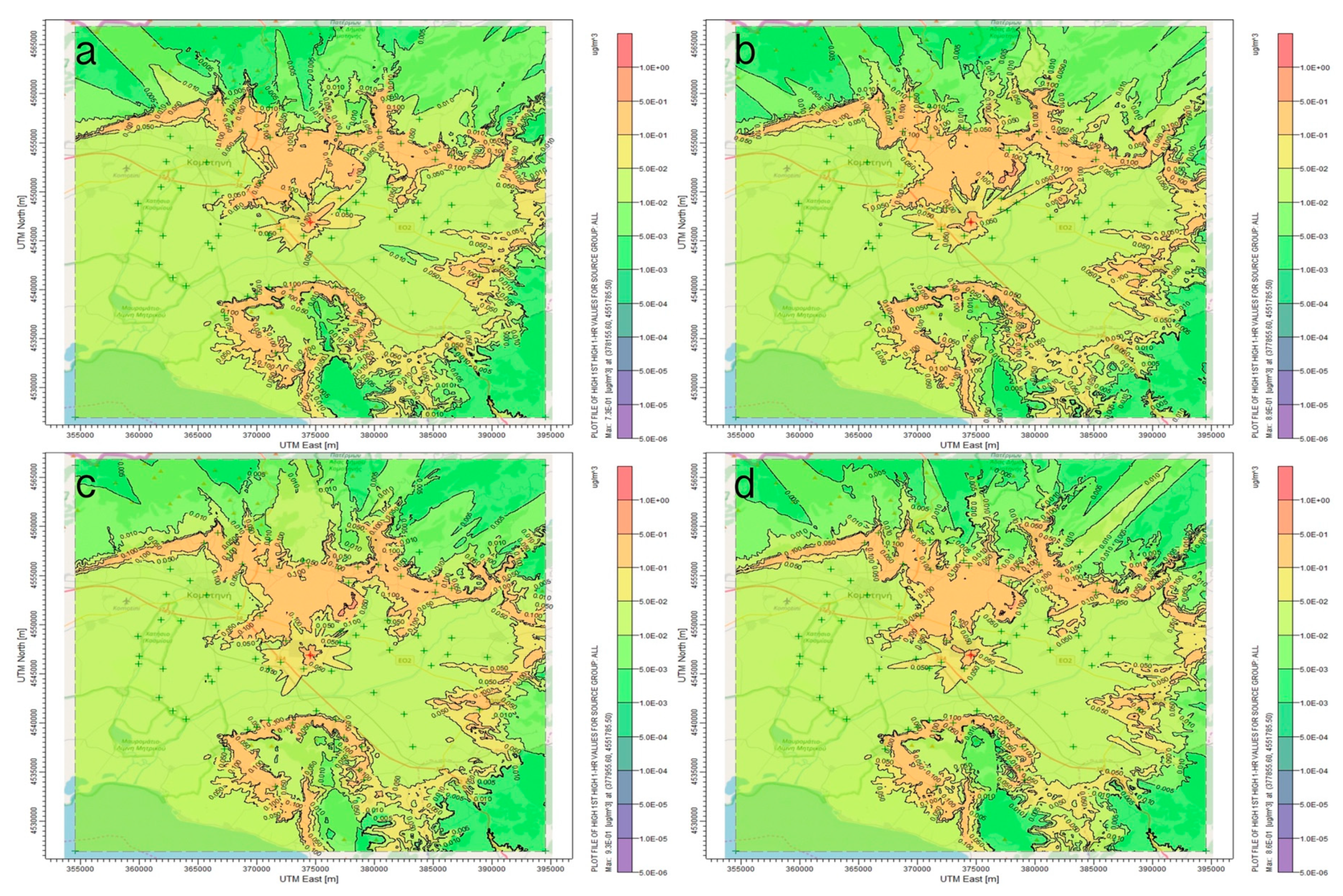
3.1. AERMOD Dispersion Results
3.2. Influence of Stack Emissions on the Topsoil of the Area
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- International Programme for Chemical Safety. Inorganic Lead; World Health Organization: Geneva, Switzerland, 1995. [Google Scholar]
- Kelektsoglou, K.; Karali, D.; Stavridis, A.; Loupa, G. Efficiency of the Air-Pollution Control System of a Lead-Acid-Battery Recycling Industry. Energies 2018, 11, 3465. [Google Scholar] [CrossRef] [Green Version]
- Eguchi, A.; Nomiyama, K.; Sakurai, K.; Trang, P.T.K.; Viet, P.H.; Takahashi, S.; Iwata, H.; Tanabe, S.; Todaka, E.; Mori, C. Alterations in urinary metabolomic profiles due to lead exposure from a lead–acid battery recycling site. Environ. Pollut. 2018, 242, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Risk Assessment Guidance for Superfund. Volume I: Human Health Evaluation Manual (Part A); EPA/540/1-89/002; Environmental Protection Agency: Washington, DC, USA, 1989.
- Järup, L. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- WHOQOL Group. WHO, The World Health Organization quality of life assessment (WHOQOL): Position paper from the World Health Organization. Soc. Sci. Med. 1995, 41, 1403–1409. [Google Scholar] [CrossRef]
- UNEP. Draft final review of scientific information on lead. In United Nations Environment Programme, Chemicals Branch; UNEP: Nairobi, Kenya, 2008. [Google Scholar]
- Johansson, K.; Bergbäck, B.; Tyler, G. Impact of Atmospheric Long Range Transport of Lead, Mercury and Cadmium on the Swedish Forest Environment. Water Air Soil Pollut. Focus 2001, 1, 279–297. [Google Scholar] [CrossRef]
- Health risks of heavy metals from long-range transboundary air pollution. In World Health Organization Regional Office for Europe; WHO: Geneva, Switzerland, 2007.
- Collivignarelli, C.; Riganti, V.; Urbini, G. Battery lead recycling and environmental pollution hazards. Conserv. Recycl. 1986, 9, 111–125. [Google Scholar] [CrossRef]
- Ettler, V.; Johan, Z.; Baronnet, A.; Jankovský, F.; Gilles, C.; Mihaljevič, M.; Šebek, O.; Strnad, L.; Bezdička, P. Mineralogy of air-pollution-control residues from a secondary lead smelter: Environmental implications. Environ. Sci. Technol. 2005, 39, 9309–9316. [Google Scholar] [CrossRef]
- Milton, B.-A.; Juan, V.M.; María, M.V.; Alejandro, A.; José, P.-C.J. Chemical characterization and local dispersion of slag generated by a lead recovery plant in Central Mexico. Afr. J. Biotechnol. 2014, 13, 1973–1978. [Google Scholar]
- Salomone, R.; Mondello, F.; Lanuzza, F.; Micali, G. An eco-balance of a recycling plant for spent lead–acid batteries. Environ. Manag. 2005, 35, 206–219. [Google Scholar] [CrossRef]
- IARC. Summaries & evaluations: Inorganic and organic lead compounds. In International Agency for Research on Cancer (IARC Monographs for the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 2006; Volume 87. [Google Scholar]
- Araiza-Aguilar, J.A.; Rojas-Valencia, M.N. Spatial modelling of gaseous emissions from two municipal solid waste dump sites. Int. J. Environ. Stud. 2019, 76, 213–224. [Google Scholar] [CrossRef]
- Matacchiera, F.; Manes, C.; Beaven, R.P.; Rees-White, T.C.; Boano, F.; Mønster, J.; Scheutz, C. AERMOD as a Gaussian dispersion model for planning tracer gas dispersion tests for landfill methane emission quantification. Waste Manag. 2019, 87, 924–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abril, G.A.; Diez, S.C.; Pignata, M.L.; Britch, J. Particulate matter concentrations originating from industrial and urban sources: Validation of atmospheric dispersion modeling results. Atmos. Pollut. Res. 2016, 7, 180–189. [Google Scholar] [CrossRef]
- Dos Santos Cerqueira, J.; de Albuquerque, H.N.; de Assis Salviano de Sousa, F. Atmospheric pollutants: Modeling with Aermod software. Air Qual. Atmos. Health 2019, 12, 21–32. [Google Scholar] [CrossRef]
- Gibson, M.D.; Kundu, S.; Satish, M. Dispersion model evaluation of PM2.5, NOx and SO2 from point and major line sources in Nova Scotia, Canada using AERMOD Gaussian plume air dispersion model. Atmos. Pollut. Res. 2013, 4, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Macêdo, M.F.M.; Ramos, A.L.D. Vehicle atmospheric pollution evaluation using AERMOD model at avenue in a Brazilian capital city. Air Qual. Atmos. Health 2020, 13, 309–320. [Google Scholar] [CrossRef]
- Makridis, M.; Lazaridis, M. Dispersion modeling of gaseous and particulate matter emissions from aircraft activity at Chania Airport, Greece. Air Qual. Atmos. Health 2019, 12, 933–943. [Google Scholar] [CrossRef]
- De Nevers, N. Air Pollution Control Engineering; Waveland Press: Long Grove, IL, USA, 2010. [Google Scholar]
- Dornbrack, A. Turbulence and diffusion in the atmosphere Lectures in Environmental Sciences; Blackadar, A.K., Ed.; Springer-Verlag GmbH & Co. KG: Berlin/Heidelberg, Germany, 1997; p. 185. ISBN 3540- 61406-0. [Google Scholar]
- Turner, D.B. Workbook of Atmospheric Dispersion Estimates: An Introduction to Dispersion Modeling; CRC Press: Boca Raton, FL, USA, 1994. [Google Scholar]
- EPA. AERMOD Implementation Guide. In Aermod Implementation Workgroup, U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards; Air Quality Assessment Division: North Carolina, NC, USA, 2009. [Google Scholar]
- EPA. AERMOD: Description of Model Formulation. In On Assignment to the Atmospheric Research And Exposure Assessment Laboratory, U.S. Environmental Protection Agency; EPA-454/R-03-004 September 2004; U.S. Environmental Protection Agency, Office of Air Quality Planning and Standards, Emissions Monitoring and Analysis Division: North Carolina, NC, USA, 2004. [Google Scholar]
- Directive 2010/75/EU of the European Parliament and of the Council of 24 November 2010 on industrial emissions (integrated pollution prevention and control). Off. J. Eur. Union L 2010, 334, 17–119.
- Brouwer, P. Theory of XRF: Getting Acquainted with the Principles; PANalytical BV: Almelo, The Netherlands, 2006. [Google Scholar]
- Jaklevic, J. Photon Induced X-ray Fluorescence Analysis Using Energy Dispersive Detector and Dichotomous Sampler; Lawrence Berkeley Laboratory University of California: Berkeley, CA, USA, 1976. [Google Scholar]
- Tarvainen, T.; Reeder, S.; Albanese, S. Database management and map production. Geochem. Atlas Eur. Part 2005, 1, 526. [Google Scholar]
- Sehmel, G.A. Particle and gas dry deposition: A review. Atmos. Environ. (1967) 1980, 14, 983–1011. [Google Scholar] [CrossRef]
- Little, P.; Wiffen, R. Emission and deposition of petrol engine exhaust Pb—I. Deposition of exhaust Pb to plant and soil surfaces. Atmos. Environ. (1967) 1977, 11, 437–447. [Google Scholar] [CrossRef]
- Crecelius, E.; Robertson, D.; Abel, K.; Cochran, D.; Weimer, W. Atmospheric deposition of 7Be and other elements on the Washington coast. In Pacific Northwest Laboratory Annual Report for 1977 to the DOE Assistant Secretary for Environment: Ecological Sciences; PNL-2500 PT 2; Pacific Northwest Laboratorv Richland: Richland, WA, USA, 1978; pp. 983–1011. [Google Scholar]
- Ballabio, C.; Panagos, P.; Monatanarella, L. Mapping topsoil physical properties at European scale using the LUCAS database. Geoderma 2016, 261, 110–123. [Google Scholar] [CrossRef]
- Directive 2008/50/EC of the European Parliament and of the Council of 21 May 2008 on ambient air quality and cleaner air for Europe. Off. J. Eur. Union 2008.
- Lado, L.R.; Hengl, T.; Reuter, H.I. Heavy metals in European soils: A geostatistical analysis of the FOREGS Geochemical database. Geoderma 2008, 148, 189–199. [Google Scholar] [CrossRef]
- Del Delumyea, R.; Kalivretenos, A. Elemental carbon and lead content of fine particles from American and French cities of comparable size and industry, 1985. Atmos. Environ. (1967) 1987, 21, 1643–1647. [Google Scholar] [CrossRef]
- Frank, J.J.; Poulakos, A.G.; Tornero-Velez, R.; Xue, J. Systematic review and meta-analyses of lead (Pb) concentrations in environmental media (soil, dust, water, food, and air) reported in the United States from 1996 to 2016. Sci. Total Environ. 2019, 694, 133489. [Google Scholar] [CrossRef] [PubMed]
- Air Quality Guidelines for Europe; WORLD HEALTH ORGANIZATION: Geneva, Switzerland, 2000.
- Diapouli, E.; Manousakas, M.; Vratolis, S.; Vasilatou, V.; Maggos, T.; Saraga, D.; Grigoratos, T.; Argyropoulos, G.; Voutsa, D.; Samara, C.; et al. Evolution of air pollution source contributions over one decade, derived by PM10 and PM2.5 source apportionment in two metropolitan urban areas in Greece. Atmos. Environ. 2017, 164, 416–430. [Google Scholar] [CrossRef]
- Grigoratos, T.; Samara, C.; Voutsa, D.; Manoli, E.; Kouras, A. Chemical composition and mass closure of ambient coarse particles at traffic and urban-background sites in Thessaloniki, Greece. Environ. Sci. Pollut. Res. 2014, 21, 7708–7722. [Google Scholar] [CrossRef]
- Karageorgos, E.; Rapsomanikis, S. Chemical Characterization of the Inorganic Fraction of Aerosols and Mechanisms of the Neutralization of Atmospheric Acidity in Athens, Greece; Copernicus Publications on behalf of the European Geosciences Union; Copernicus Publications: Göttingen, Germany, 2007. [Google Scholar]
- Manoli, E.; Chelioti-Chatzidimitriou, A.; Karageorgou, K.; Kouras, A.; Voutsa, D.; Samara, C.; Kampanos, I. Polycyclic aromatic hydrocarbons and trace elements bounded to airborne PM10 in the harbor of Volos, Greece: Implications for the impact of harbor activities. Atmos. Environ. 2017, 167, 61–72. [Google Scholar] [CrossRef]
- Terzi, E.; Argyropoulos, G.; Bougatioti, A.; Mihalopoulos, N.; Nikolaou, K.; Samara, C. Chemical composition and mass closure of ambient PM10 at urban sites. Atmos. Environ. 2010, 44, 2231–2239. [Google Scholar] [CrossRef]
- Grivas, G.; Cheristanidis, S.; Chaloulakou, A.; Koutrakis, P.; Mihalopoulos, N. Elemental Composition and Source Apportionment of Fine and Coarse Particles at Traffic and Urban Background Locations in Athens, Greece. Aerosol Air Qual. Res. 2018, 18, 1642–1659. [Google Scholar] [CrossRef] [Green Version]
- Remoundaki, E.; Kassomenos, P.; Mantas, E.; Mihalopoulos, N.; Tsezos, M. Composition and Mass Closure of PM2.5 in Urban Environment (Athens, Greece). Aerosol Air Qual. Res. 2013, 13, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Theodosi, C.; Tsagkaraki, M.; Zarmpas, P.; Grivas, G.; Liakakou, E.; Paraskevopoulou, D.; Lianou, M.; Gerasopoulos, E.; Mihalopoulos, N. Multi-year chemical composition of the fine-aerosol fraction in Athens, Greece, with emphasis on the contribution of residential heating in wintertime. Atmos. Chem. Phys. 2018, 18, 14371–14391. [Google Scholar] [CrossRef] [Green Version]
- Directive 86/278/EEC of 12 June 1986. Protection of the Environment, and in Particular of the Soil, When Sewage Sludge is Used in Agriculture. 1986. Available online: http://gov.wales/topics/environmentcountryside/farmingandcountryside/farming/farm-regulations-wales/farmregs-wales-waste/directive-86-278-eec-sewage-sludgesoil (accessed on 20 September 2020).
- Langenkamp, H.; für Geowissenschaften, B.; Düwel, O.; Utermann, J. Progress Report Trace Element and Organic Matter Contents of European Soils. In First Results of the Second Phase of the “Short Term Action”; Ispra JRC: Ispra, Italy, 2001. [Google Scholar]


| Scenario | Typical Operating Conditions | Legal Limit Operating Conditions |
|---|---|---|
| Source | Point | |
| X (UTM35) | 374555.62 | |
| Y (UTM35) | 4546885.51 | |
| Height (m) | 19.7 m | |
| Diameter (m) | 2.5 m | |
| Stack exit gas temperature (°C) | 80 °C | |
| Gas exit flow rate (m3 s−1) | 44.4 m3 s−1 | |
| Gas exit velocity (m s−1) | 9.053 m s−1 | |
| Emission rates of the Pb particulate matter (g s−1) | 0.00016 g s−1 | 0.0222 g s−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karali, D.; Stavridis, A.; Loupa, G.; Rapsomanikis, S. Dispersion Effects of Particulate Lead (Pb) from the Stack of a Lead Battery Recycling Plant. Energies 2020, 13, 5690. https://doi.org/10.3390/en13215690
Karali D, Stavridis A, Loupa G, Rapsomanikis S. Dispersion Effects of Particulate Lead (Pb) from the Stack of a Lead Battery Recycling Plant. Energies. 2020; 13(21):5690. https://doi.org/10.3390/en13215690
Chicago/Turabian StyleKarali, Dimitra, Alexandros Stavridis, Glykeria Loupa, and Spyridon Rapsomanikis. 2020. "Dispersion Effects of Particulate Lead (Pb) from the Stack of a Lead Battery Recycling Plant" Energies 13, no. 21: 5690. https://doi.org/10.3390/en13215690
APA StyleKarali, D., Stavridis, A., Loupa, G., & Rapsomanikis, S. (2020). Dispersion Effects of Particulate Lead (Pb) from the Stack of a Lead Battery Recycling Plant. Energies, 13(21), 5690. https://doi.org/10.3390/en13215690







